Neutralizing human monoclonal antibody aiming at novel coronavirus and application thereof
A coronavirus, cloned antibody technology, applied in applications, antibodies, antiviral agents, etc., can solve the problems of no effective preventive vaccines, no specific antiviral drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Expression and purification of novel coronavirus receptor binding domain (RBD) protein
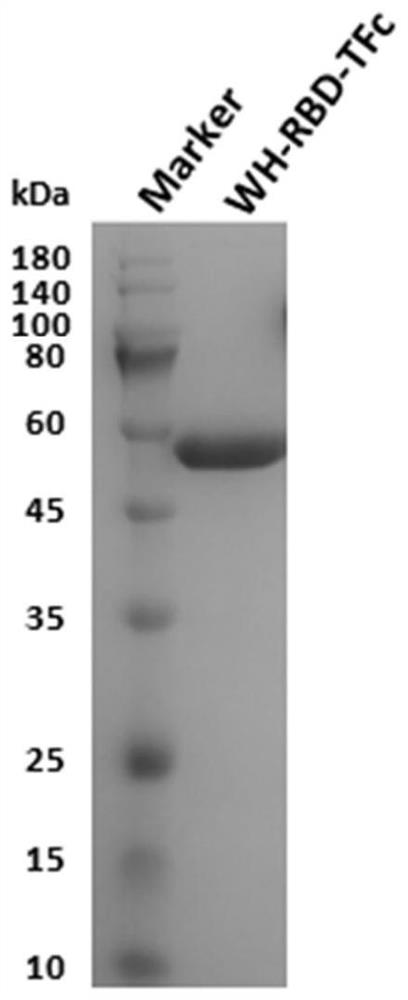
[0043]The amino acid sequence (319-541) of the extracellular domain of the spike glycoprotein (S protein) gene sequence (GenBank: QHR63250.2) of the new coronavirus SARS-CoV-2, the signal peptide from albumin at the N-terminal, and the thrombin site at the C-terminal The point and the Fc fragment tag of human IgG1 were constructed on the pSectag2A vector. The specific process was to convert the receptor binding domain (RBD, amino acid from 319 After fusion of 541) (WH-RBD), thrombin site (T) and the Fc fragment (Fc) of human IgG1, the enzyme was constructed into the pSecTag2A vector (ThermoFisherScientific, catalog number: V90020) by homologous recombination At the cutting site SfiI, the vector pSectag2A-WH-RBD-TFc was successfully obtained. Expressed in mammalian cell 293F expression system. 1 day before transfection, 293F cells (control cell density was 5×10 5 cells / ...
Embodiment 2
[0046] Example 2: Screening using phage Fab display library
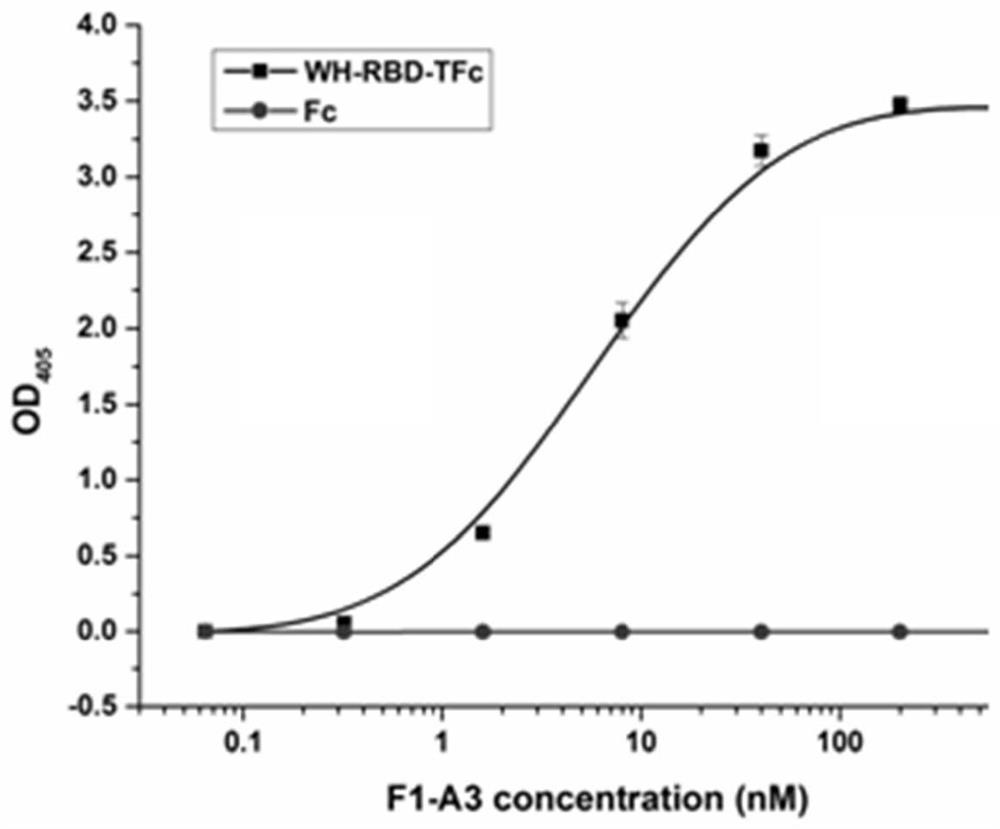
[0047] Using the antibody Fab phage display library, the receptor binding domain of the novel coronavirus spike glycoprotein (S) expressed in the mammalian cell 293F expression system was used as the antigen, and the phage Fab library was panned by the immunomagnetic bead method, and the specific phage was screened. Antigen capture, washed with PBS+0.05% Tween-20, after 4 rounds of screening, and monoclonal identification and sequencing, a clone with neutralizing activity on live virus in vitro was obtained, named Fab-F1-A3.
[0048] Sequence listing of Fab-F1-A3
[0049]
Embodiment 3
[0050] Example 3: Expression and purification of Fab-F1-A3
[0051] Fab-F1-A3 was expressed and purified according to existing literature (Zhu Z, Dimitrov DS. Methods Mol Biol. 2009; 525:129-4). The Fab-F1-A3 prokaryotic expression vector was constructed and transformed into E.coli HB2151. Re-inoculate the strains in SB medium containing 100 μg / ml ampicillin (1L medium contains 30g tryptone, 20g yeast extract and 10g MOPS, the pH value is adjusted to 7.0 with NaOH), and when the OD600 reaches 0.7-1.0, add The final concentration of IPTG was 200 μg / ml, and the expression was induced for 14-16 hours at 37° C. and 220 rpm. The cells were collected by centrifugation at 4°C, 6000 rpm, and 15 min, the medium was discarded, the pellet was resuspended in 1×PBS, and the supernatant was collected by centrifugation after being treated with polymyxin B for 45 min. Purified with Ni-NTA filler and verified its purity by SDS-PAGE. The replacement buffer was then ultrafiltered using an ult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com