Method for improving pichia pastoris recombinant expression hyaluronidase
A technology of hyaluronidase and Pichia pastoris, applied in the field of bioengineering, can solve the problem of low expression of hyaluronidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Construction of expression vector containing signal peptide hyaluronidase gene (nsB-HaseA3887)
[0024] 1. Signal peptide nsB
[0025] By PCR, a 6×His-tag tag is fused upstream of the leech hyaluronidase whose nucleotide sequence is shown in SEQ ID NO.2 to obtain the hyaluronidase gene HaseA3887. According to the sequence of the signal peptide nsB gene and the hyaluronidase Fusion primers were designed for the full-length gene sequence of HaseA3887, which were divided into three sections for fusion, and the signal peptide nsB with the nucleotide sequence shown in SEQ ID NO.1 was gradually added to the N-terminal of the HaseA3887 gene sequence. Primers were designed as follows:
[0026] nsB1:
[0027] ACTTGCGTTGCAGCCACTCCTTTGGTGAAGCGTCACCACCACCACCACATGAAAGAnsB2:
[0028] TACTCTCTCTGACCGGTGTGGCTGGTGTGCTTGCGACTTGCGTTGCAGCCACTCCTTTGnsB3:
[0029]CGCGGATCCAAACGATGAAGCTACTCTCTCTGACCGGTGTGGCT
[0030] nsB1, nsB2, and nsB3 are used as upstream primers, and the up...
Embodiment 2
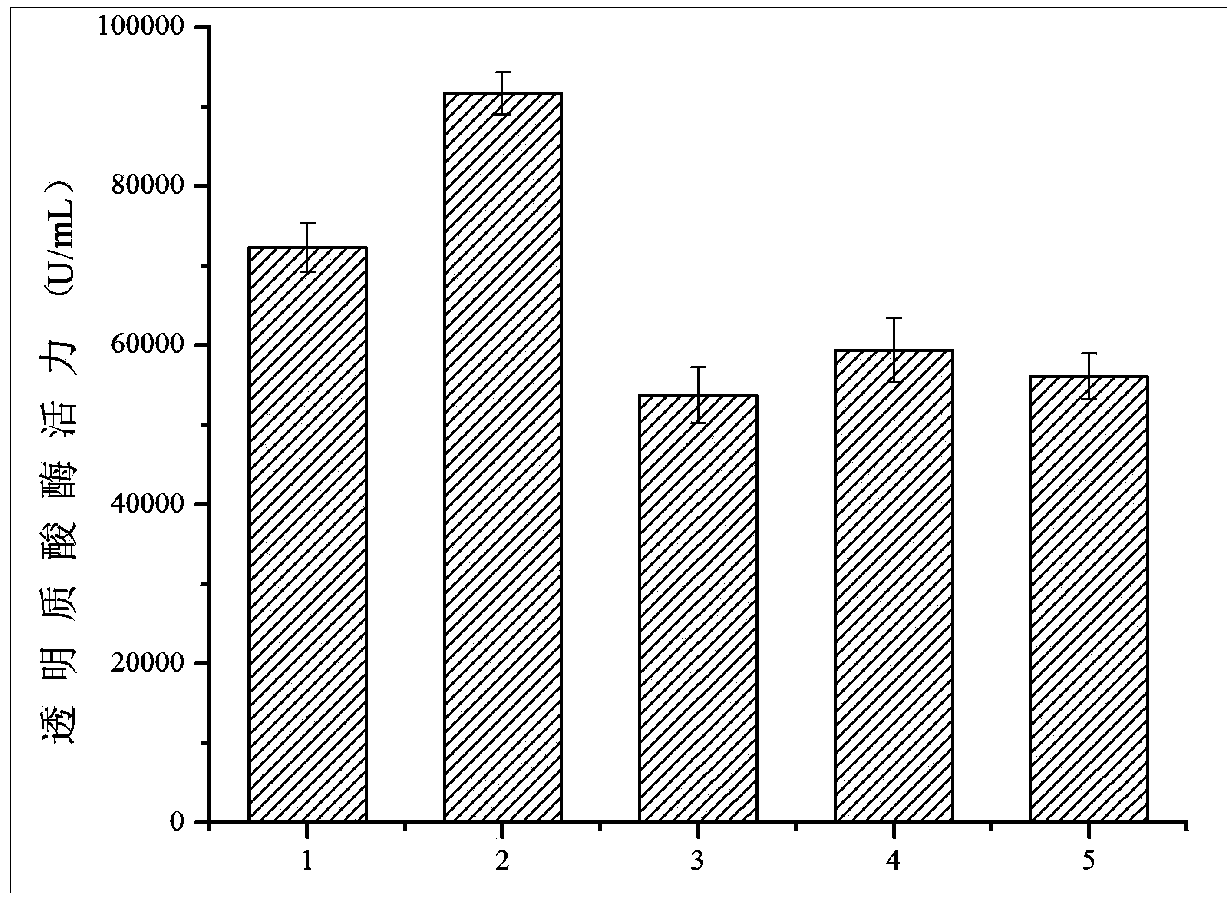
[0048] Embodiment 2 Shake flask fermentation
[0049] Recombinant ɑ-factor-HaseA3887HF-pPIC9K-P.pastoris GS115 / NT-his, nsB-HaseA3887HF-pPIC9K-P.pastoris GS115 / NT-his, YTP1-HaseA3887HF-pPIC9K-P.pastoris GS115 / NT-his, HKR1 -HaseA3887HF-pPIC9K-P. pastoris GS115 / NT-his, SCS3-HaseA3887HF-pPIC9K-P. pastoris GS115 / NT-his were fermented. Single clones were inoculated in 10 mL of YPD medium (yeast extract 10 g / L, peptone 20 g / L, glucose 20 g / L), and cultured at 30°C and 200 rpm for 24 hours. Transfer to 50ml induction expression medium BMGY (yeast extract 10g / L, peptone 20g / L, 3g / L K 2 HPO 4 , 11.8g / L KH 2 PO 4 , 10×YNB100ml / L (13.4g / L), 500×biotin 1mL (4×10 -4 g / L), glycerol 10mL), cultured at 25°C 200rpm to OD 600 When the value is between 4, the thalline is collected by centrifugation, and replaced with 50ml induction expression medium BMMY (yeast extract 10g / L, peptone 20g / L, 3g / L K 2 HPO 4 , 11.8g / L KH 2 PO 4 , 100×YNB100mL / L (13.4g / L), 500×biotin 1mL (4×10 -4 g / L), met...
Embodiment 3
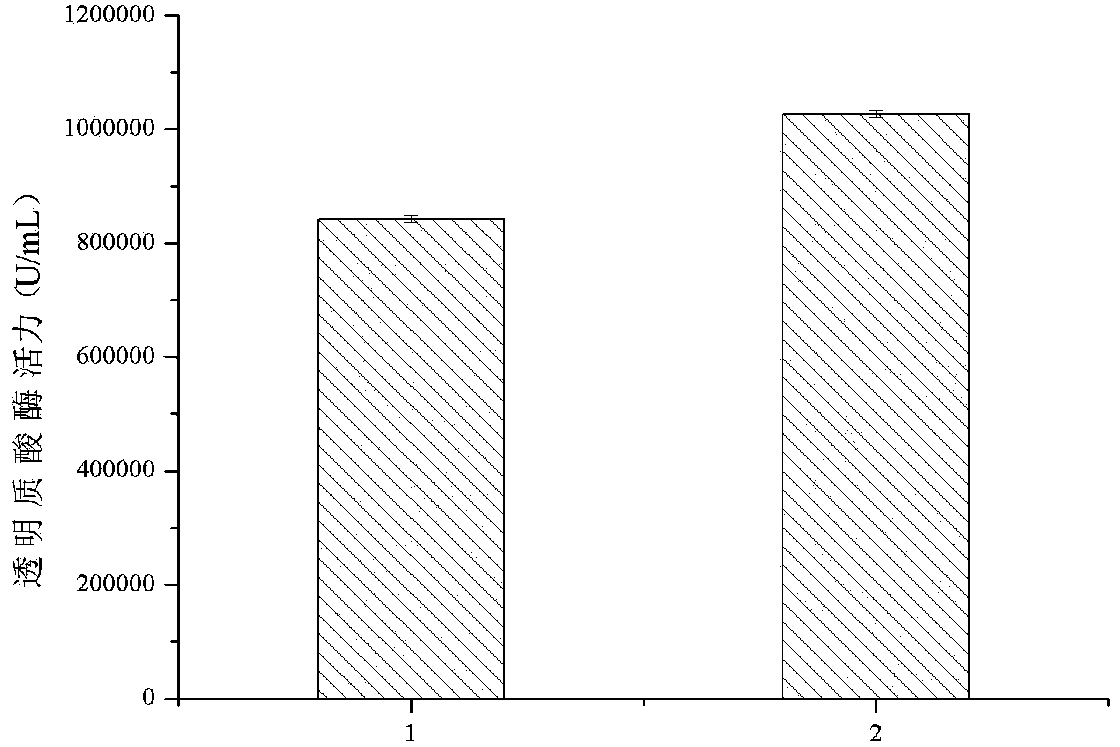
[0051] Example 3 Induced Expression of Recombinant Bacteria nsB-HaseA3887HF-pPIC9K-P.pastoris GS115 / NT-his on a 3L Fermenter
[0052] The recombinant nsB-HaseA3887HF-pPIC9K-P.pastoris GS115 / NT-hiss clone was expressed in fermentation culture. Single clones were inoculated in 50 mL of YPD medium (yeast extract 10 g / L, peptone 20 g / L, glucose 20 g / L), and cultured at 30°C and 200 rpm for 24 hours. Inoculated with 800mL BSM medium (85% phosphoric acid 26.7mL / L, CaSO 4 ·H 2 O0.93g / L, K 2 SO 4 18.2g / L, MgSO 4 ·7H 2 O14.9g / L, KOH4.13g / L, glycerol 40.0g / L, PTM1 4.35mL / L) 3L fermenter, the fermentation conditions are: temperature is 30 ℃, 200rpm, pH is 5.5 and is cultivated to OD 600 is 80, start feeding culture, add 12mL / LPTM1 (CuSO 4 5H 2 O 6g / L, KI0.09g / L, MnSO 4 h 2 O 3g / L, H 3 BO 3 0.02g / L, MoNa 2 o 4 2H 2 O 0.2g / L, CoCl 2 0.5g / L, ZnCl 2 20g / L, FeSO 4 7H 2 O 65g / L, Biotin 0.2g / L, H 2 SO 4 5.0mL / L) of glycerol, feed volume (mL) is as follows in Table 1:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com