Anti-filovirus monoclonal neutralizing antibody as well as preparation method and application thereof
A filovirus, monoclonal technology, applied in chemical instruments and methods, antiviral immunoglobulin, botanical equipment and methods, etc., to achieve the effect of high homology and good neutralizing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
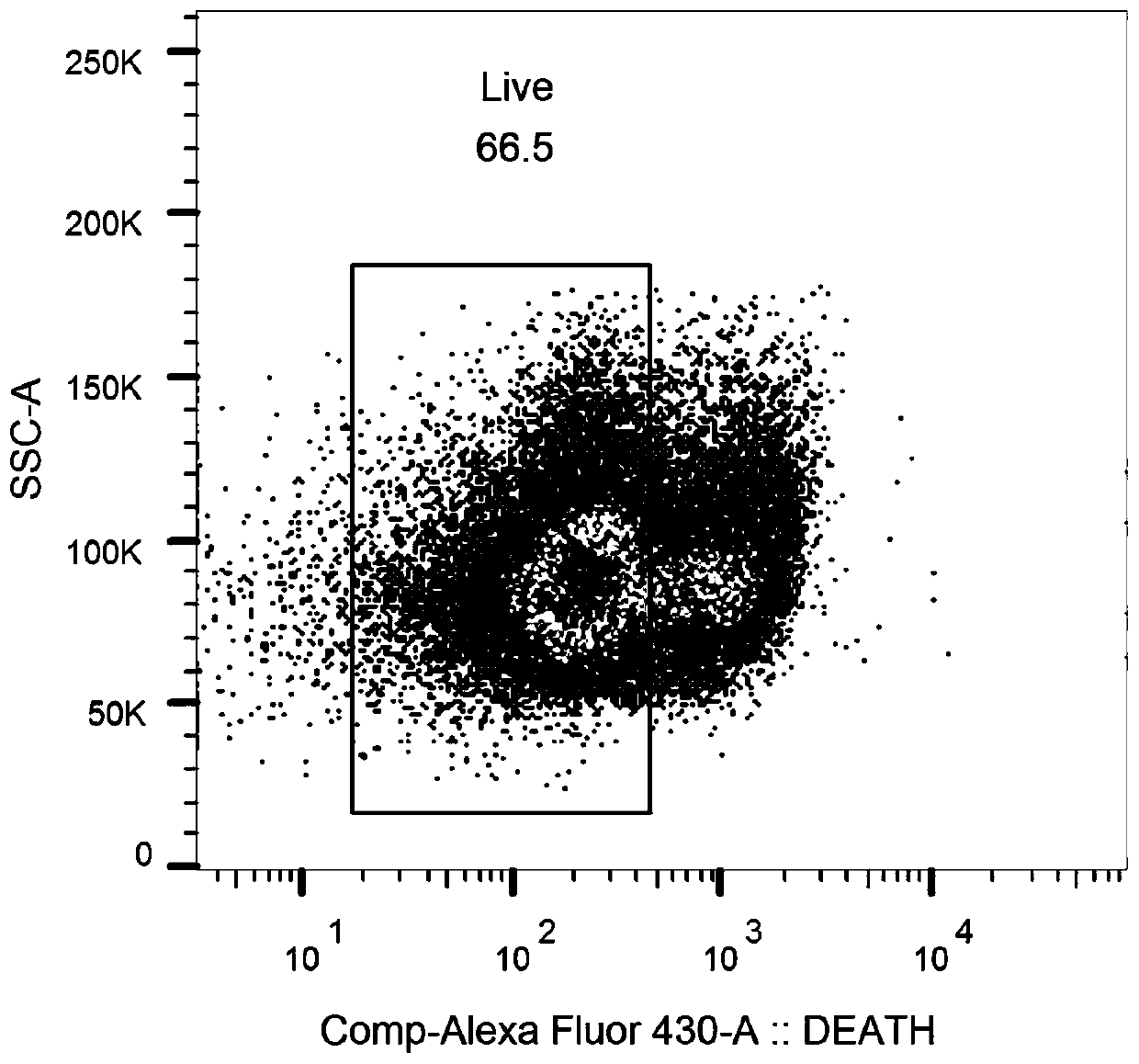
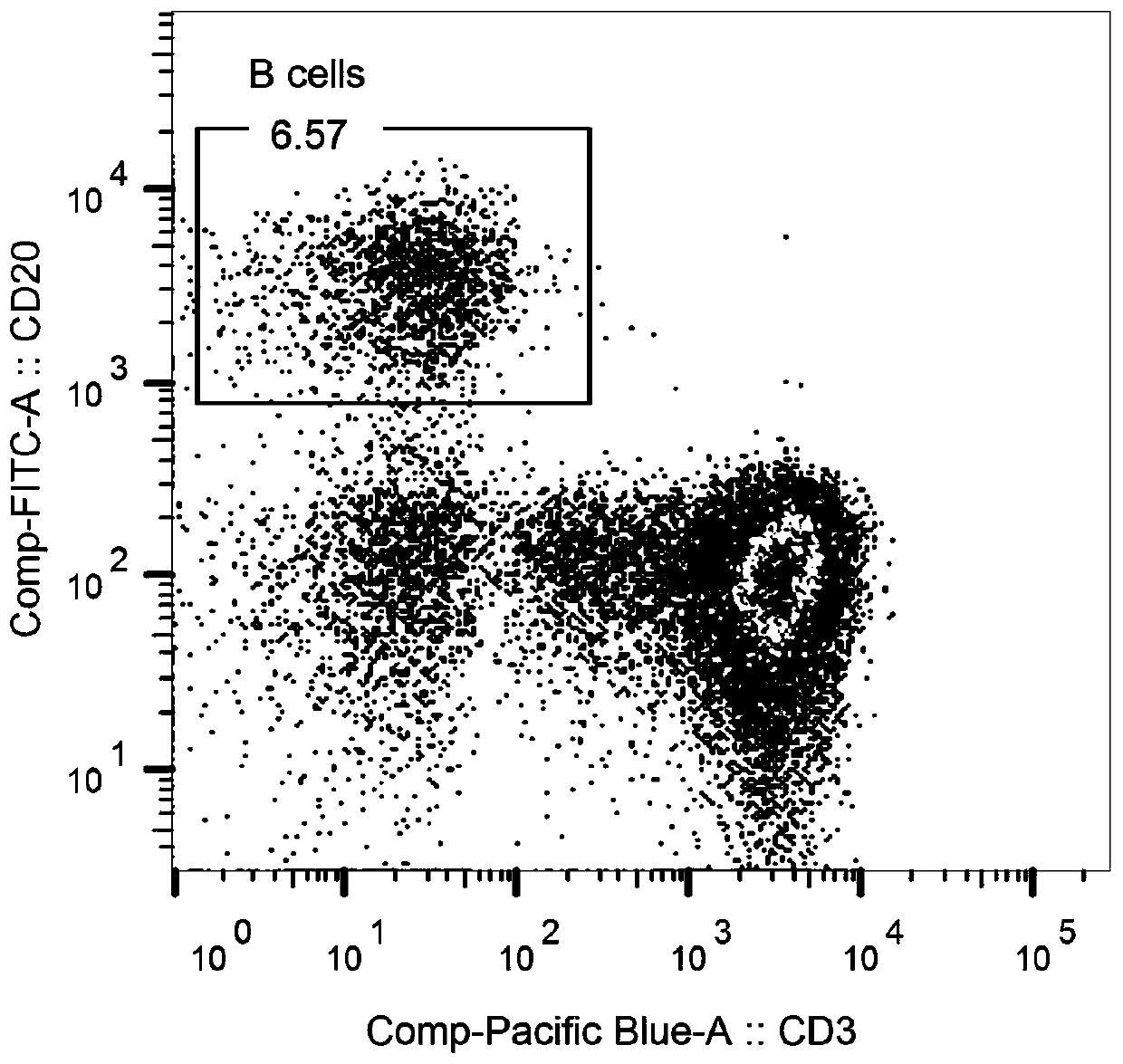
[0096] Example 2 Marking and sorting of antigen-specific B cells
[0097] The obtained rhesus monkey PBMC was labeled with antibody labeling technology, CD3 antibody, CD20 antibody, CD27 antibody, IgG antibody and anti-histidine antibody, and a single GP protein-specific B cell was sorted by flow cytometry. Proceed as follows:
[0098] (1) Centrifuge the prepared PBMCs to discard the incubation supernatant, add 1 mL of PBS buffer, centrifuge at 400×g for 5 min, and discard the supernatant;
[0099] (2) First add 1 μL Aqua, incubate at 4°C for 20 minutes, then add 1 mL PBS, centrifuge at 400×g for 5 minutes, and discard the supernatant;
[0100] (3) Add the fluorescently labeled antibodies shown in Table 1, stain at 4°C for 30 minutes, add 1 mL of PBS after staining, centrifuge at 400×g for 5 minutes, and finally add 300 μL of PBS to resuspend, incubate at 4°C in a refrigerator, and perform sorting on the machine.
[0101] Table 1 Antibody labeling system
[0102]
[0103...
Embodiment 3
[0104] Example 3 Isolation of Antibody Variable Region Genes from Single B Cells Using RT-PCR
[0105] The reverse transcription kit produced by Promega was used to prepare the cell lysate, which was divided into 96-well plates, and the cells were centrifuged. After adding the reverse transcription reagent, they were reversed into cDNA. After the reverse transcription was completed, they were stored at -80°C;
[0106] Using rhesus monkey-specific primers to amplify the heavy and light chain variable region genes of the antibody through nested PCR using B cell cDNA as a template, the 50 μL system contains 5 μL cDNA, HotStarTaq Plus enzyme, dNTPs, and 0.5 μM specific primers, Carry out PCR amplification according to the following conditions: pre-denaturation at 94°C for 5min; 94°C for 30s, 55°C for 30s, 72°C for 50s, 35 cycles; 72°C for 7min; the obtained PCR products were identified by 1% agarose gel electrophoresis, recombinant antibodies The electrophoresis results of 7D10 he...
Embodiment 4
[0108] Embodiment 4 constructs the expression vector of monoclonal neutralizing antibody
[0109] Use homologous recombination primers to add homologous recombination arms at the two ends of the antibody heavy chain variable region gene and the two ends of the light variable region gene, and use double enzymes to linearize the expression plasmid containing the human antibody heavy and light chain IgG1 constant regions Homologous recombination arm is generated; the variable region gene fragment added to the homologous recombination arm and the linearized plasmid are connected by homologous recombination to form a complete expression vector, in which the recombinant antibody heavy chain humanized plasmid The map is shown in Figure 4 (A) or Figure 4 (C), and the light chain humanized plasmid map is shown in Figure 4 (B) or Figure 4 (D). The recombinant product was transformed into TOP10 Escherichia coli competent, Amplify the plasmid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com