Fusion protein and coding gene and application thereof
A technology of fusion protein and coding gene, which is applied in the field of preparation of drugs for treating inflammation and autoimmune diseases, can solve problems such as complex process, low recovery rate, and short half-life, and achieve broad application prospects, short production cycle, large-scale practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
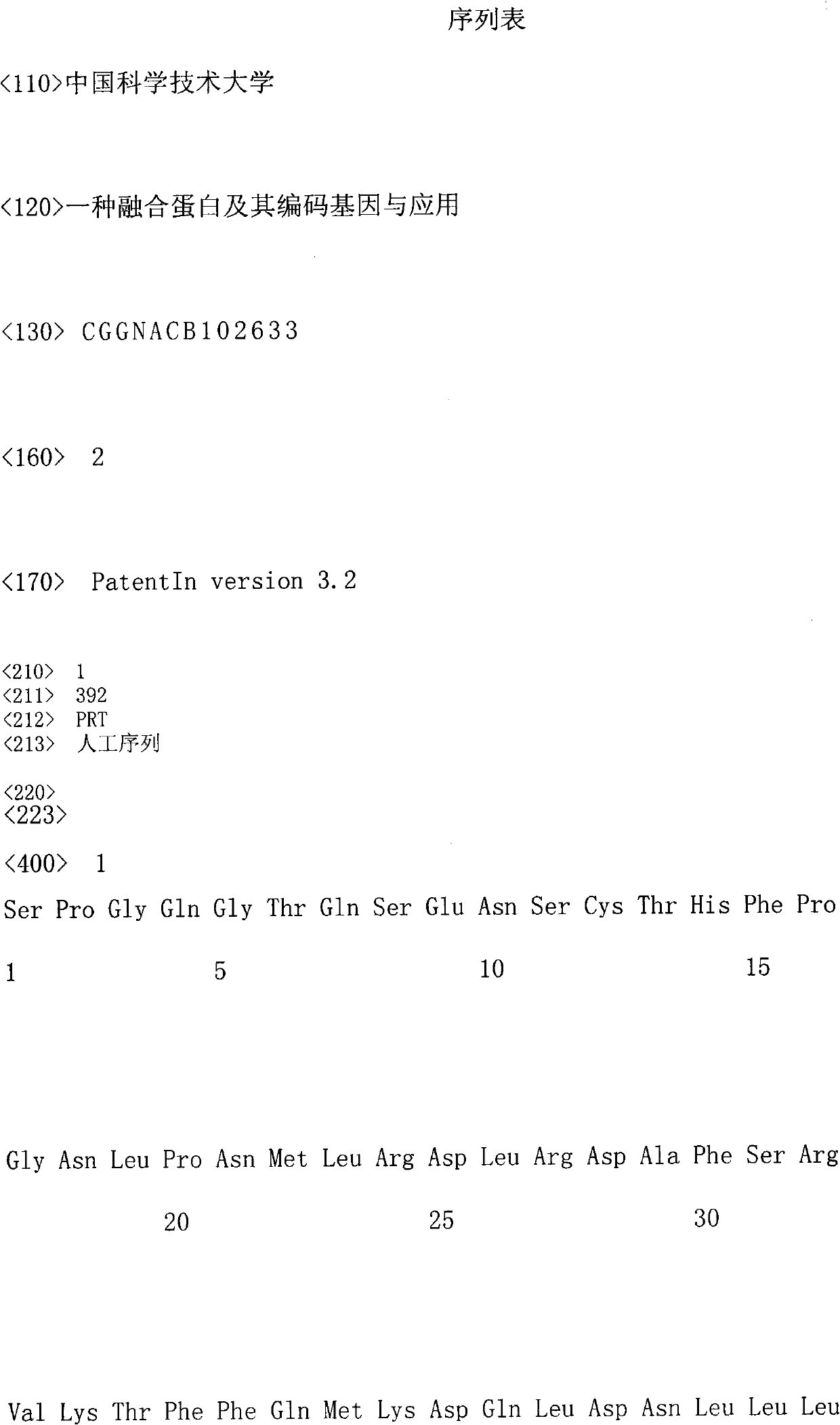
[0048] Example 1. Acquisition of a gene encoding a fusion protein with interleukin-10 activity
[0049] Amplify the coding gene of the fusion protein with interleukin-10 activity of the present invention by PCR method, and the specific process comprises the following steps:
[0050] 1. Amplification of human IL-10 gene
[0051] Use TRIzol (purchased from Invitrogen Company, Cat. No. 15596-018) and extract the total RNA of mononuclear cells from isolated healthy human peripheral blood (purchased from Hefei Blood Bank) according to its instructions, and use TRIzol (purchased from Invitrogen Company, Cat. No. 15596-018) , Cat. No. PC108), oligo dT (purchased from Shanghai Sangong, Cat. No. B0181) was used as a primer to synthesize human cDNA. Using the synthesized cDNA as a template, the forward primer 5'-ATGCACAGCTCAGCACTGCTCTG-3' and the reverse primer 5 PCR was carried out under the guidance of '-GTTTCGTATCTTCATTGTCATG-3', and the PCR product was inserted into the TA cloning ...
Embodiment 2
[0062] Example 2, Expression of α-Factor-IL-10-Fc-γ1 in Pichia pastoris and purification of expression products
[0063] 1. Construction of Pichia pastoris expression vector containing α-Factor-IL-10-Fc-γ1
[0064] see figure 1Construct the Pichia pastoris expression vector containing α-Factor-IL-10-Fc-γ1, the specific method is: use restriction endonuclease BamH I (purchased from Promega, product number R6021) and EcoR I (purchased from Promega, product number R6011 ) the PCR product obtained in Example 1 (α-Factor-IL-10-Fc-γ1) was double digested, and then the digested fragment was digested with the same enzyme double digested plasmid pPIC9K with T4 DNA ligase (purchased from Shanghai Sangong, Cat. No. EL0015) were ligated, the ligated products were transformed into E. coli DH5α competent cells, positive recombinants were screened, plasmids were extracted, and sequenced. The sequencing results showed that the plasmid was inserted between the recognition sites of BamHI and E...
Embodiment 3
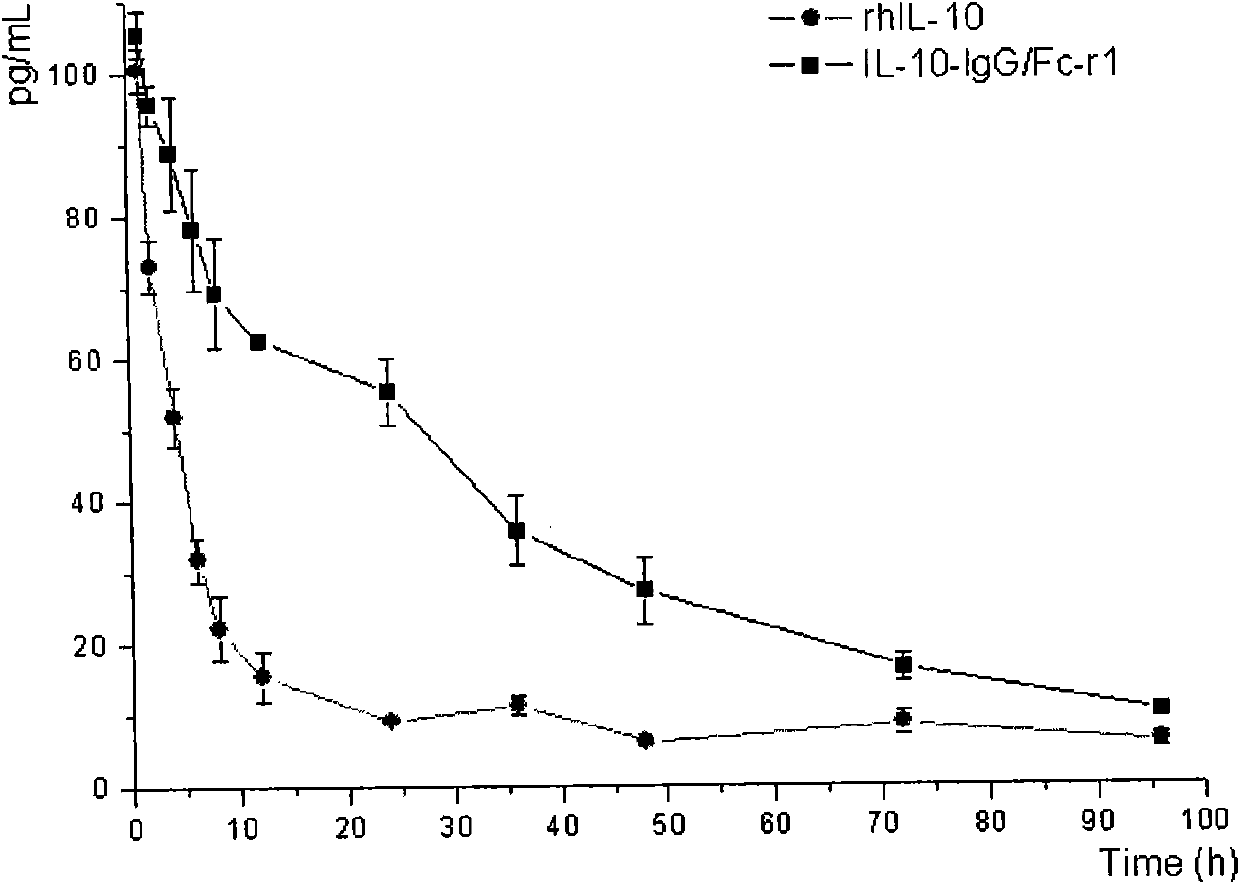
[0089] Example 3, IL-10 activity detection of fusion protein
[0090] Peripheral blood lymphocyte (PBMC) medium RPMI-1640 (purchased from HyClone, product number SH30809.01B);
[0091] Peripheral blood (purchased from Hefei Blood Bank) was taken from healthy people, and peripheral blood lymphocytes (PBMCs) were separated using Ficoll (purchased from Tianjin Haoyang Biological Products Technology Co., Ltd., product number: LTS1077) according to its instructions. Add 0.1ml human PBMC (2×10 5 cells), were added with different dilution concentrations of the fusion protein (IL-10-IgG / Fc-γ1) purified by S200 molecular sieve chromatography obtained in Example 2 and commercial rhIL-10 (purchased from Rochy Hill Company, article number: 200-10), each dilution set three duplicate wells, at 37 ° C 5% CO 2 After culturing for 24 hours, phytohemagglutinin PHA-P (purchased from sigma, product number: L8754) with a final concentration of 0.1ug / ml was added, and no PHA-P was added to the bl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com