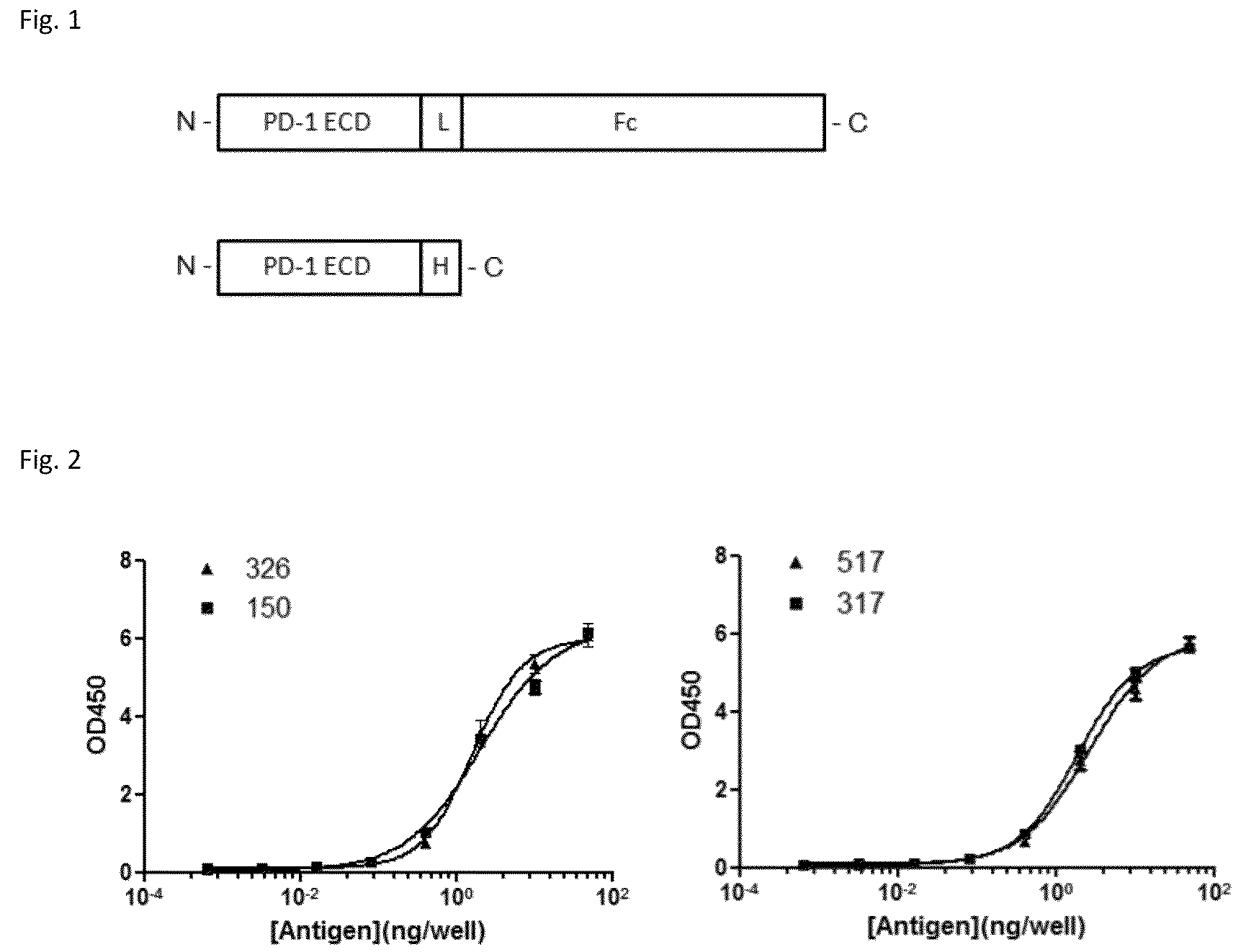
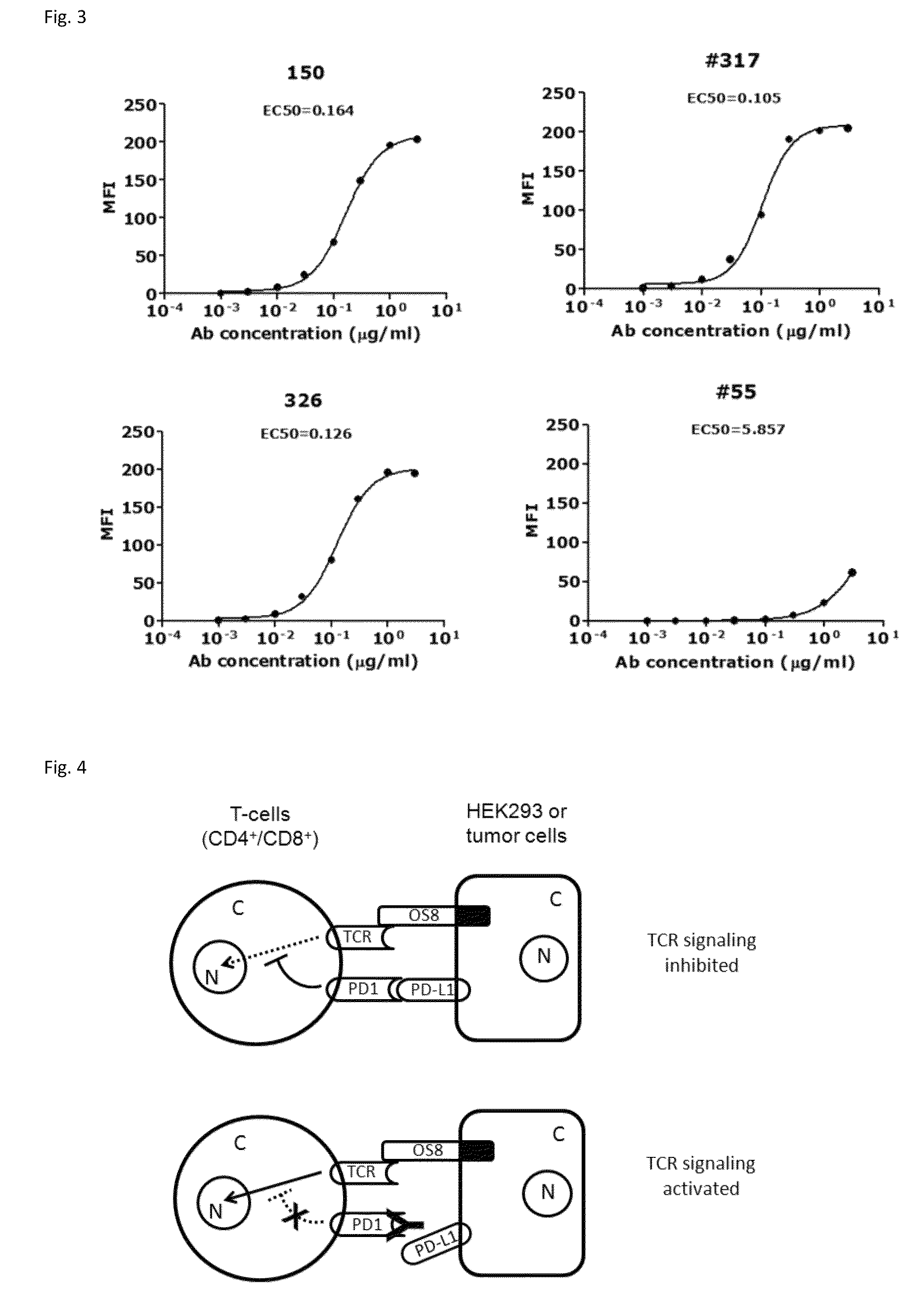
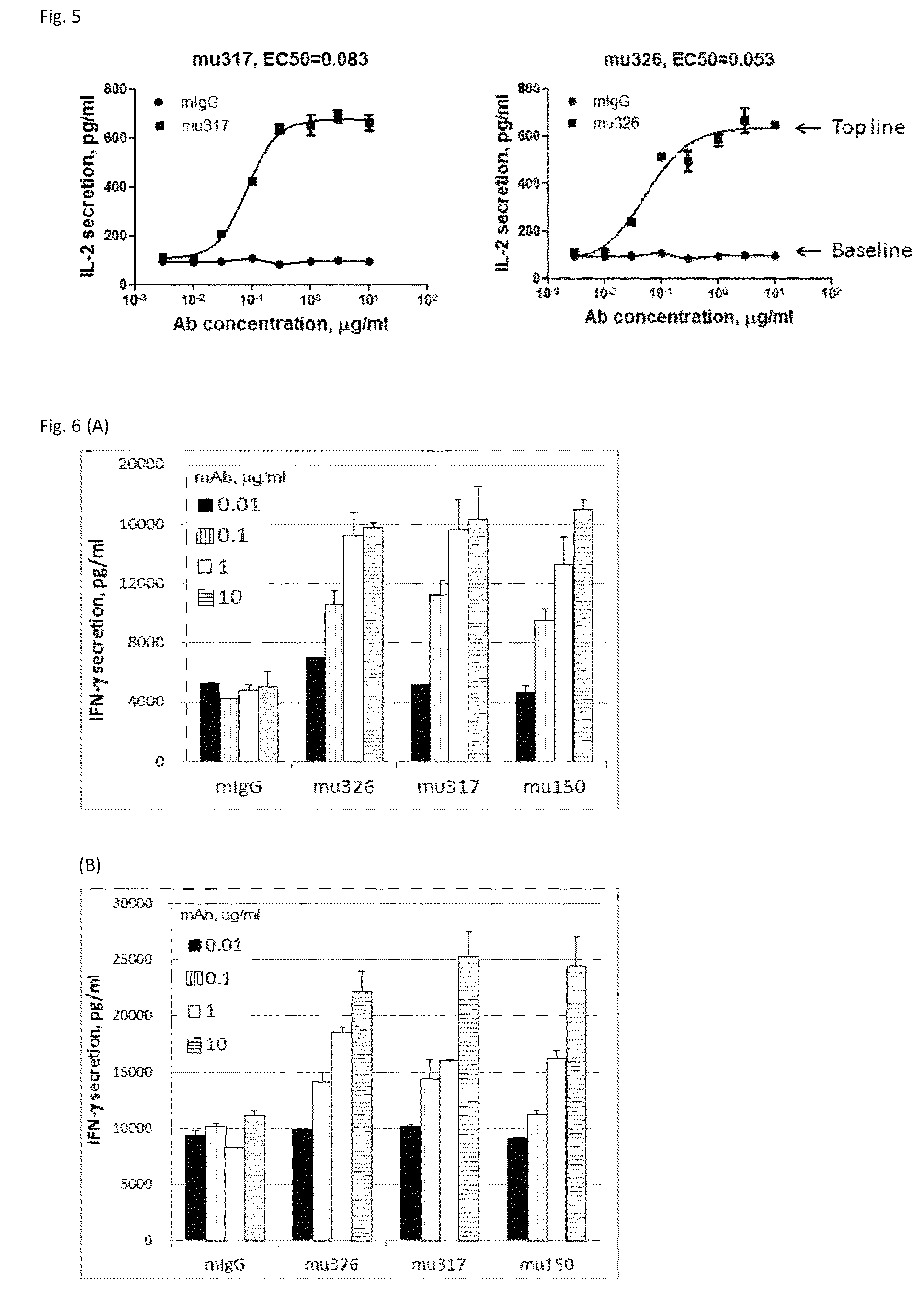
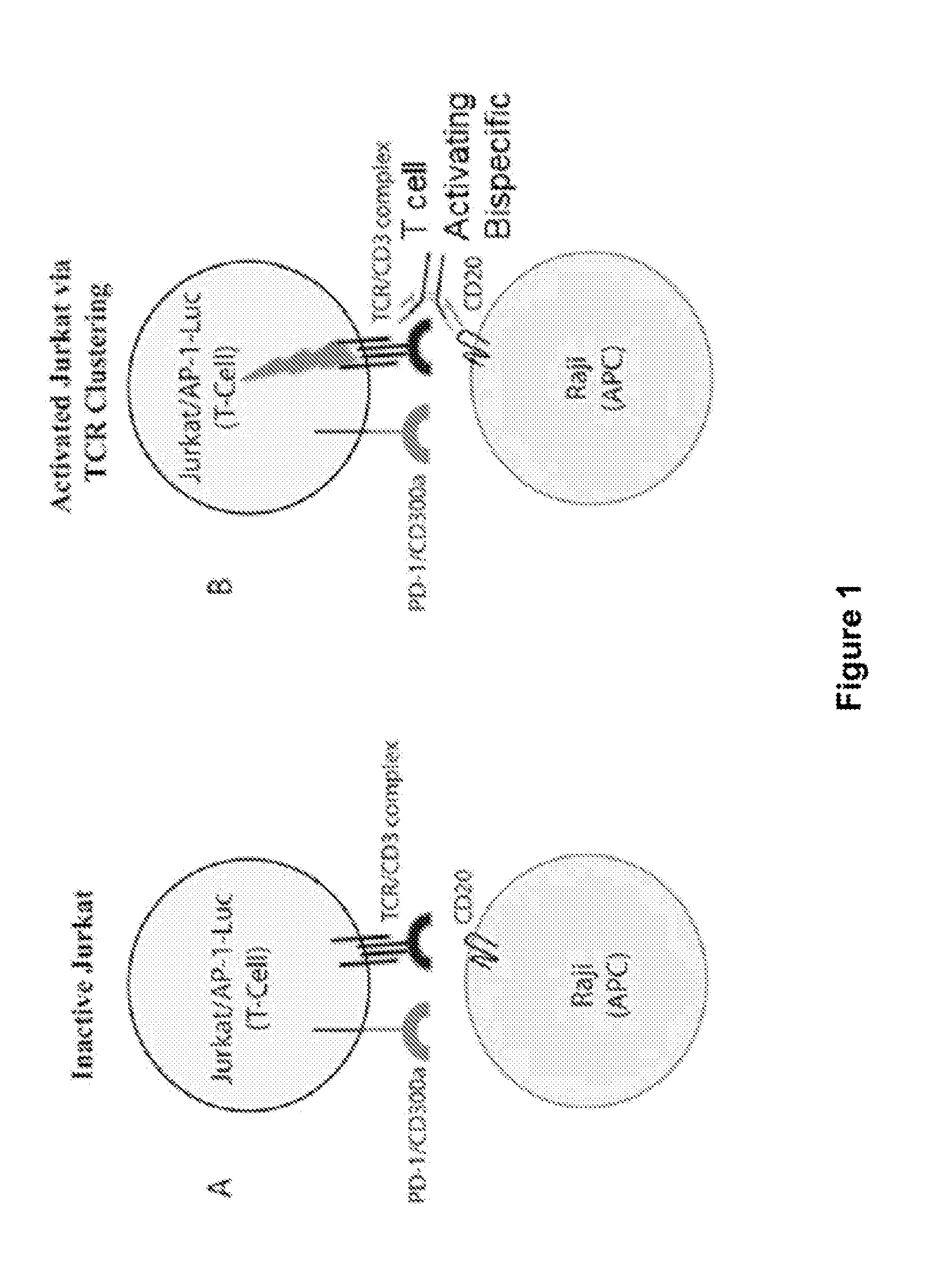
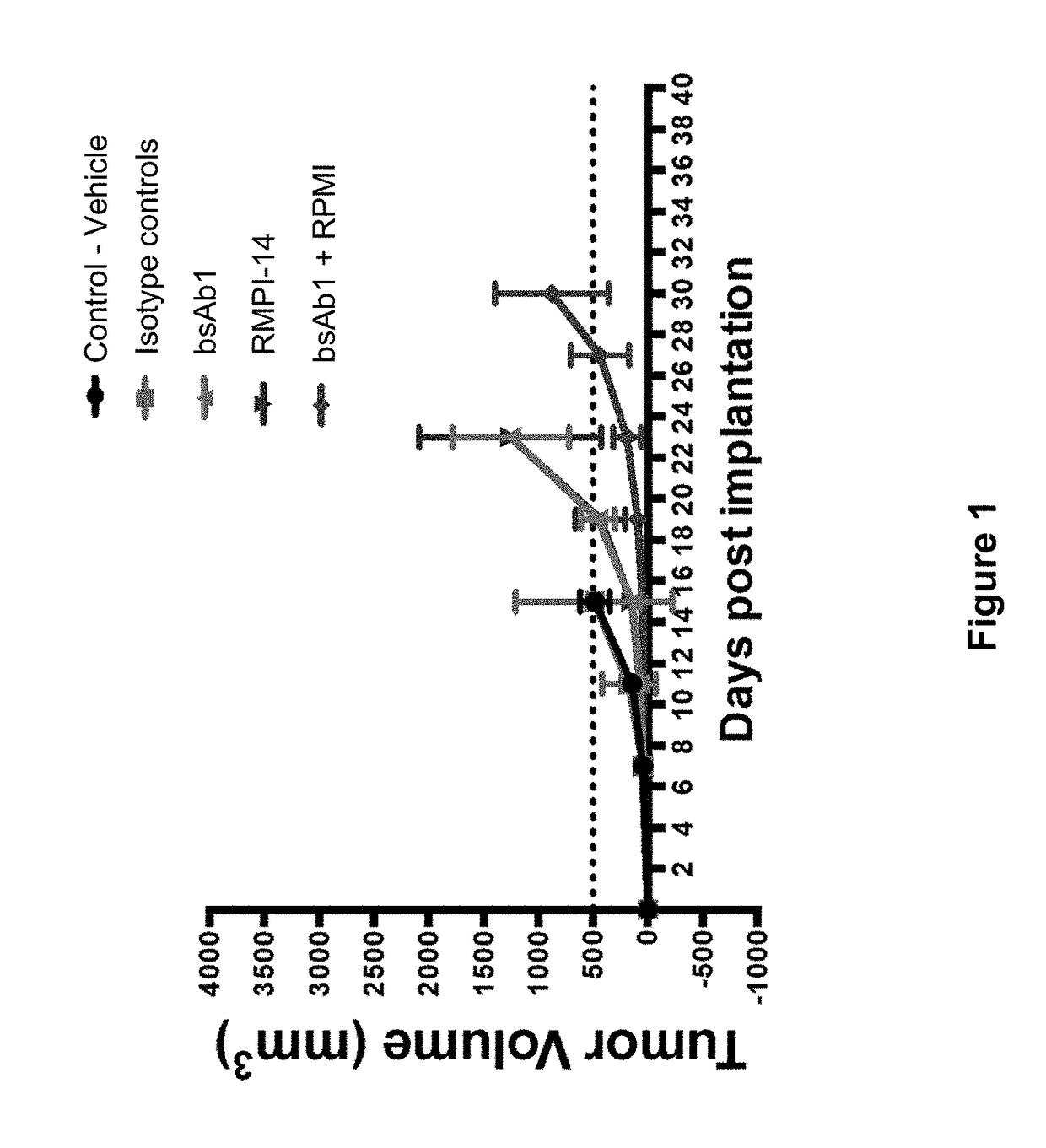
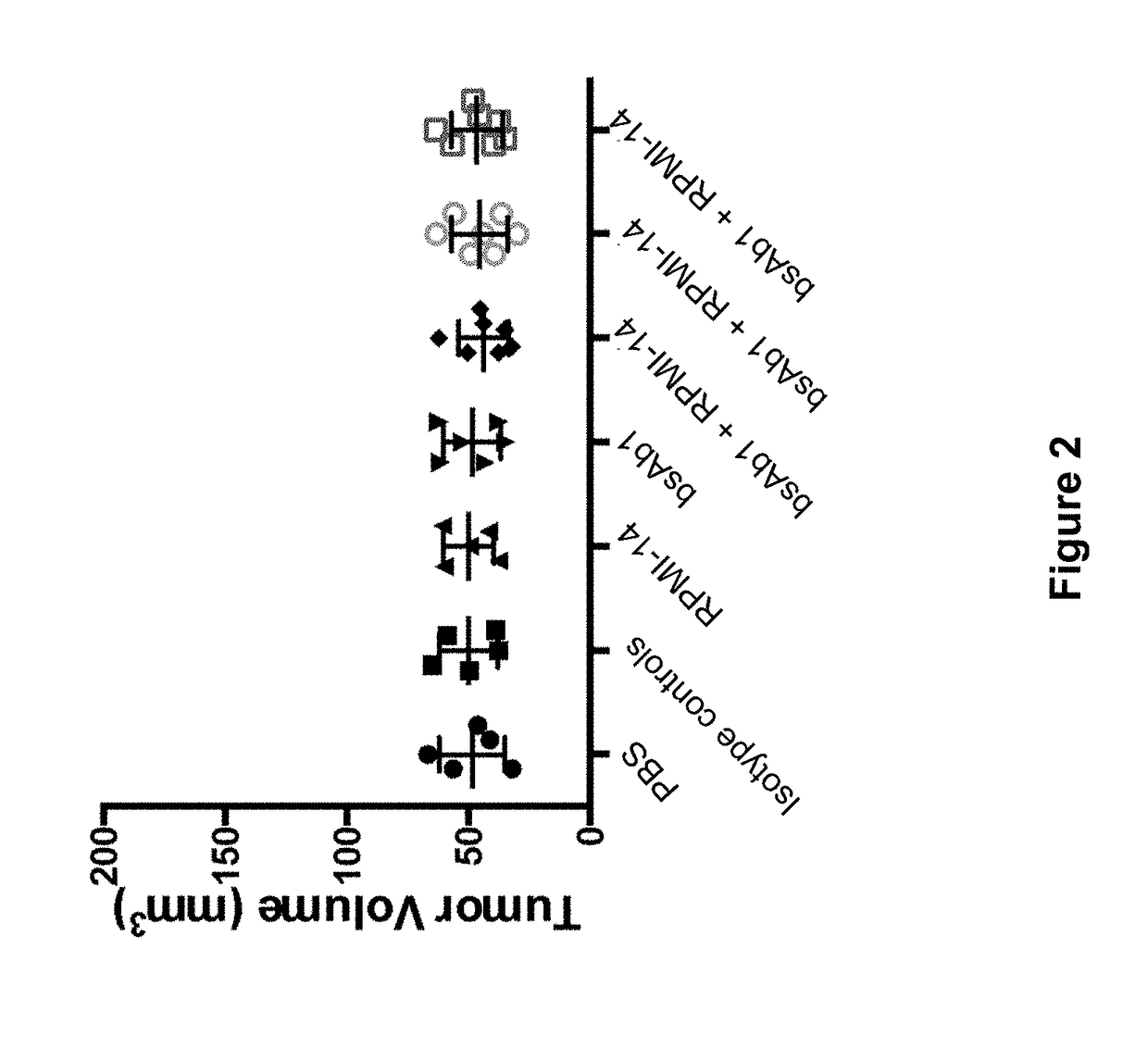
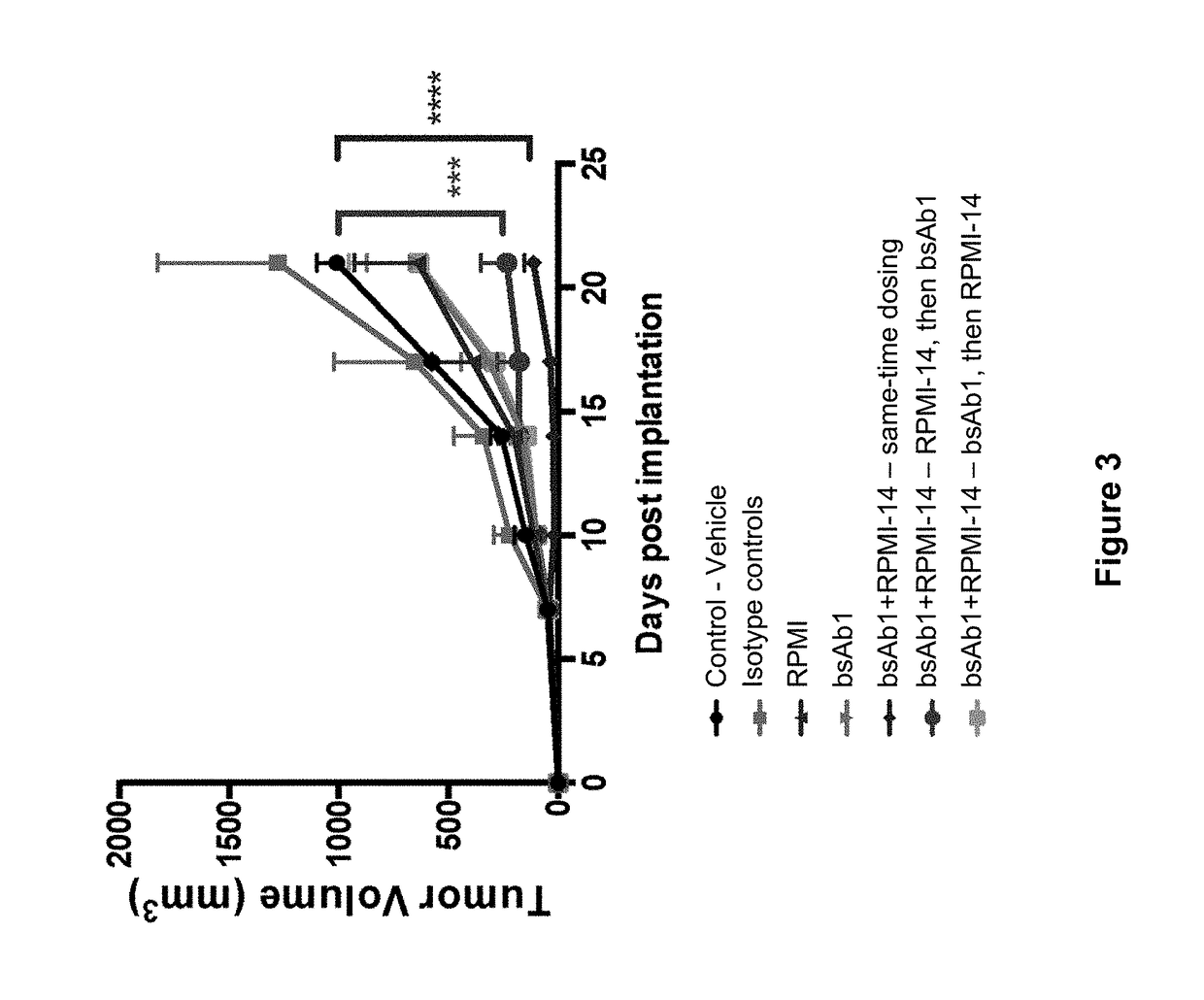
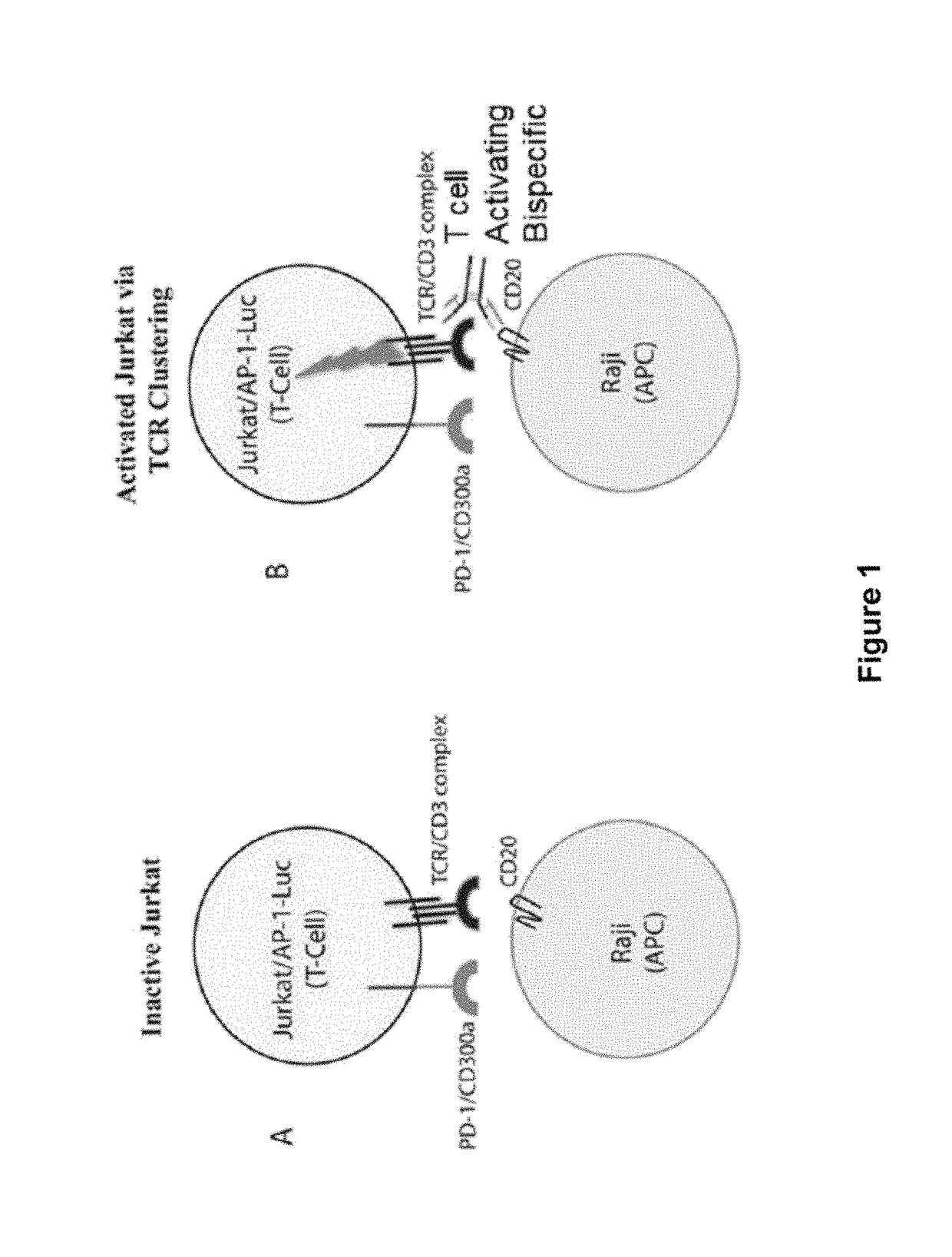
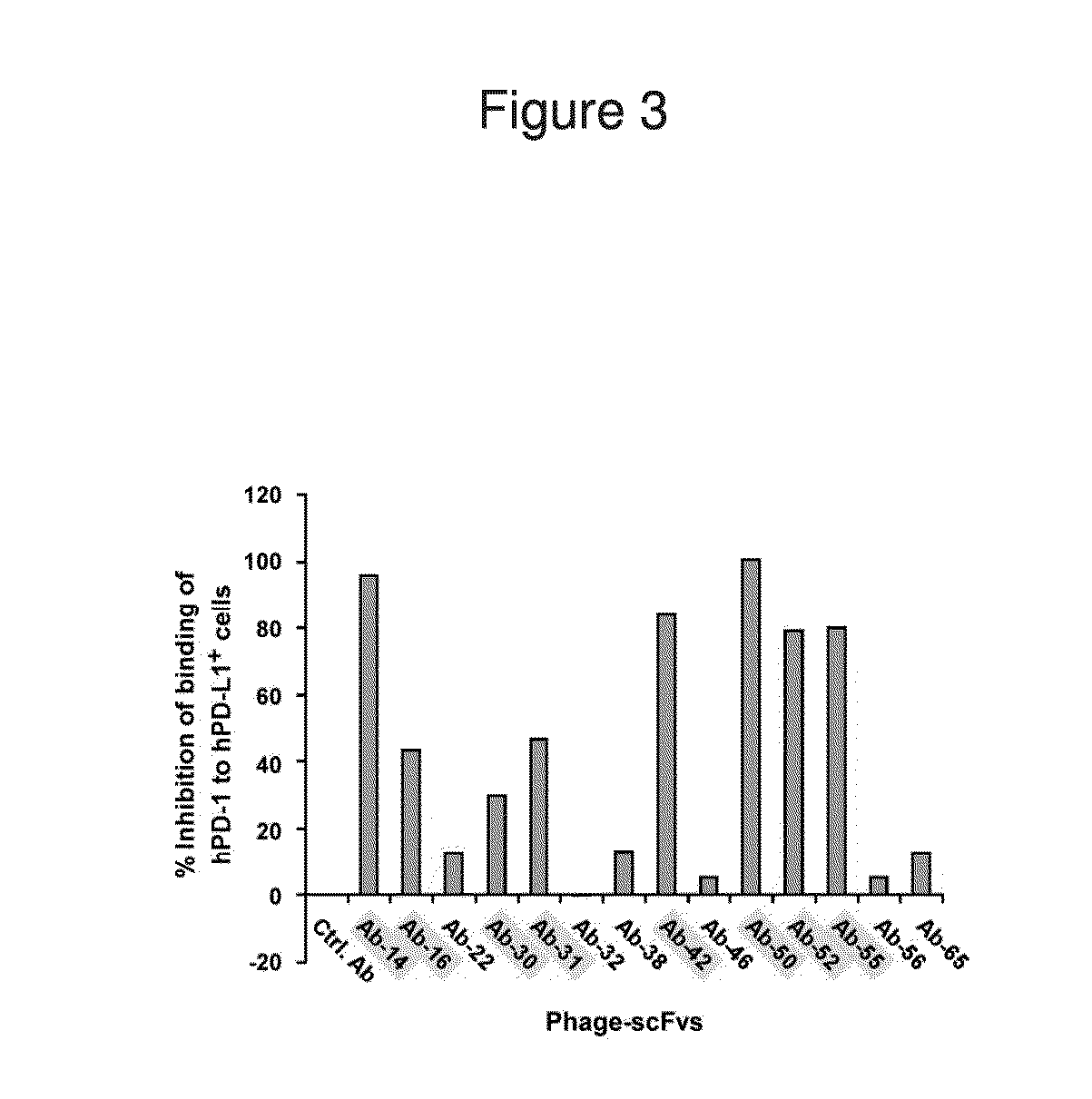
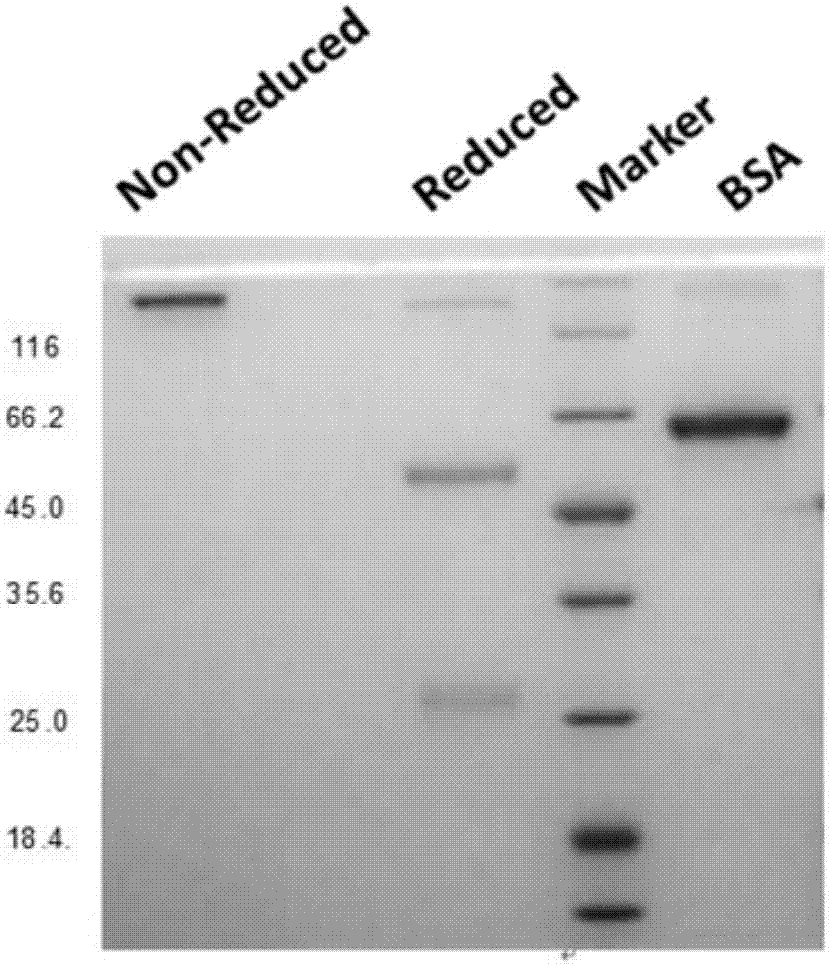
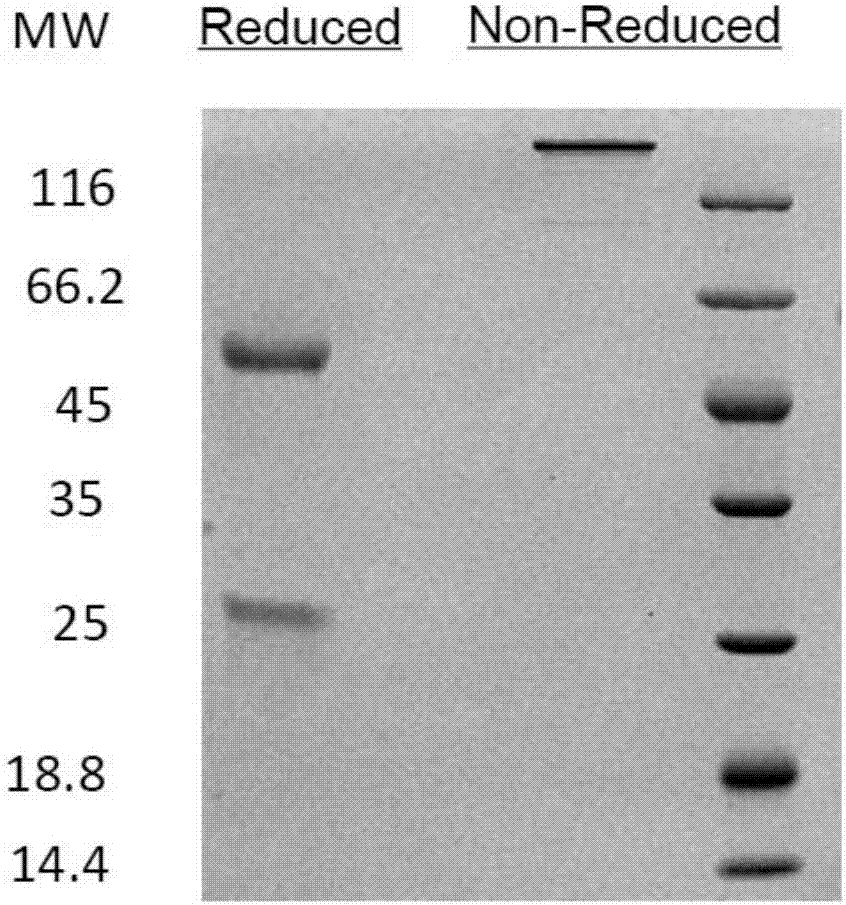
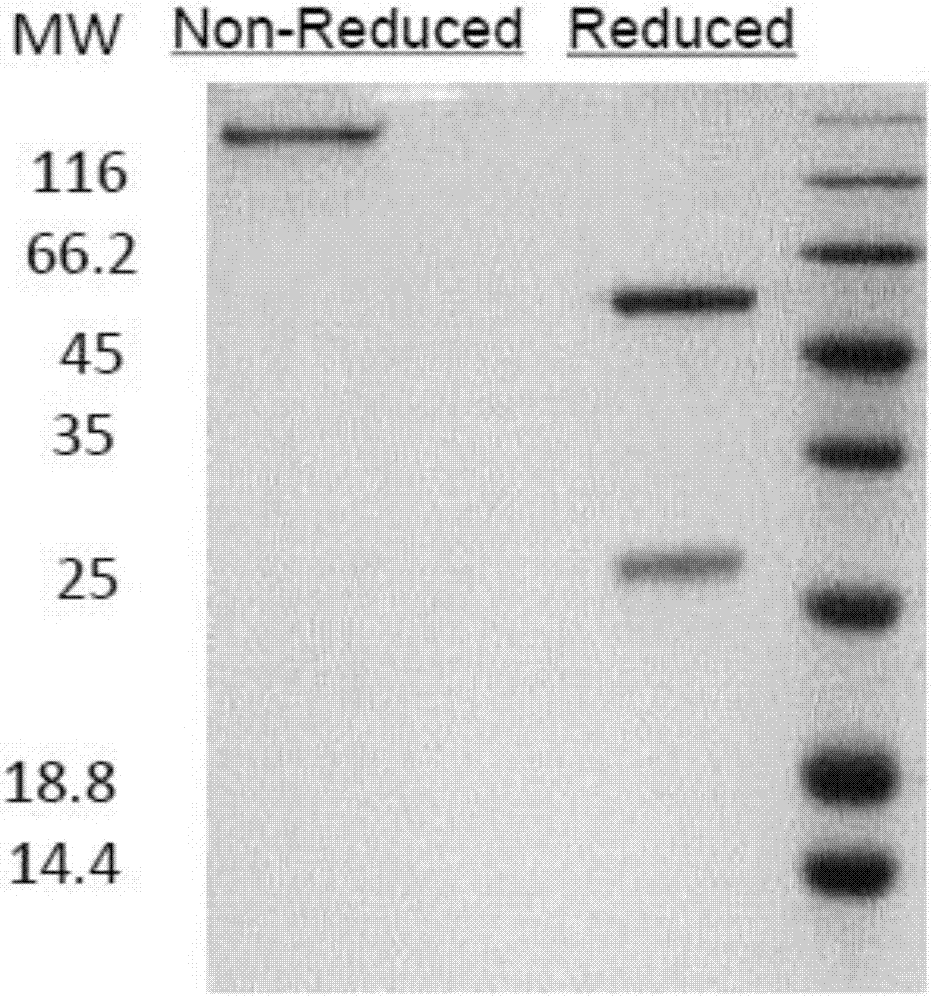
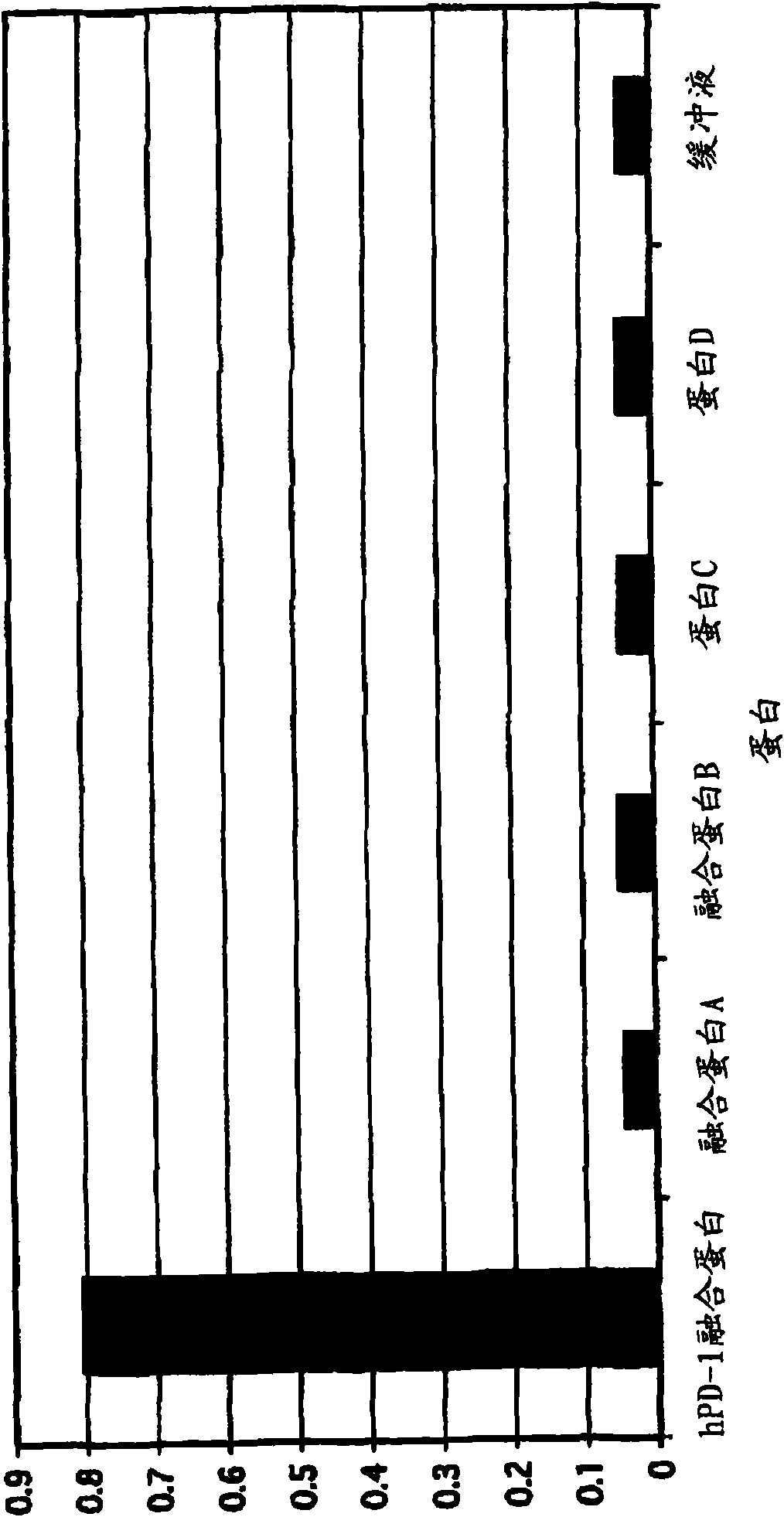
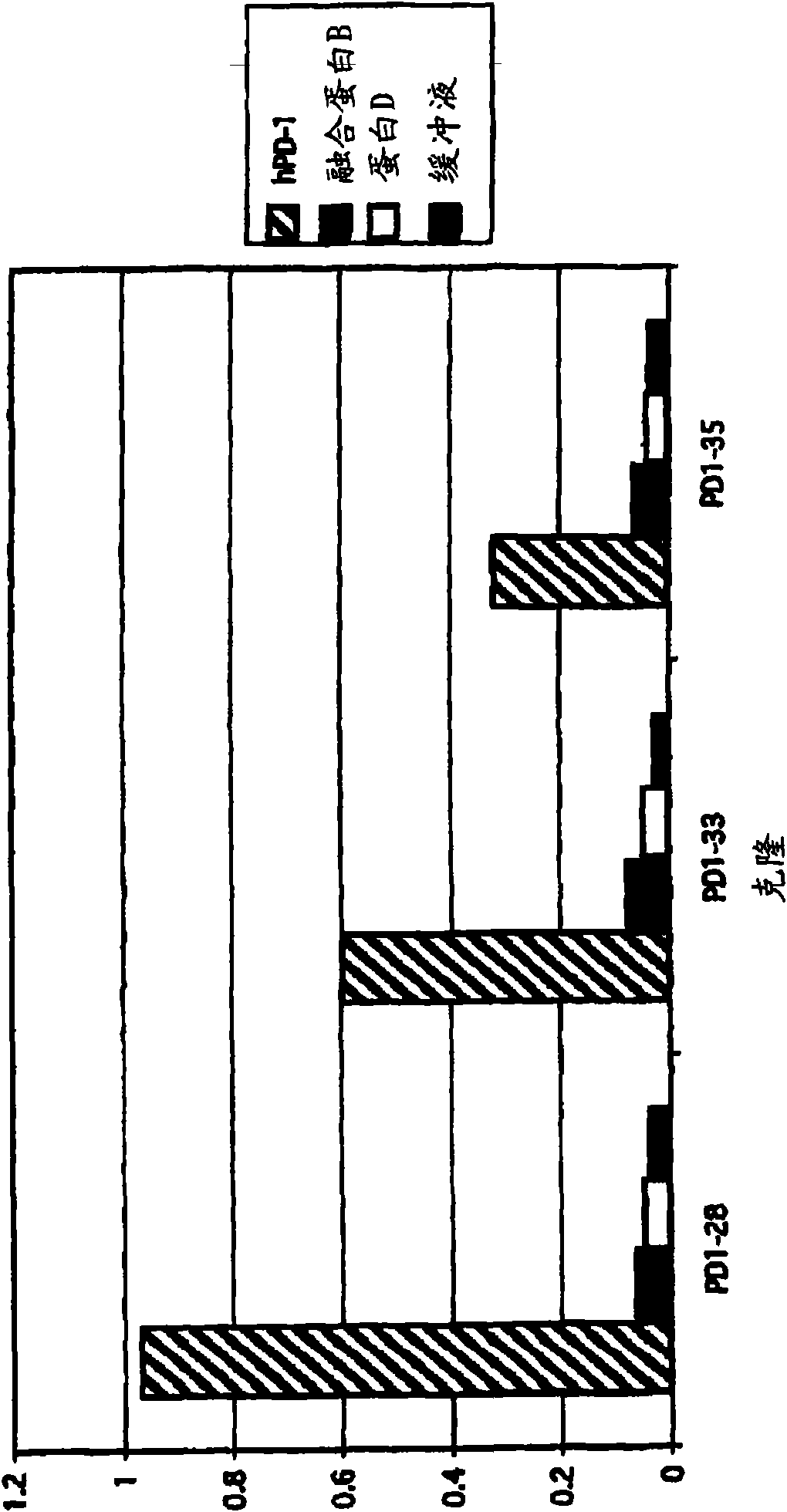
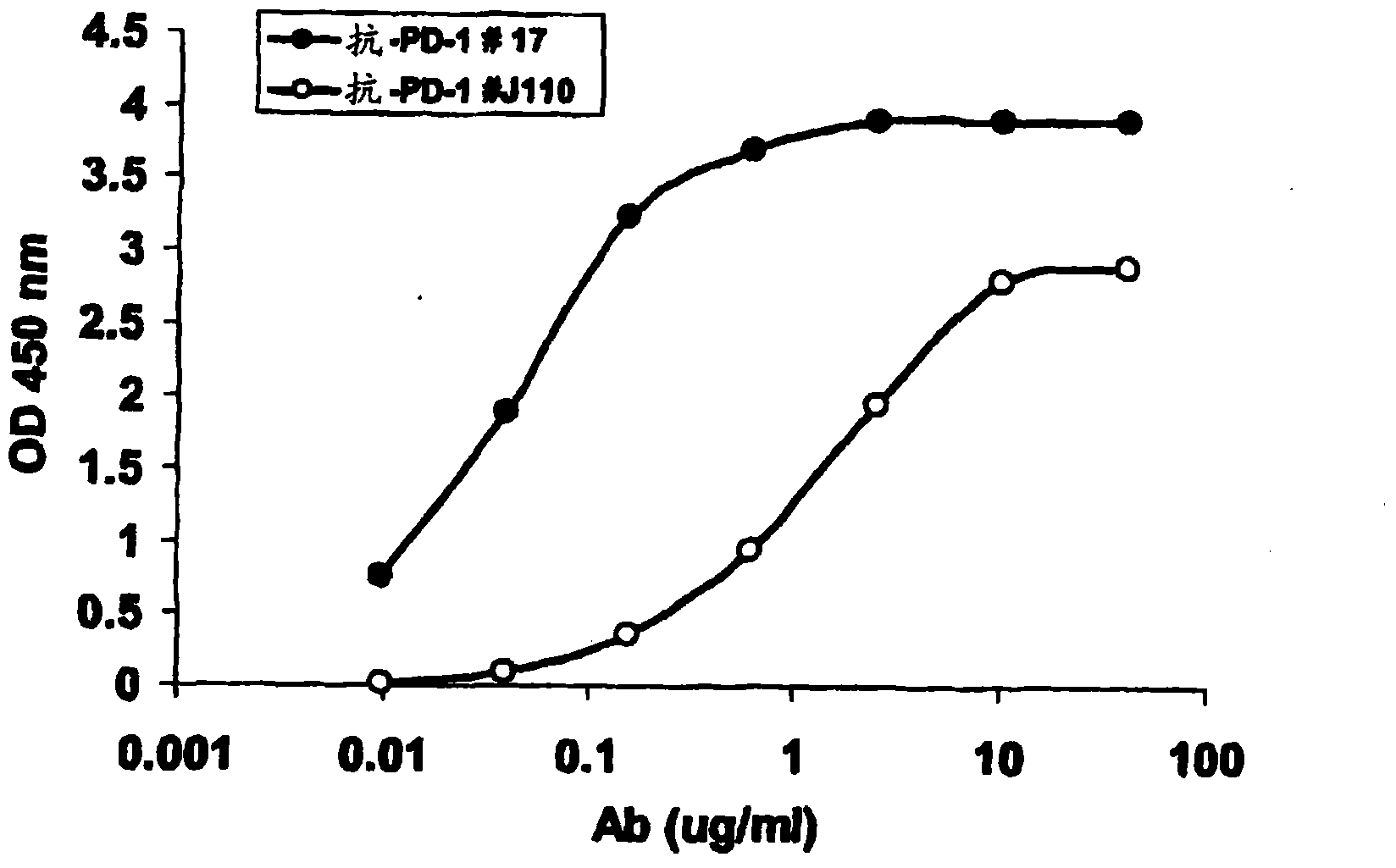
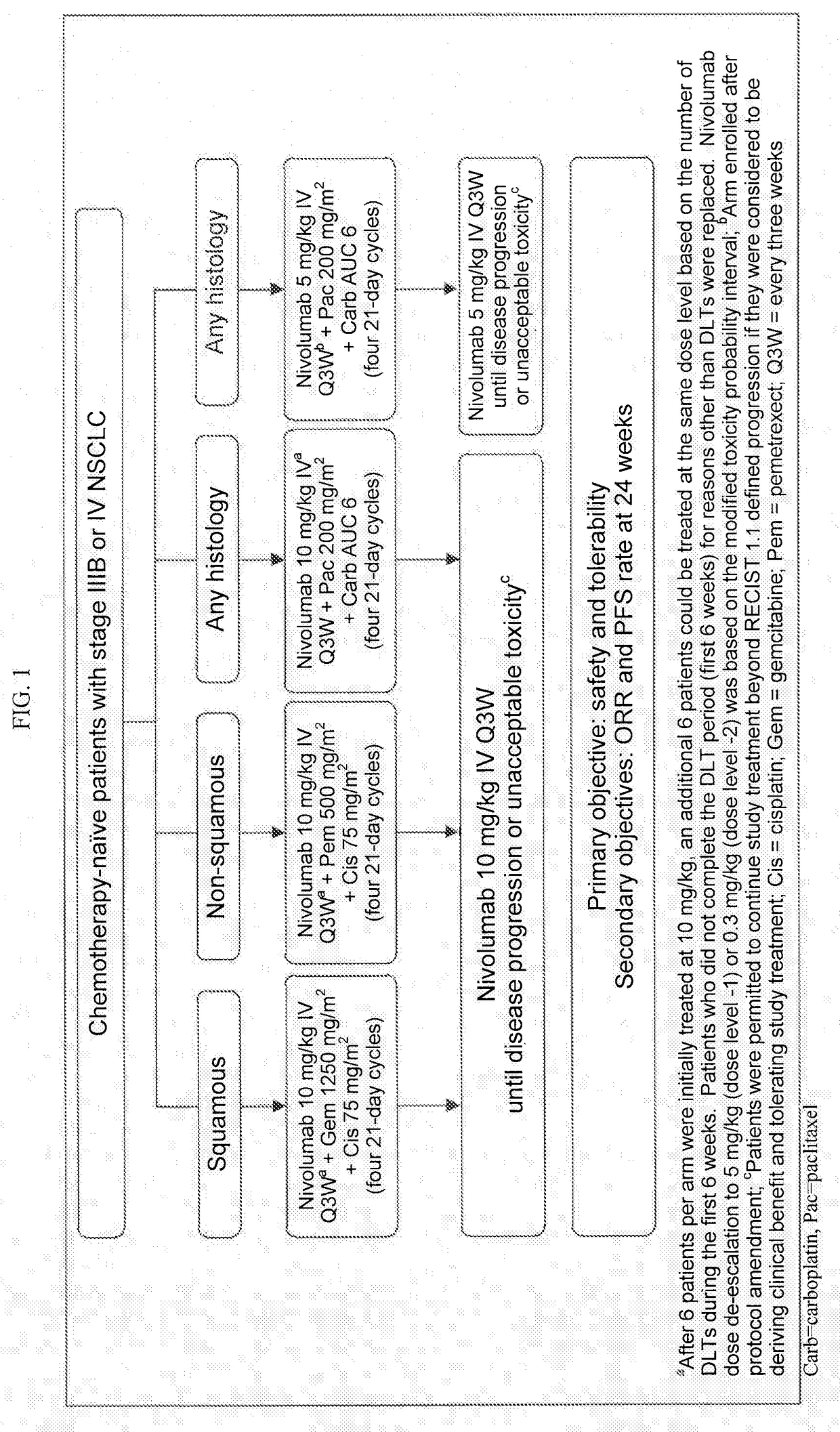
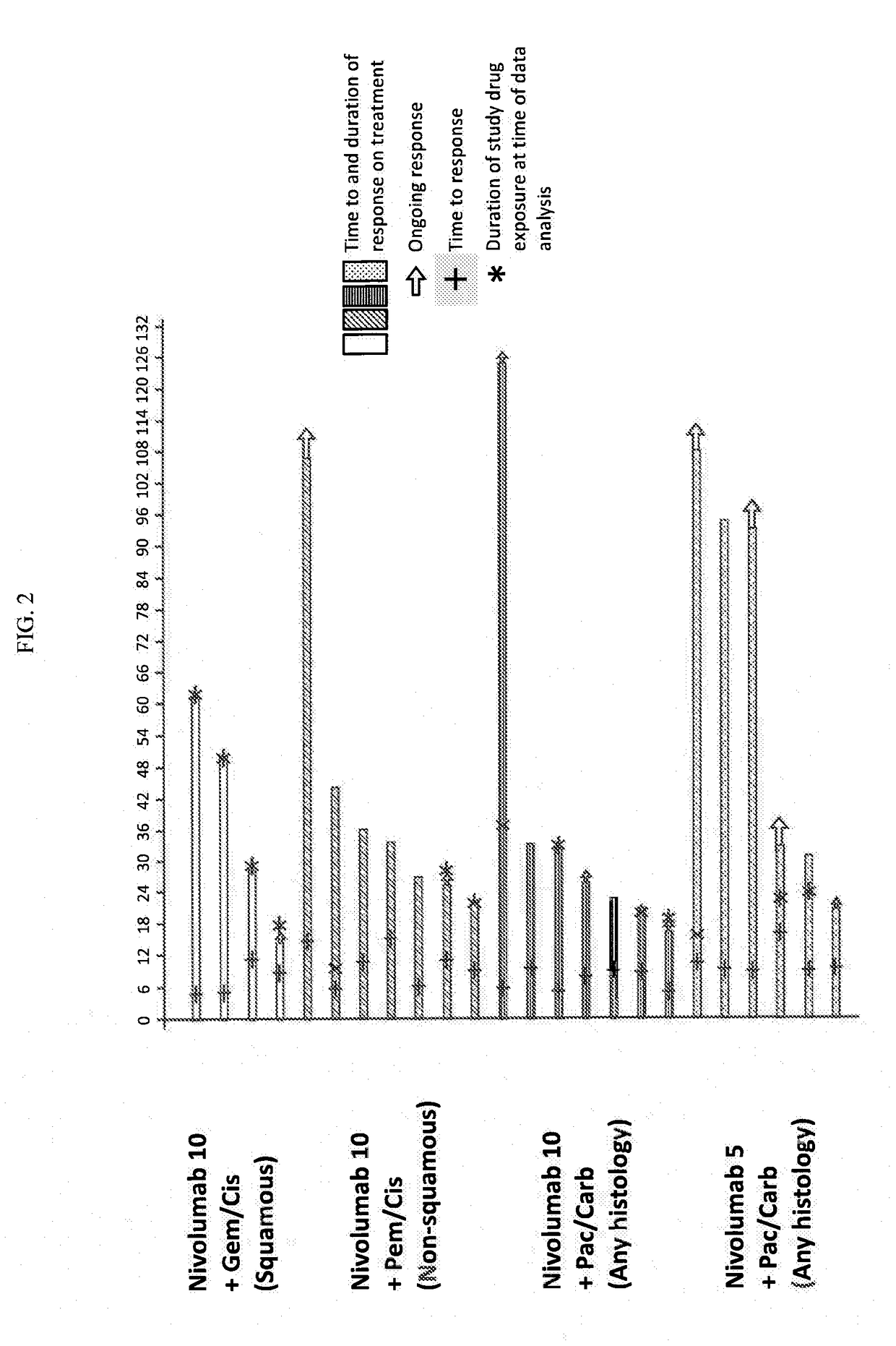
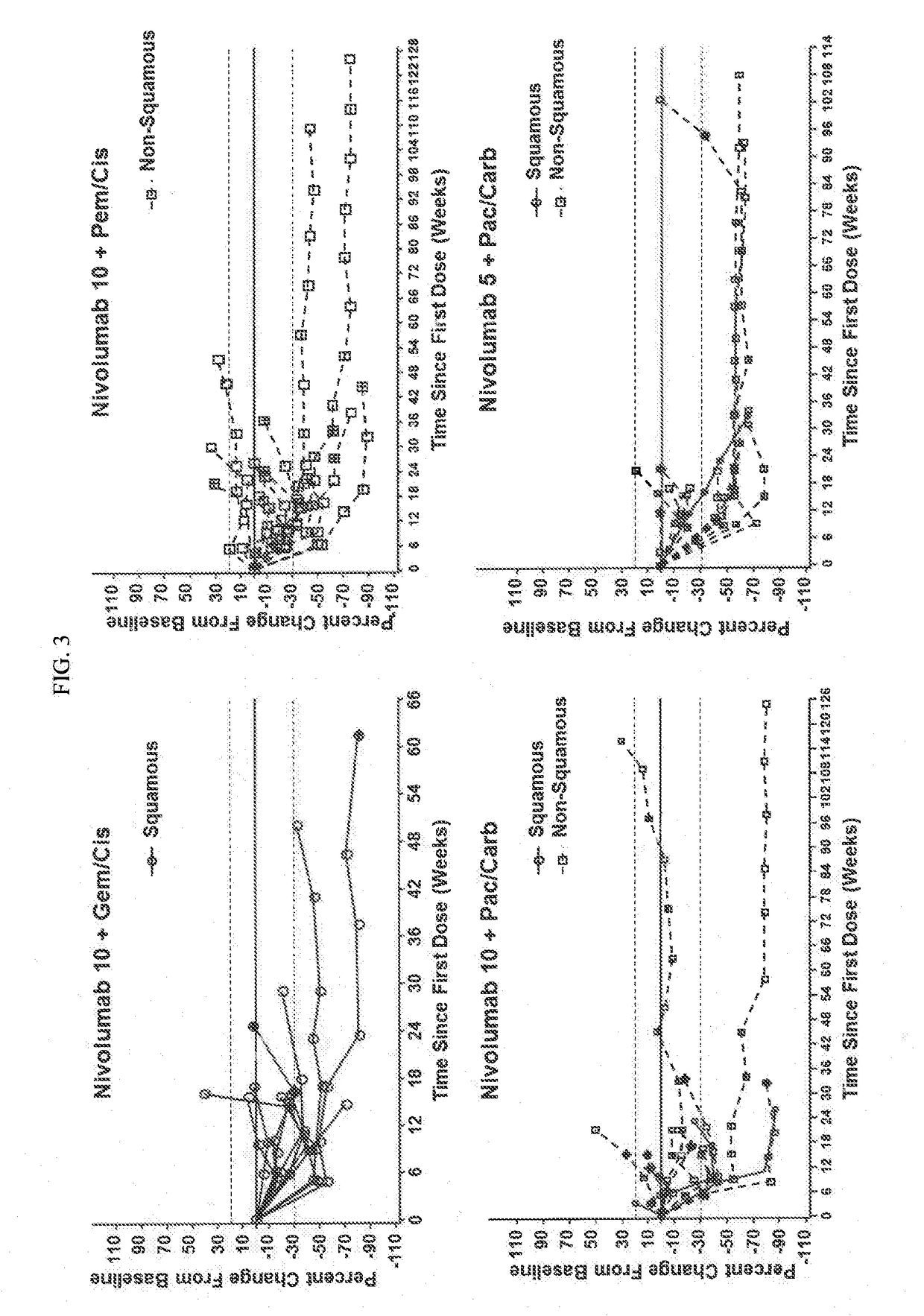
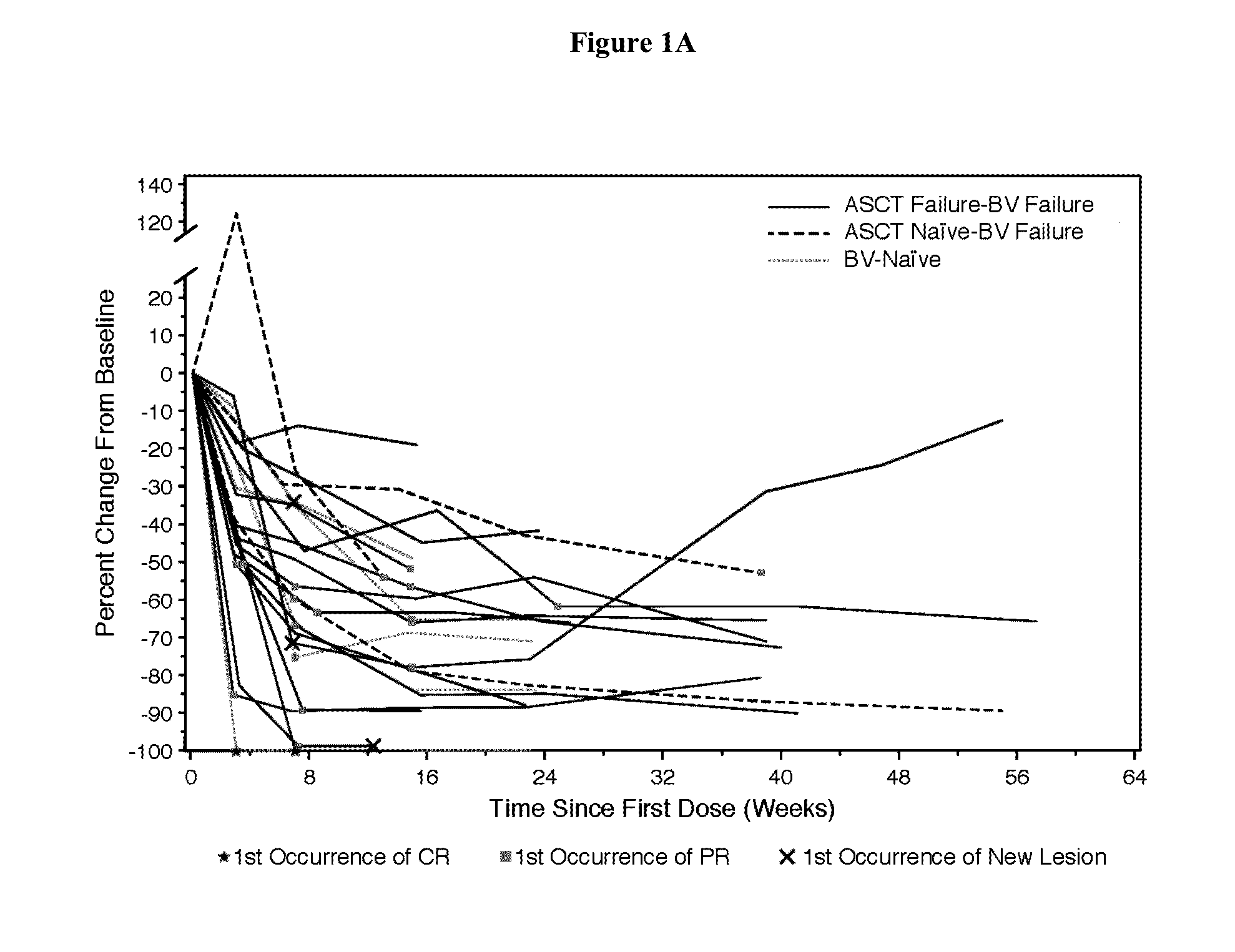
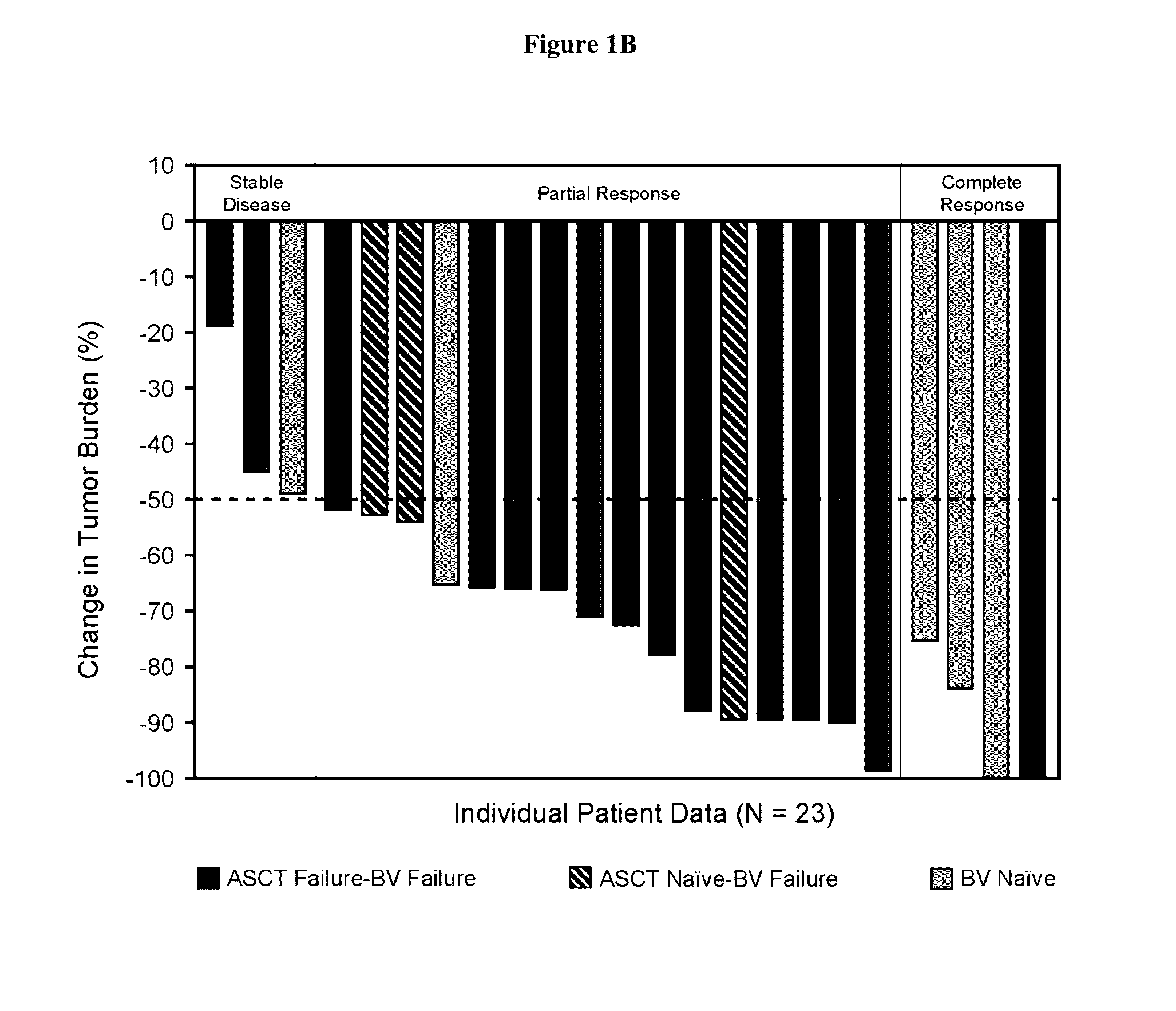
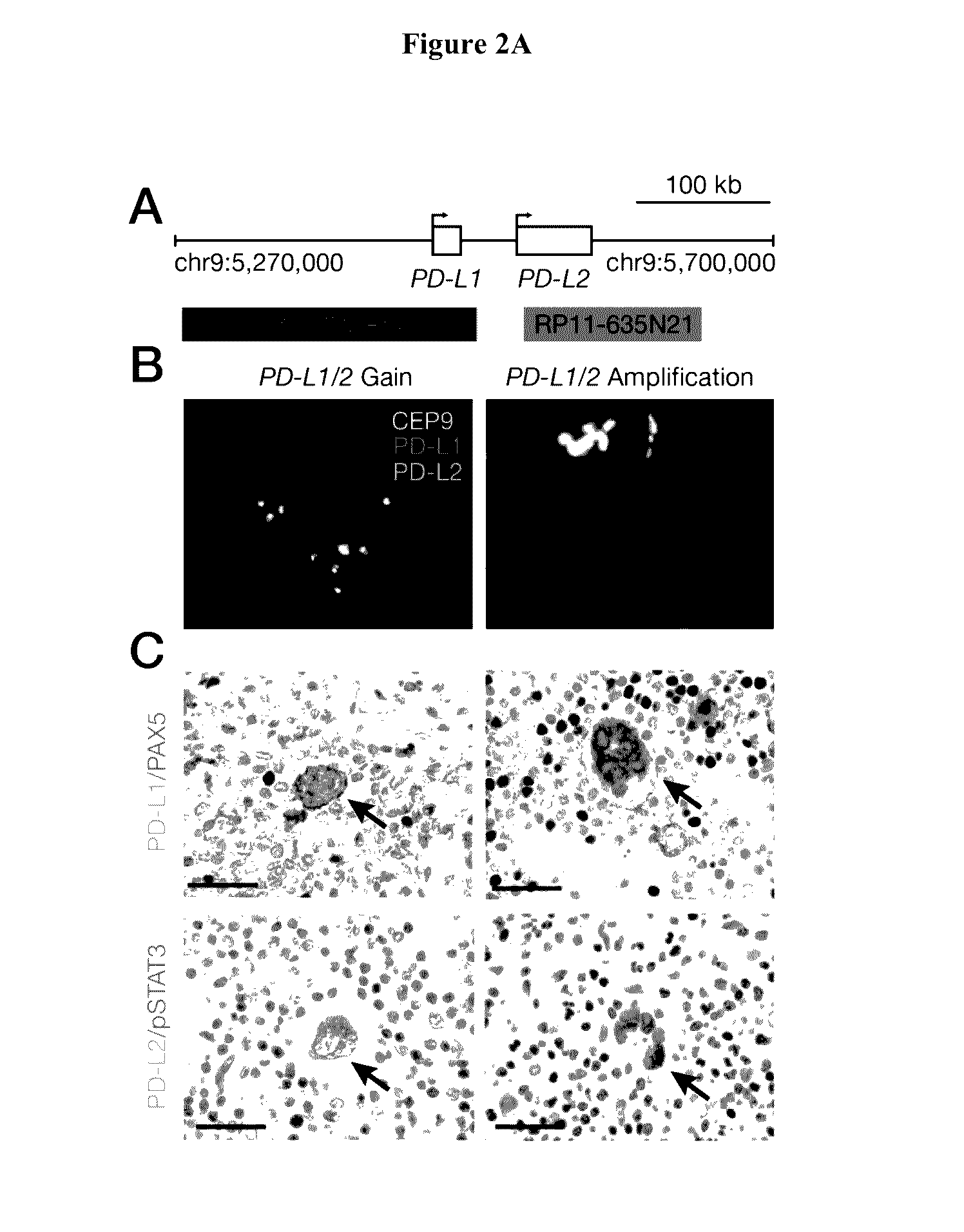
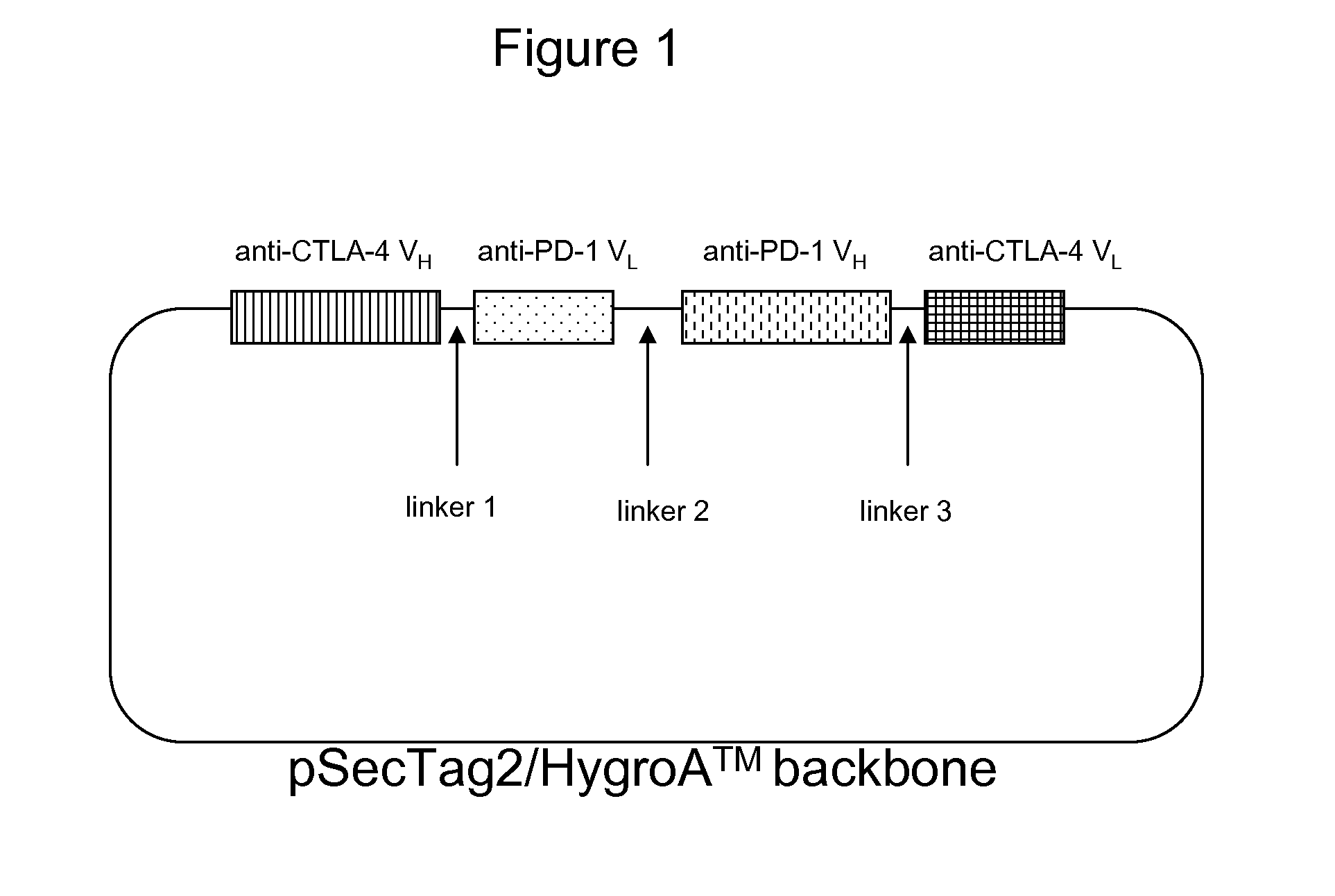
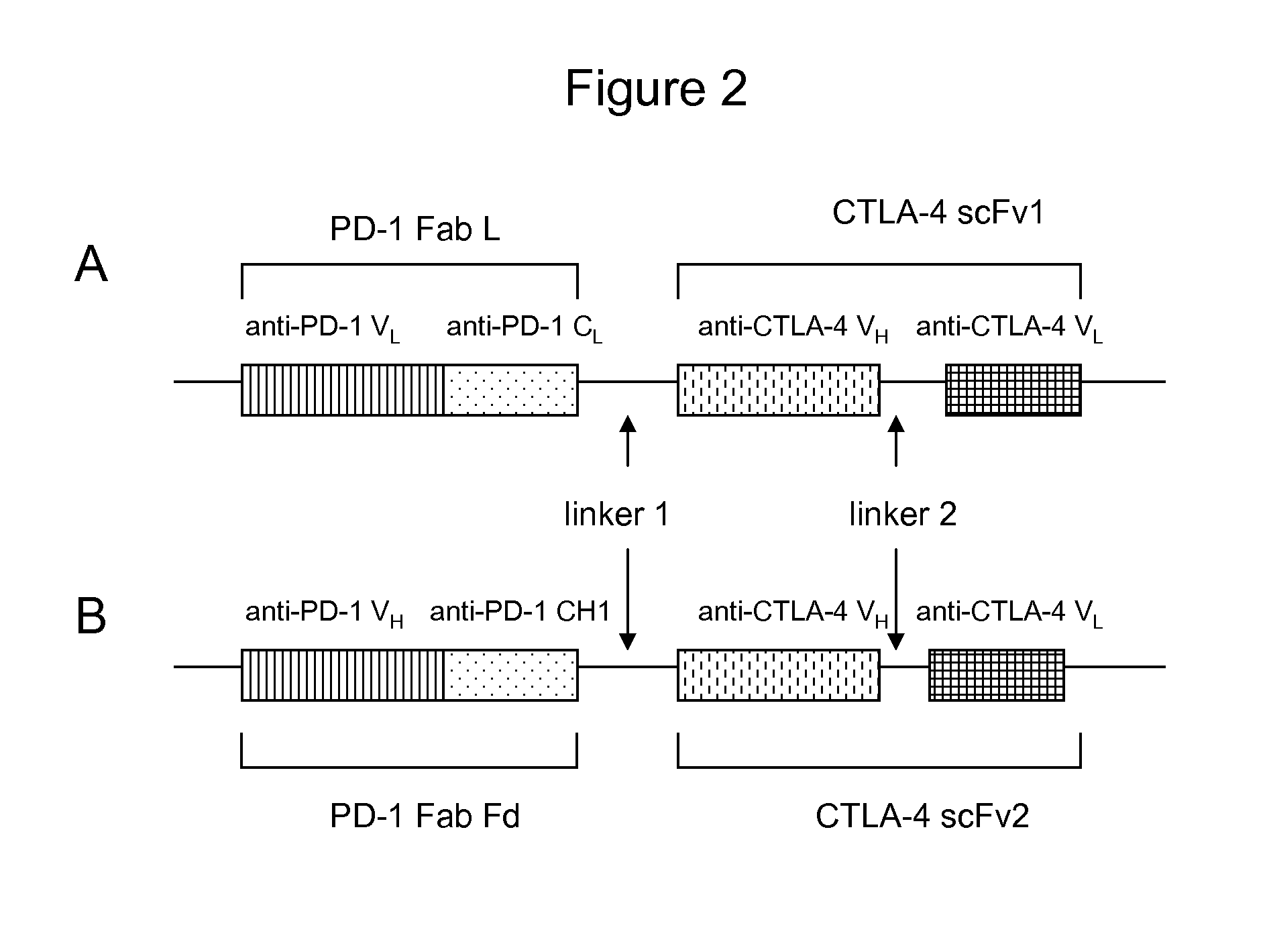
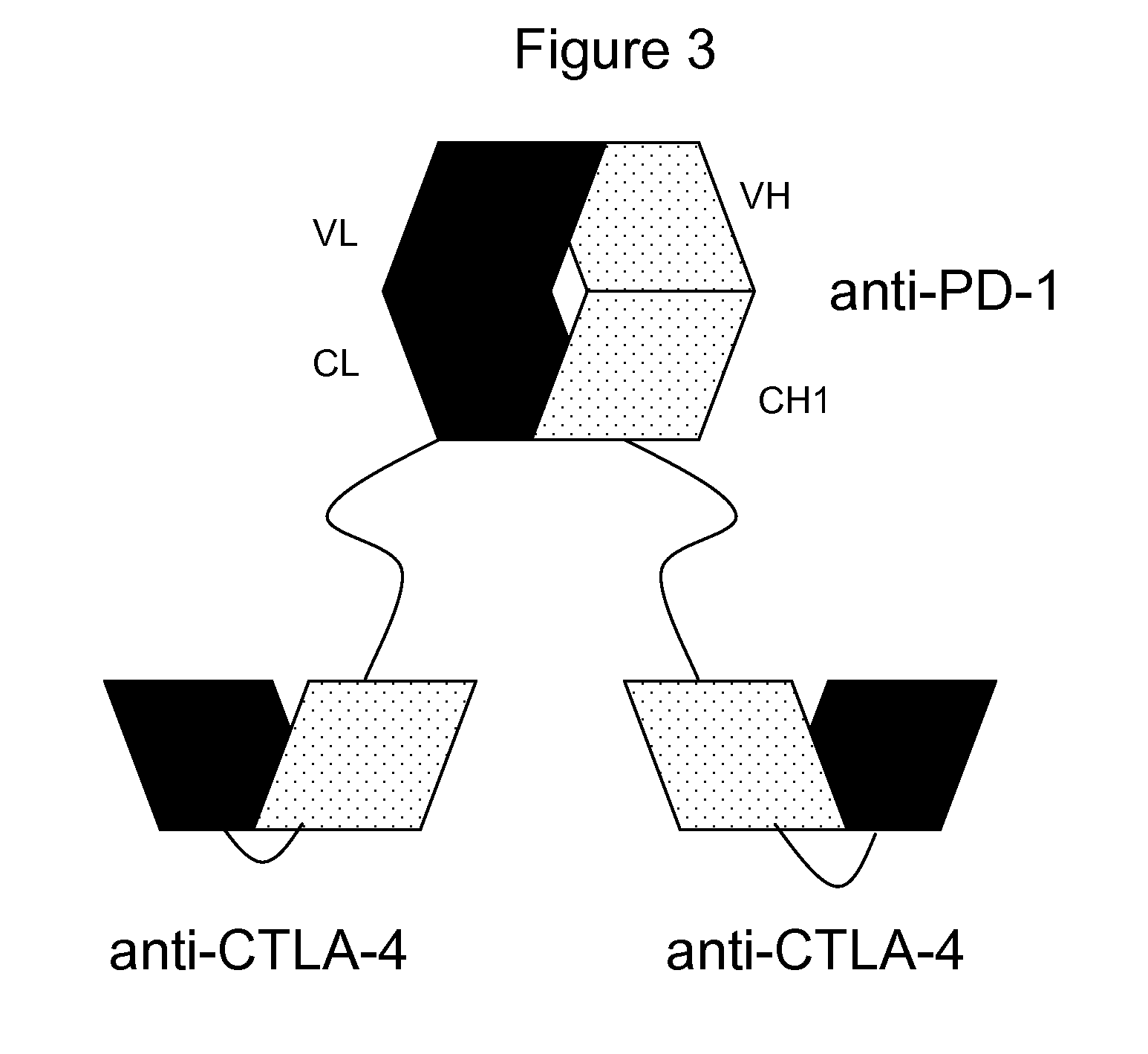
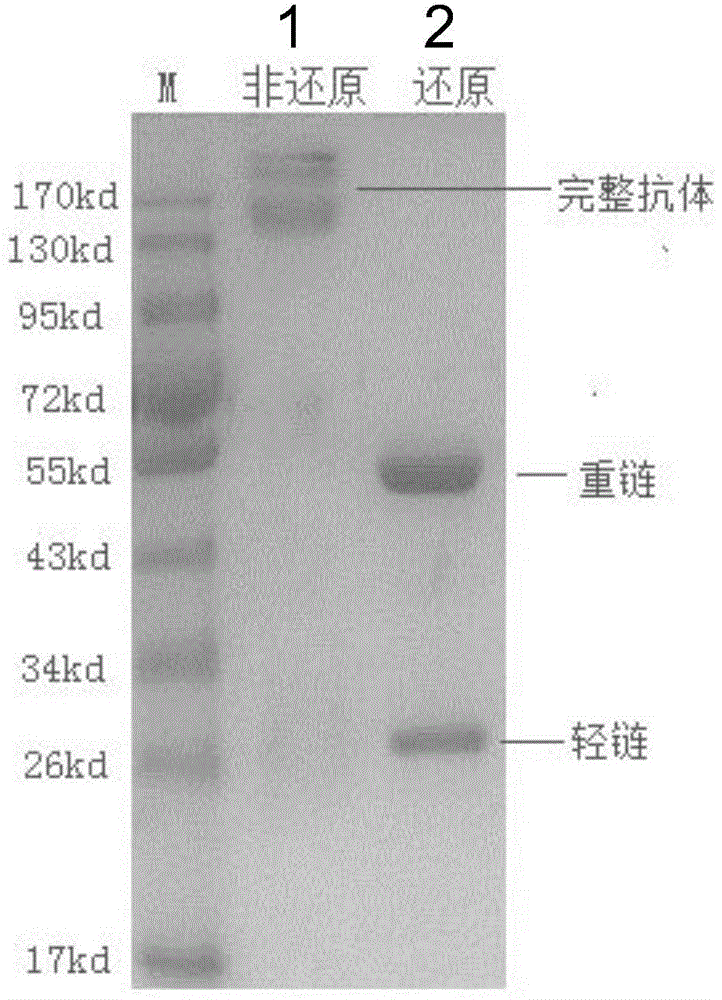
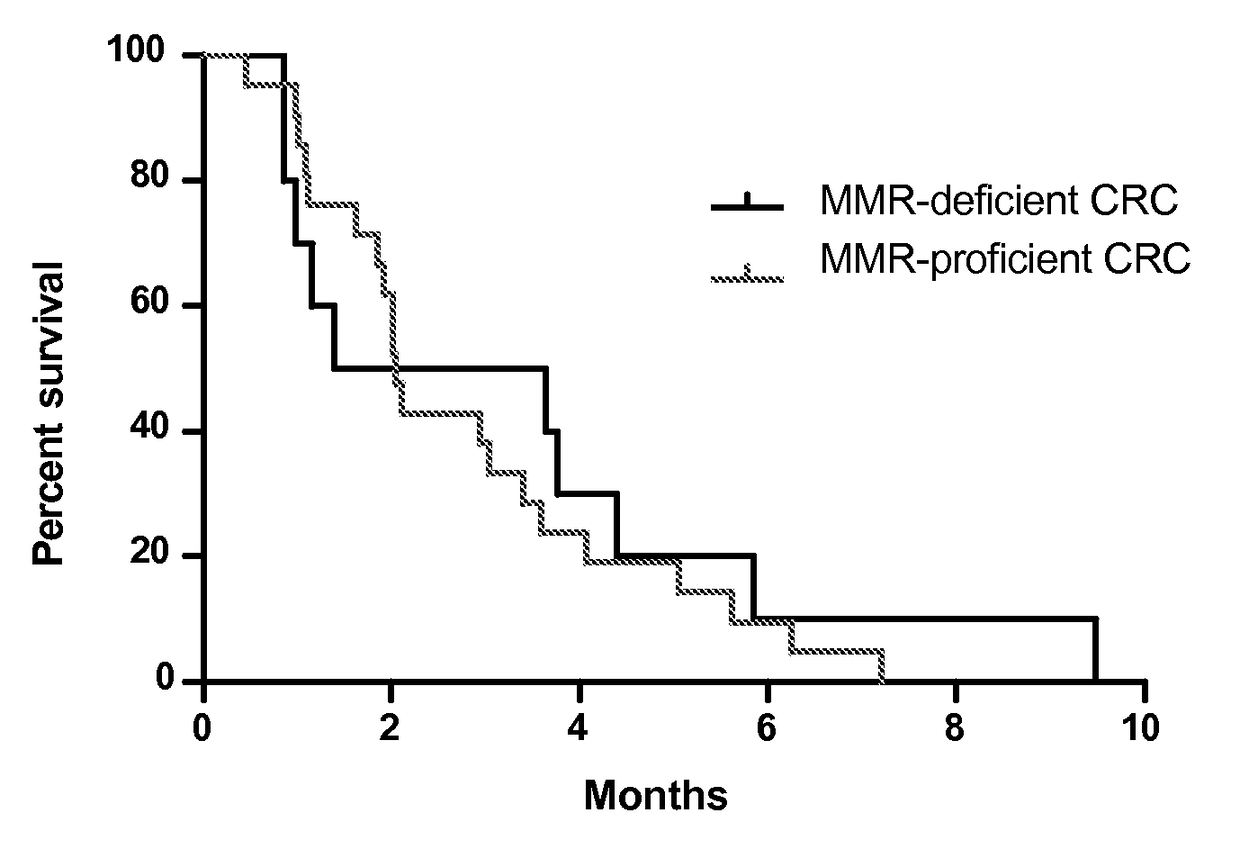
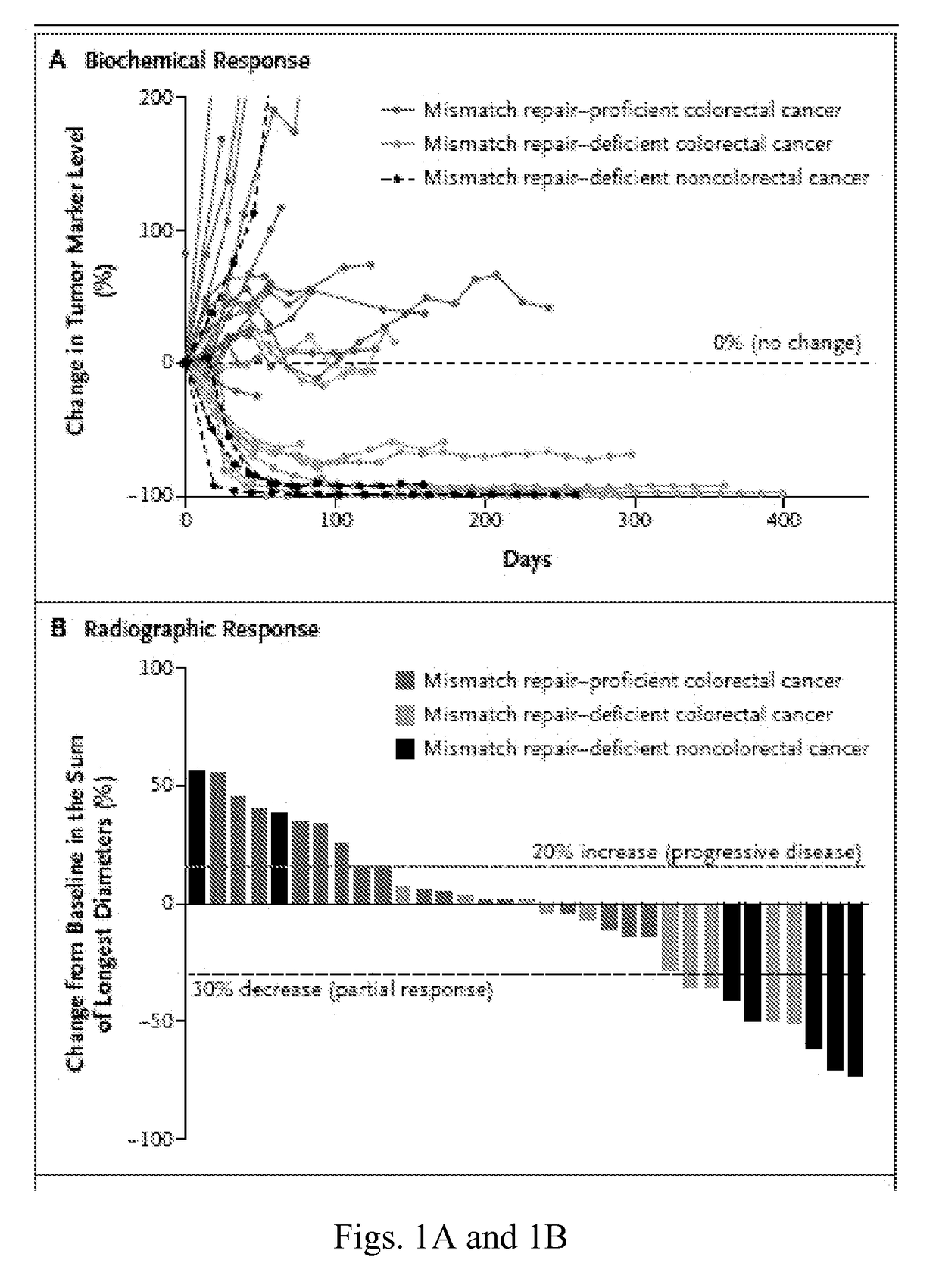
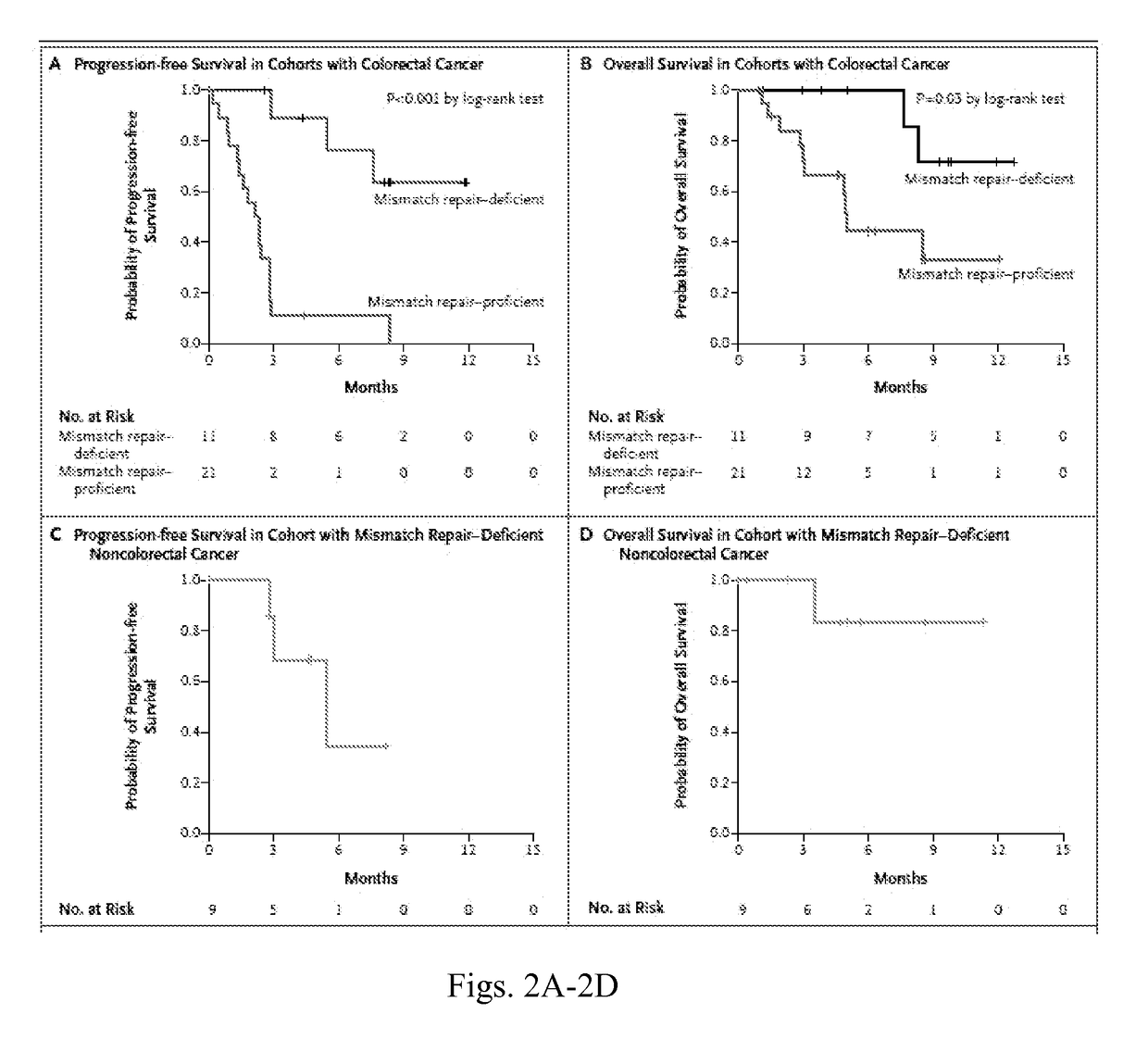
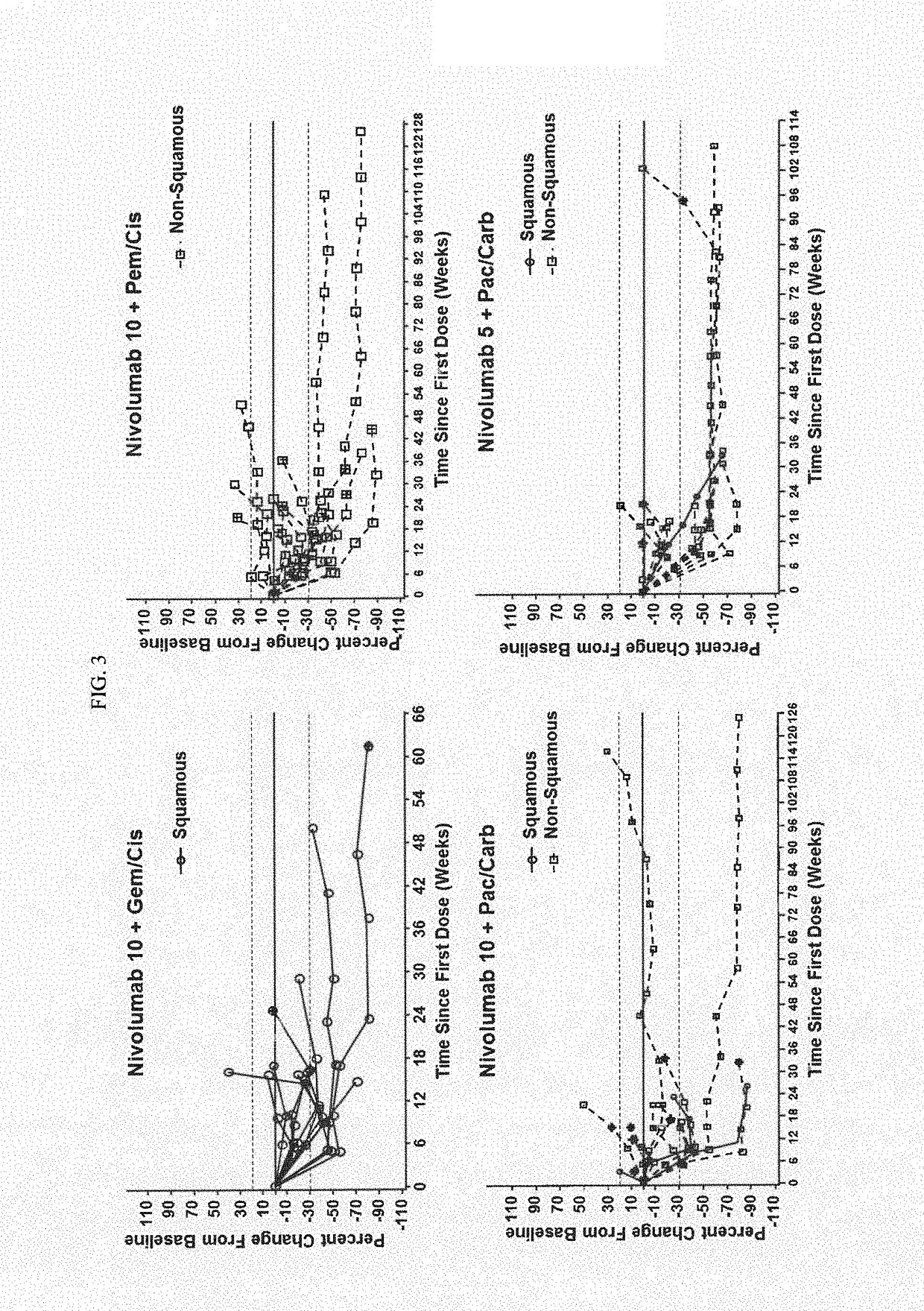
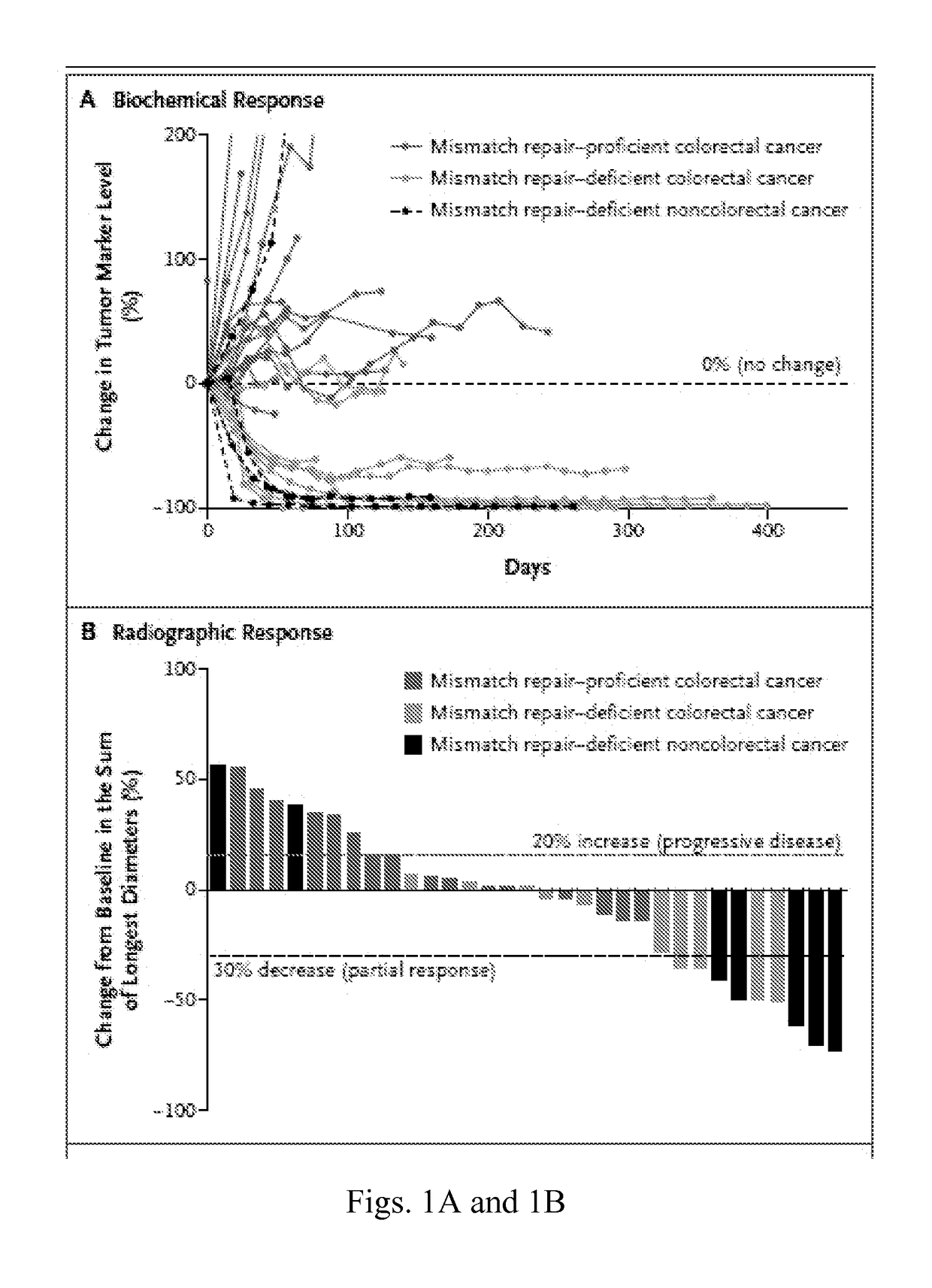
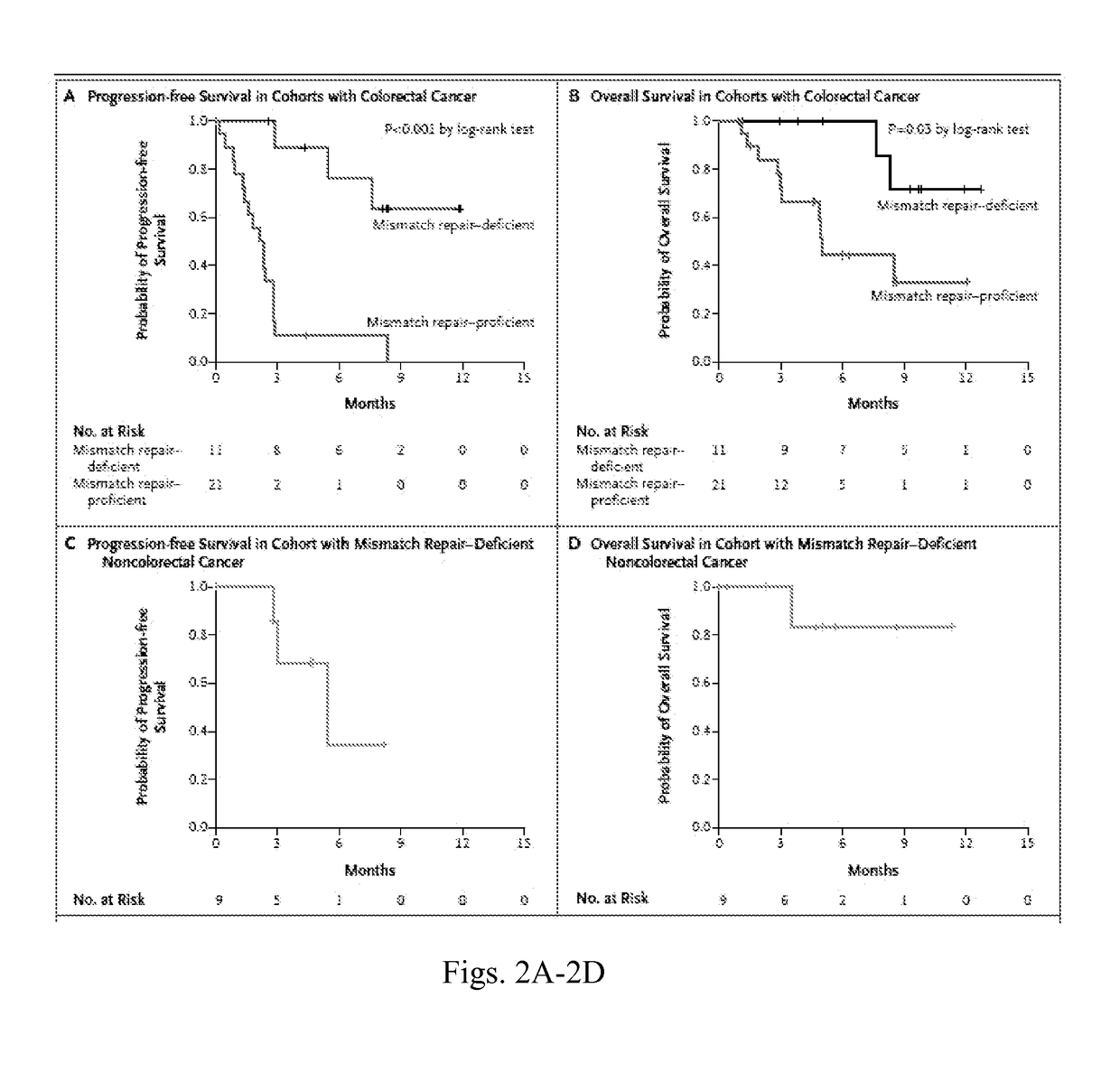
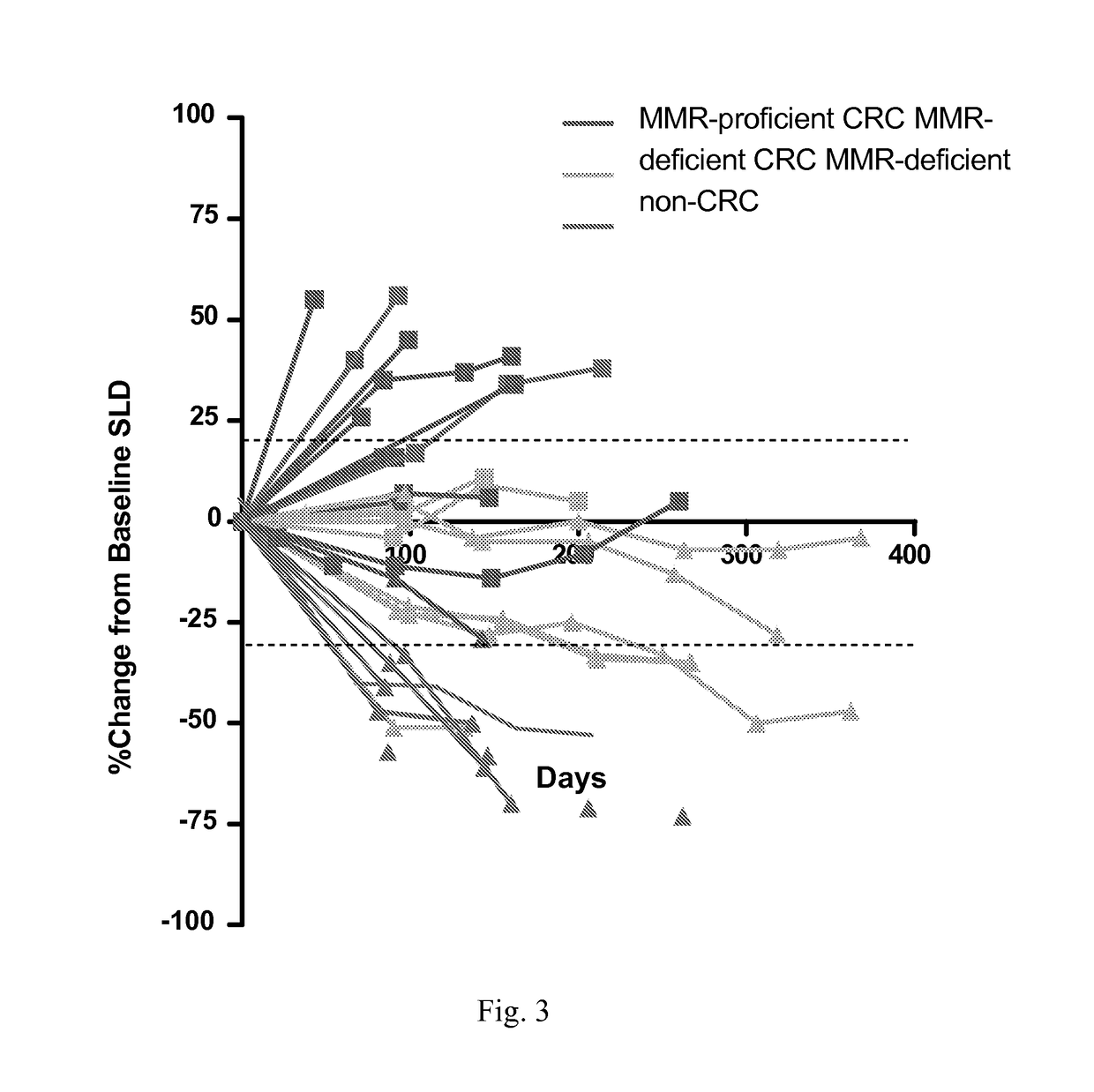
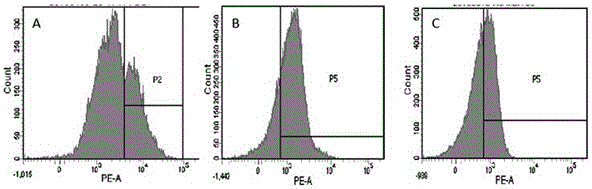
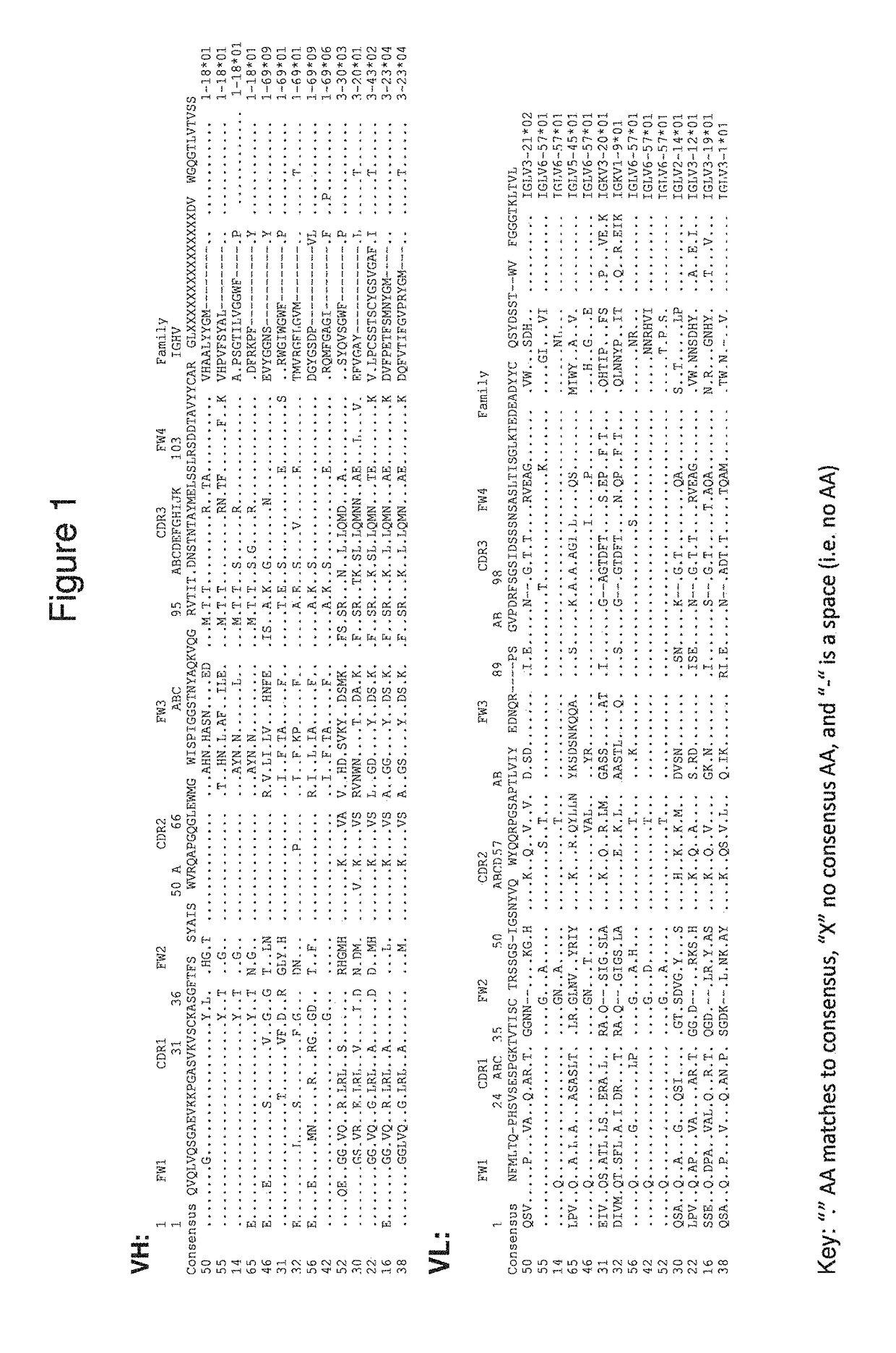
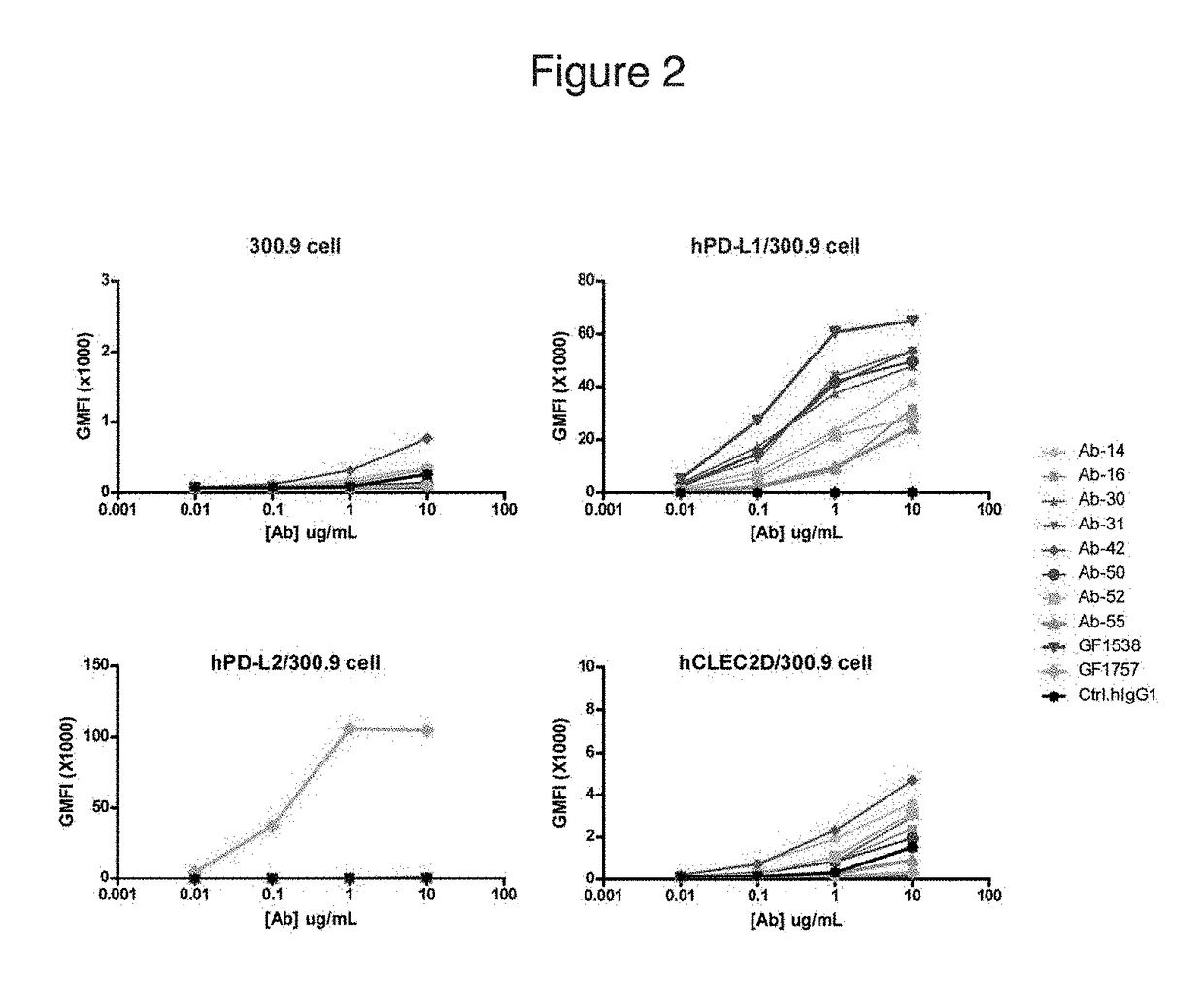
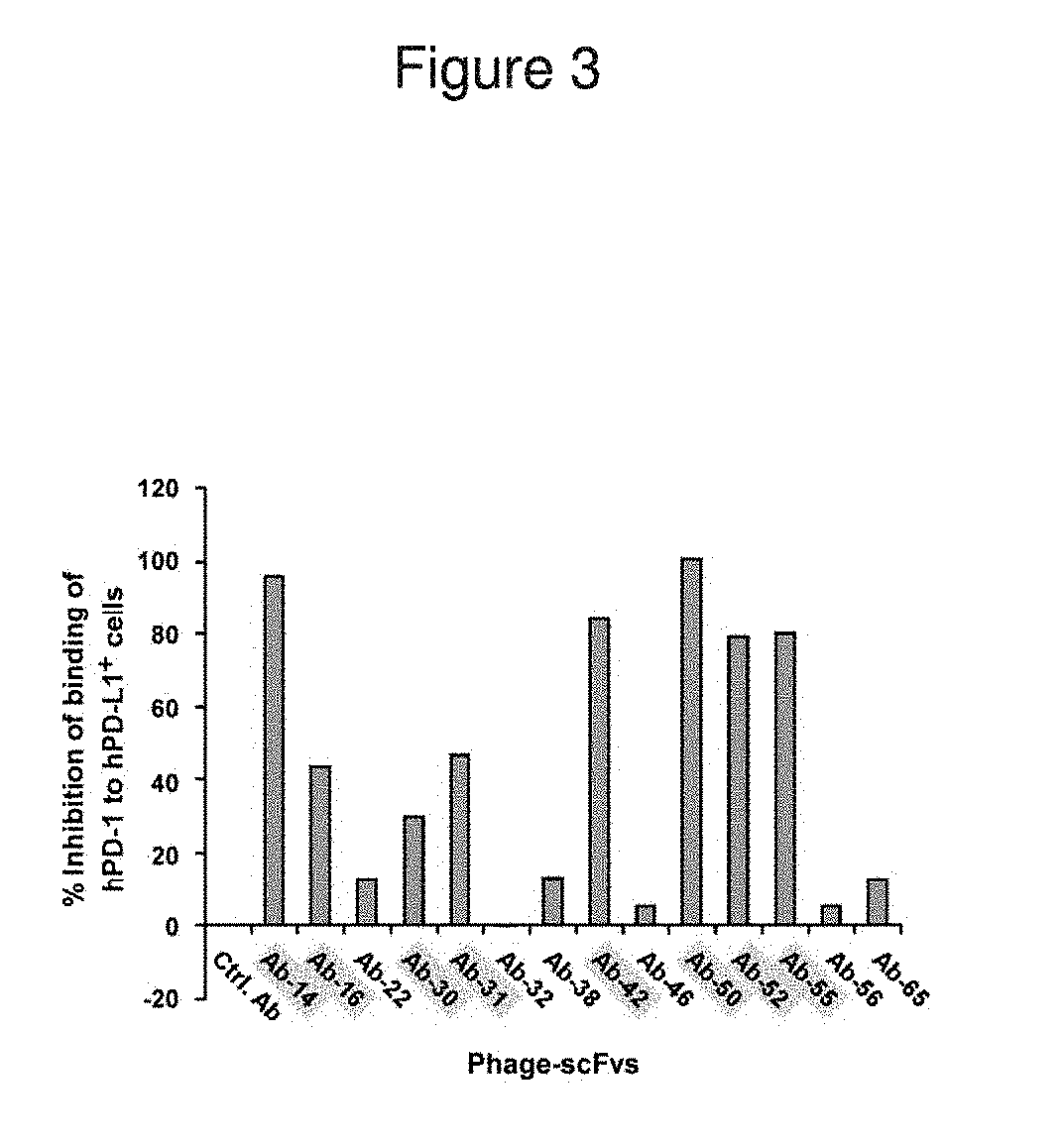
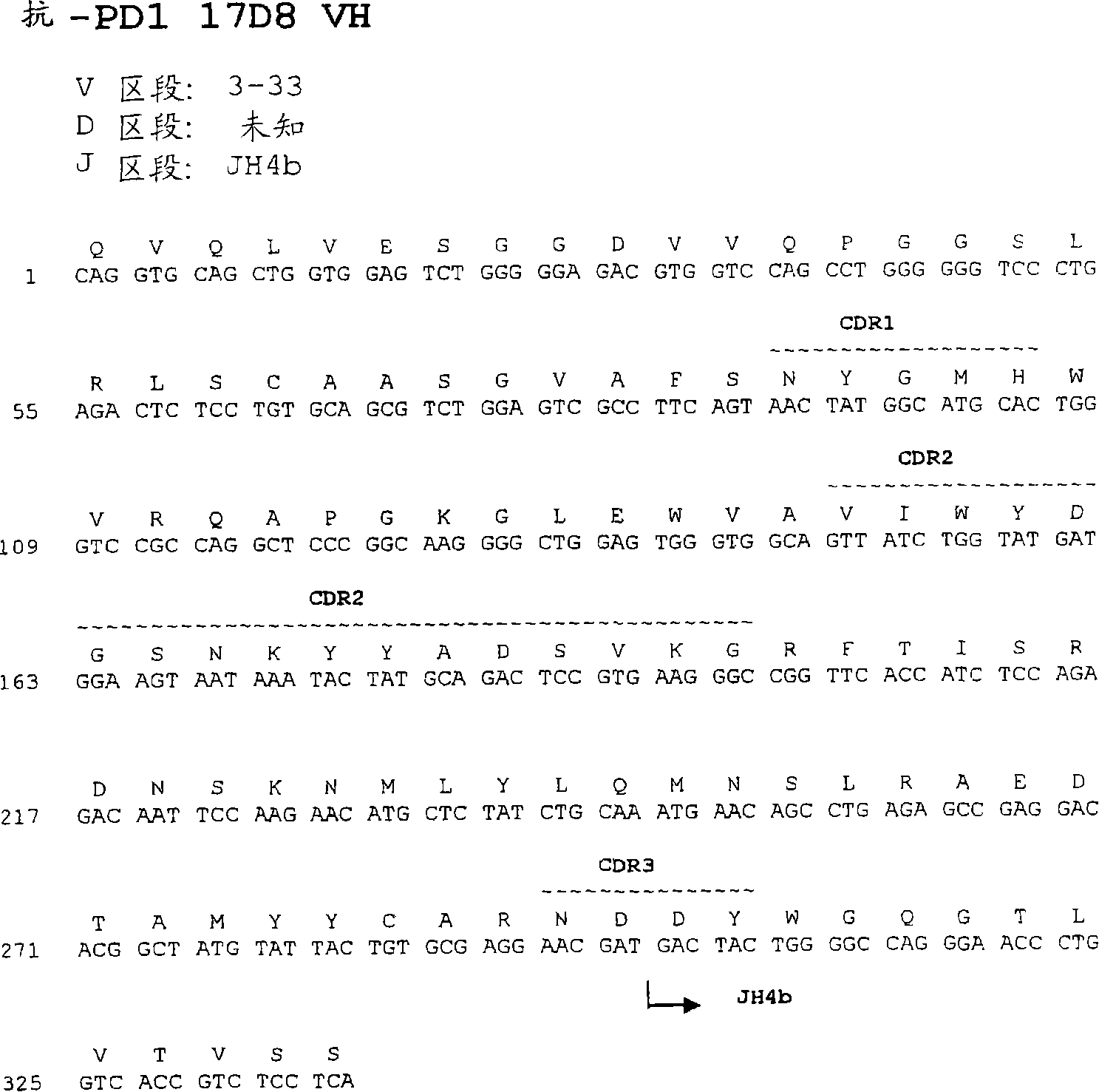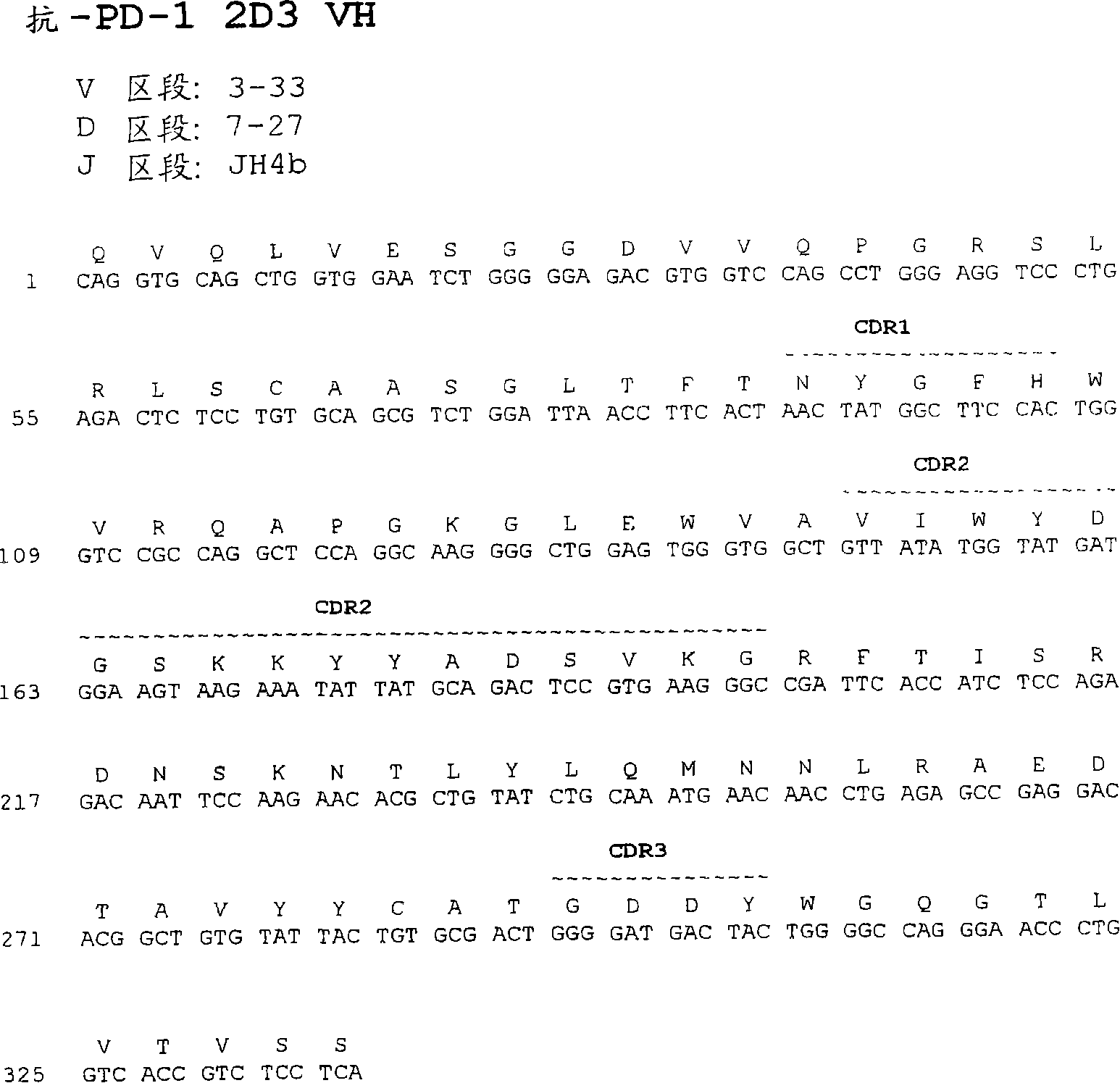Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
89 results about "Programmed death 1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Programmed death 1 (PD-1) is involved in the development of proliferative diabetic retinopathy by mediating activation-induced apoptosis.
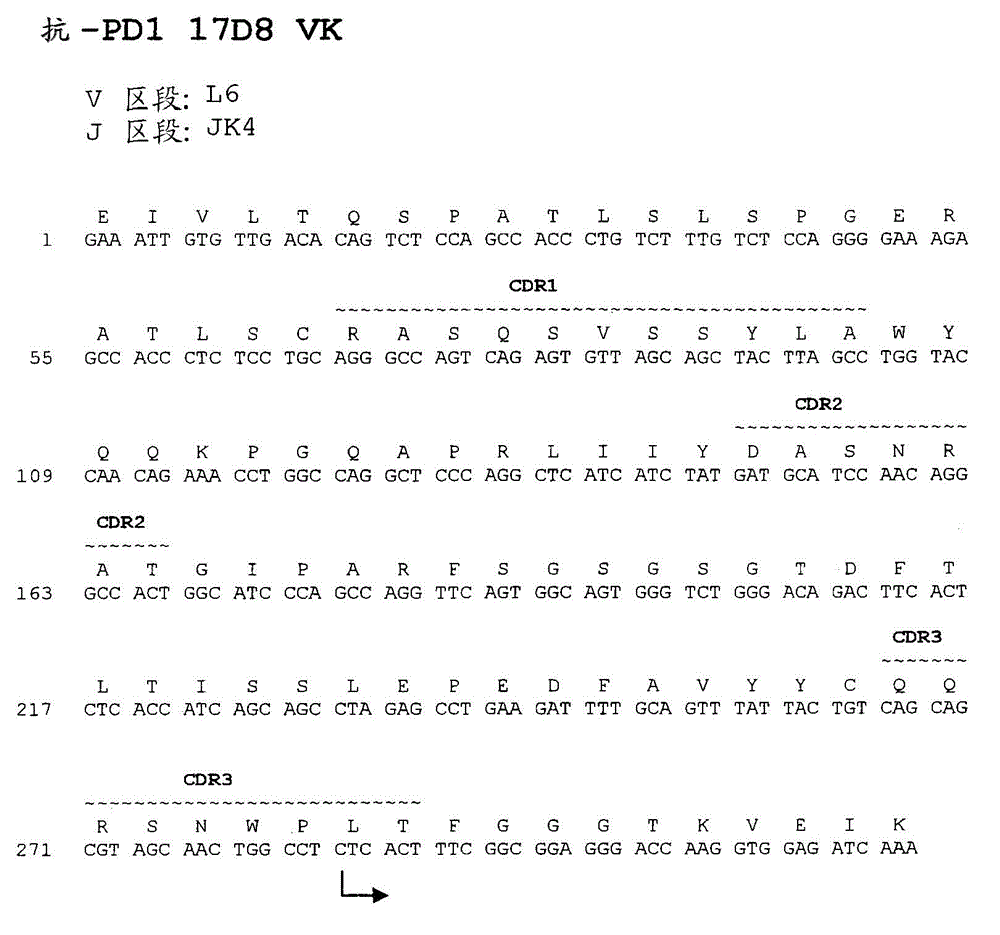
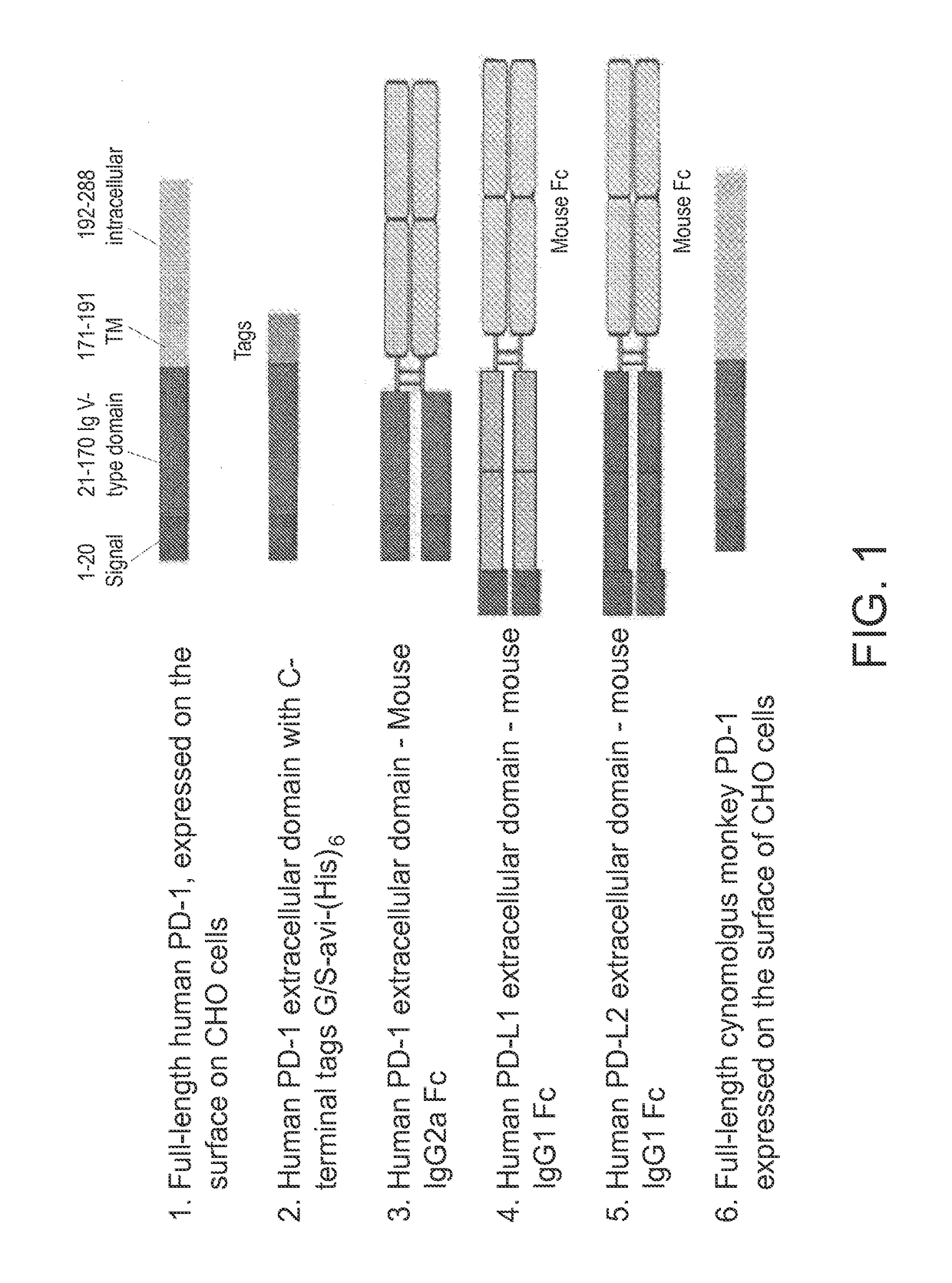
Anti-PD1 antibodies and their use as therapeutics and diagnostics
ActiveUS8735553B1Inhibition of secretionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathAnti pd1
Owner:BEIGENE SWITZERLAND GMBH
Human Antibodies to PD-1
ActiveUS20150203579A1Rescue T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
Human monoclonal antibodies to programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN101213297AMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsImmunotherapeutic agentProgrammed death
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
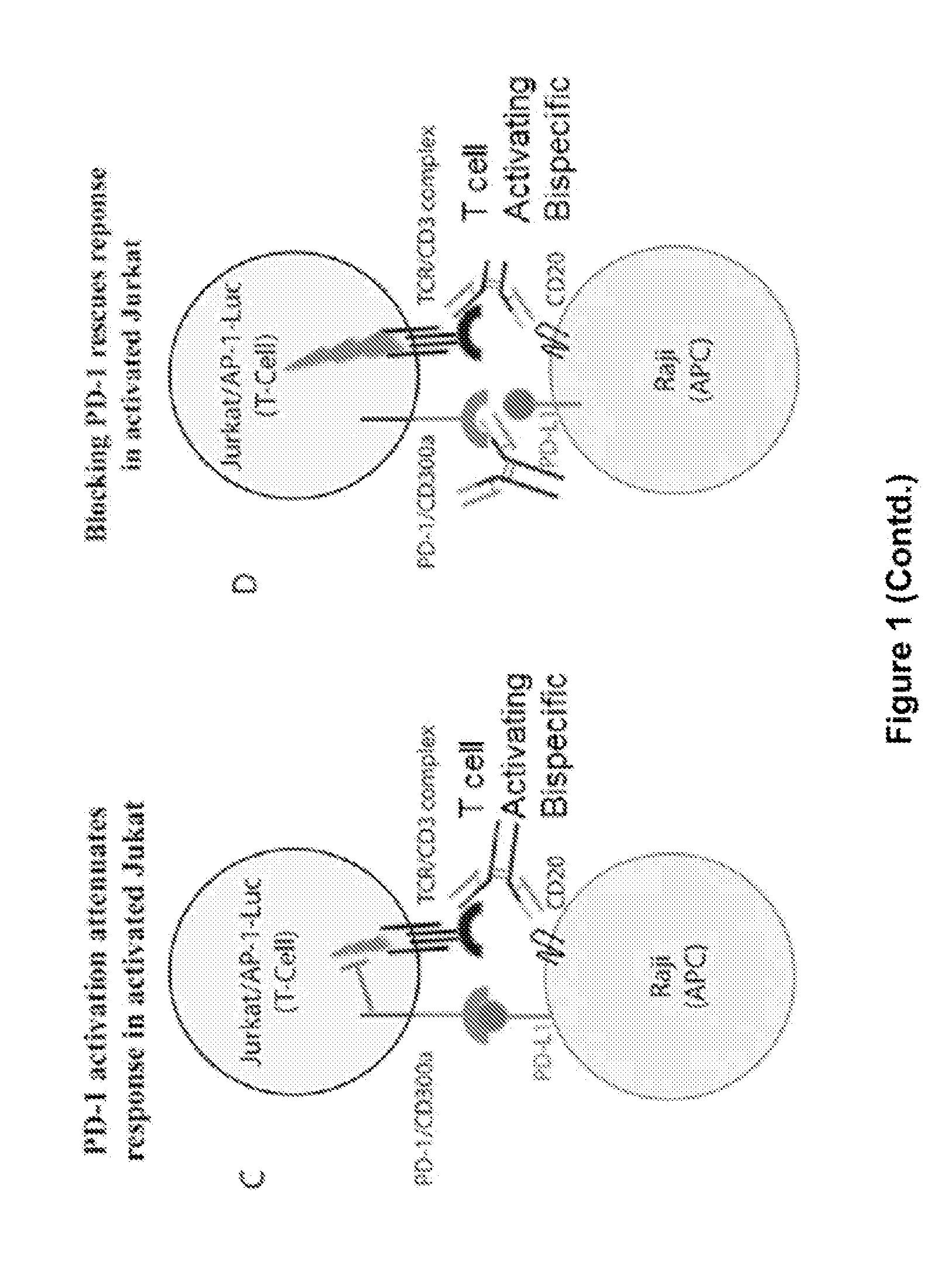
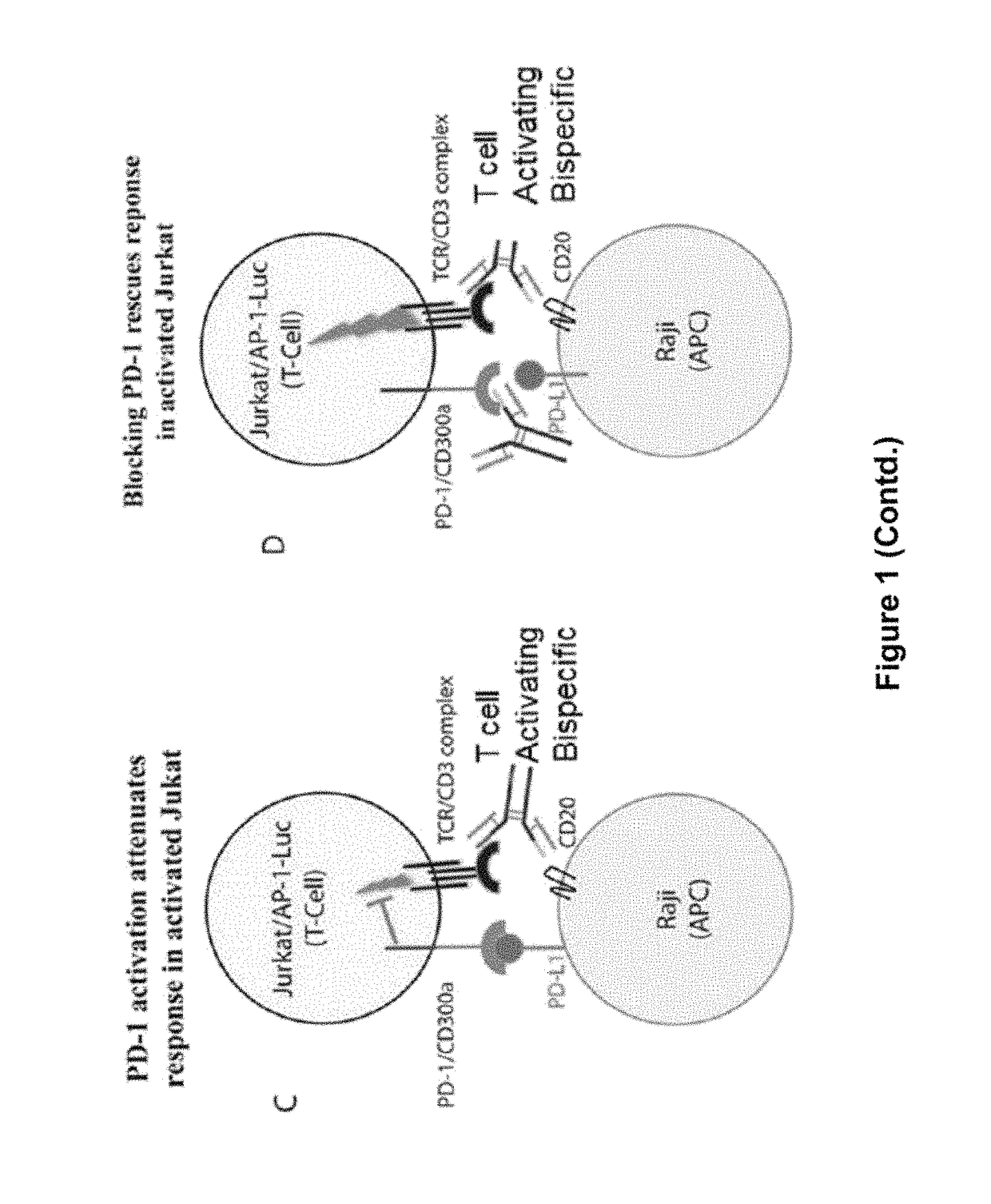
Combination of Anti-PD-1 Antibodies and Anti-CD20/Anti-CD3 Antibodies to Treat Cancer
InactiveUS20170174779A1Growth inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCD20
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of cancer (e.g., a B-cell cancer such as Hodgkin's lymphoma or acute lymphoblastic leukemia). The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of an antibody or antigen-binding fragment thereof that specifically binds to programmed death 1 (PD-1) receptor in combination with a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
Human antibodies to PD-1
ActiveUS9987500B2Rescues T-cell signalingInhibit tumor growthNervous disorderAntipyreticFc(alpha) receptorDisease
The present invention provides antibodies that bind to the T-cell co-inhibitor programmed death-1 (PD-1) protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to PD-1. In certain embodiments, the present invention provides multi-specific antigen-binding molecules comprising a first binding specificity that binds to PD-1 and a second binding specificity that binds to an autoimmune tissue antigen, another T-cell co-inhibitor, an Fc receptor, or a T-cell receptor. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing PD-1 activity, thus providing a means of treating a disease or disorder such as cancer or a chronic viral infection. In other embodiments, the antibodies are useful for enhancing or stimulating PD-1 activity, thus providing a means of treating, for example, an autoimmune disease or disorder.
Owner:REGENERON PHARM INC
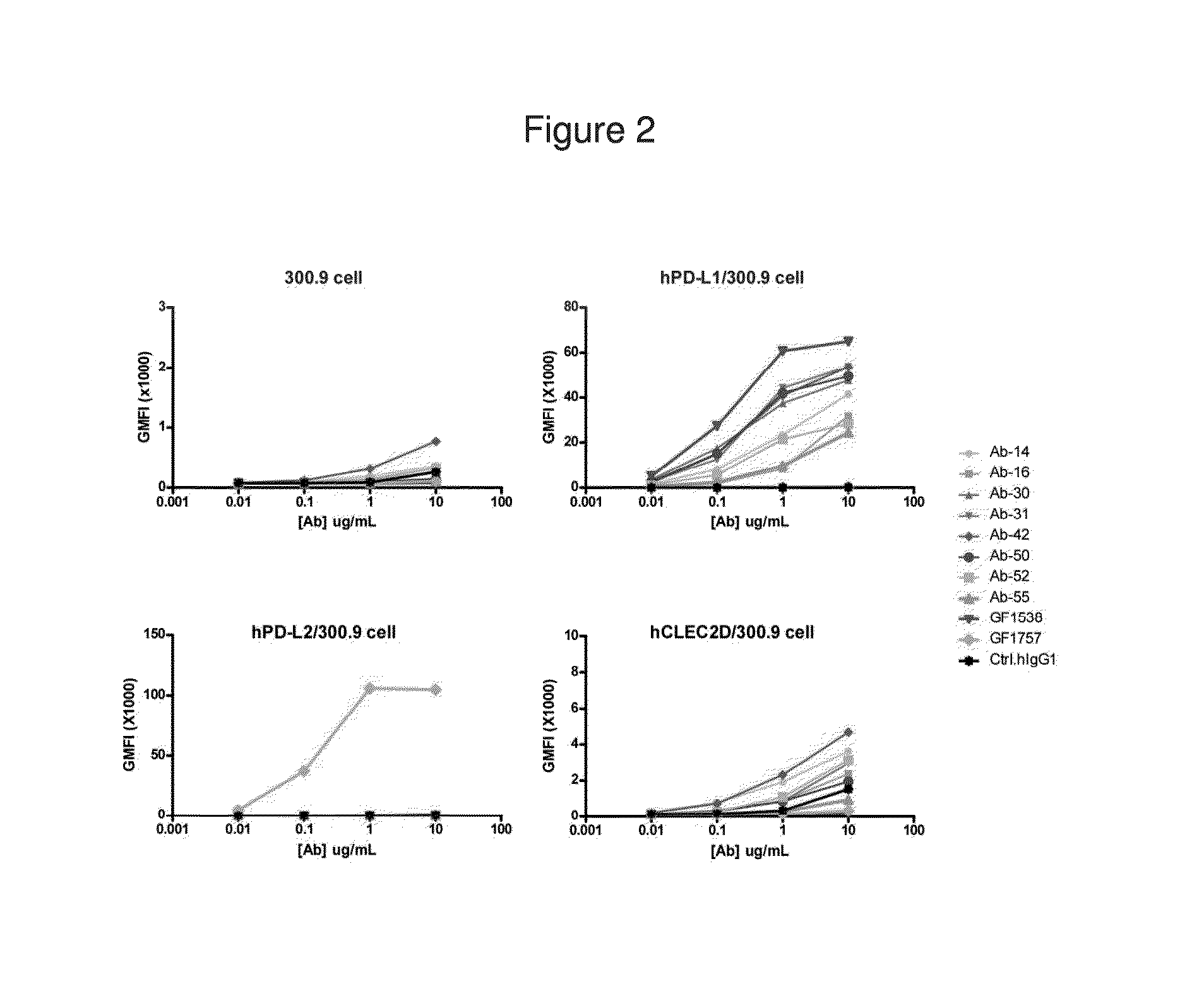
Human monoclonal Anti-pd-l1 antibodies and methods of use
ActiveUS20150274835A1Enhance immune responseIncrease in antigen specific T cell activityImmunoglobulins against cytokines/lymphokines/interferonsAntiviralsPD-L1Programmed death
The present invention comprises human monoclonal antibodies that bind to PD-L1 (also known as programmed death ligand 1 or B7H1). Binding of the invented antibody to PD-L1 inhibits binding to its receptor, PD1 (programmed death 1), and ligand-mediated activities and can be used to treat cancer and chronic viral infections.
Owner:DANA FARBER CANCER INST INC
Anti-CTLA4-anti-PD-1 bifunctional antibody, pharmaceutical composition and use thereof
ActiveCN106967172ABinding blockRelief of immunosuppressionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyT lymphocyte
Belonging to the field of tumor treatment and molecular immunology, the invention relates to an anti-CTLA4-anti-PD-1 bifunctional antibody, a pharmaceutical composition and use thereof. Specifically, the anti-CTLA4-anti-PD-1 bifunctional antibody comprises: a PD-1 (programmed death-1) targeted first protein functional region, and a CTLA4 (cytotoxic T lymphocyte sociated antigen 4) targeted second protein functional region. The bifunctional antibody provided by the invention can well bind to CTLA4 and PD-1 specifically, specifically remove the immunosuppression of CTLA4 and PD-1 on the body, and activate T lymphocytes, thus having good application prospect.
Owner:AKESO PHARMA INC
Compositions comprising a combination of an Anti-pd-1 antibody and another antibody
InactiveUS20160304607A1Change is minimalSenses disorderNervous disorderAnticarcinogenProgrammed death
This provides pharmaceutical compositions that comprise a combination of an anti-cancer agent which is an first antibody and a second antibody. In some embodiments, the first antibody is an anti-Programmed Death-1 (PD-1) antibody. In certain embodiments, the composition is a fixed dose formulation. In certain embodiments, the composition is administered as a flat-dose. The disclosure also provides a kit for treating a subject afflicted with a disease, the kit comprising a dosage of any composition disclosed herein and instructions for using the composition in any of the disclosed methods for treating a disease.
Owner:BRISTOL MYERS SQUIBB CO
Human monoclonal antibodies to programmed death 1 (pd-1) and methods for treating cancer using anti-pd-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN103059138ARadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesInfectious Disorder
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
Anti-PD-1 (Programmed Death-1) antibody and application thereof
ActiveCN106519034AHigh binding affinityIncrease productionAntibacterial agentsAntipyreticInfective disorderAntibody
The invention provides an antibody specifically combined with PD-1 (Programmed Death-1) with high affinity, and also provides a nucleic acid molecule for coding the antibody, an expression vector and a host cell for expressing the antibody, and a production method of the antibody. In addition, the invention also provides an immune conjugate and a medicinal composition containing the antibody, and application of the antibody to the preparation of a medicine for treating multiple diseases (including a cancer, an infectious disease and an inflammatory disease).
Owner:LUNAN PHARMA GROUP CORPORATION
Antibodies against PD-1 and uses therefor
This disclosure provides antibodies and antigen-binding fragments that can act as agonists and / or antagonists of PD-1 (Programmed Death 1), thereby modulating immune responses in general, and those mediated by TcR and CD28, in particular. The disclosed compositions and methods may be used for example, in treating autoimmune diseases, inflammatory disorders, allergies, transplant rejection, cancer, and other immune system disorders.
Owner:WYETH LLC +1
Treatment of lung cancer using a combination of an Anti-pd-1 antibody and another Anti-cancer agent
InactiveUS20170158776A1Durable clinical responseReliable responseHeavy metal active ingredientsImmunoglobulins against growth factorsAnticarcinogenTyrosine-kinase inhibitor
This disclosure provides a method for treating a subject afflicted with a lung cancer, which method comprises administering to the subject therapeutically effective amounts of: (a) an anti-cancer agent which is an antibody or an antigen-binding portion thereof that specifically binds to a Programmed Death-1 (PD-1) receptor and inhibits PD-1 activity; and (b) another anti-cancer agent. The other anti-cancer agent can be a platinum-based doublet chemotherapy, an EGFR-targeted tyrosine kinase inhibitor, bevacizumab, an anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) antibody, or any other therapy used to treat lung cancer in the art or disclosed herein.
Owner:BRISTOL MYERS SQUIBB CO
Treatment of hodgkin's lymphoma using an Anti-pd-1 antibody
InactiveUS20160347836A1Inhibitory activityMicrobiological testing/measurementBiological material analysisAnticarcinogenCTLA4 Protein
This disclosure provides methods for treating Hodgkin's lymphoma in a subject comprising administering to the subject an anti-Programmed Death-1 (PD-1) antibody. In some embodiments, this invention relates to methods for treating Hodgkin's lymphoma in a subject comprising administering to the subject a combination of an anti-cancer agent which is an anti-Programmed Death-1 (PD-1) antibody and another anti-cancer agent such as an anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) antibody.
Owner:BRISTOL MYERS SQUIBB CO
Disruption of programmed death 1 (pd-1) ligand to adjuvant adeno-associated virus vector vaccines
InactiveUS20100035973A1Enhance immune responseImprove efficiencyPeptide/protein ingredientsGenetic material ingredientsVector vaccineProgrammed death
The invention provides for methods of modulating an immune response against a therapeutic polypeptide or an antigenic polypeptide delivered via rAAV comprising administering a modulator of programmed death-1 (PD-1) signaling.
Owner:NATIONWIDE CHILDRENS HOSPITAL
Bispecific antibodies
InactiveUS20160145355A1Hybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeBispecific antibody
The present invention provides, inter alia, bispecific antibodies containing a first antigen binding moiety that specifically binds an epitope on human cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and a second antigen binding moiety that specifically binds an epitope on a human programmed death 1 (PD-1) receptor. Also provided are pharmaceutical compositions containing such bispecific antibodies, as well as methods and kits for treating cancer using such bispecific antibodies and pharmaceutical compositions.
Owner:BIOMED VALLEY DISCOVERIES INC
Stable antibody formulation
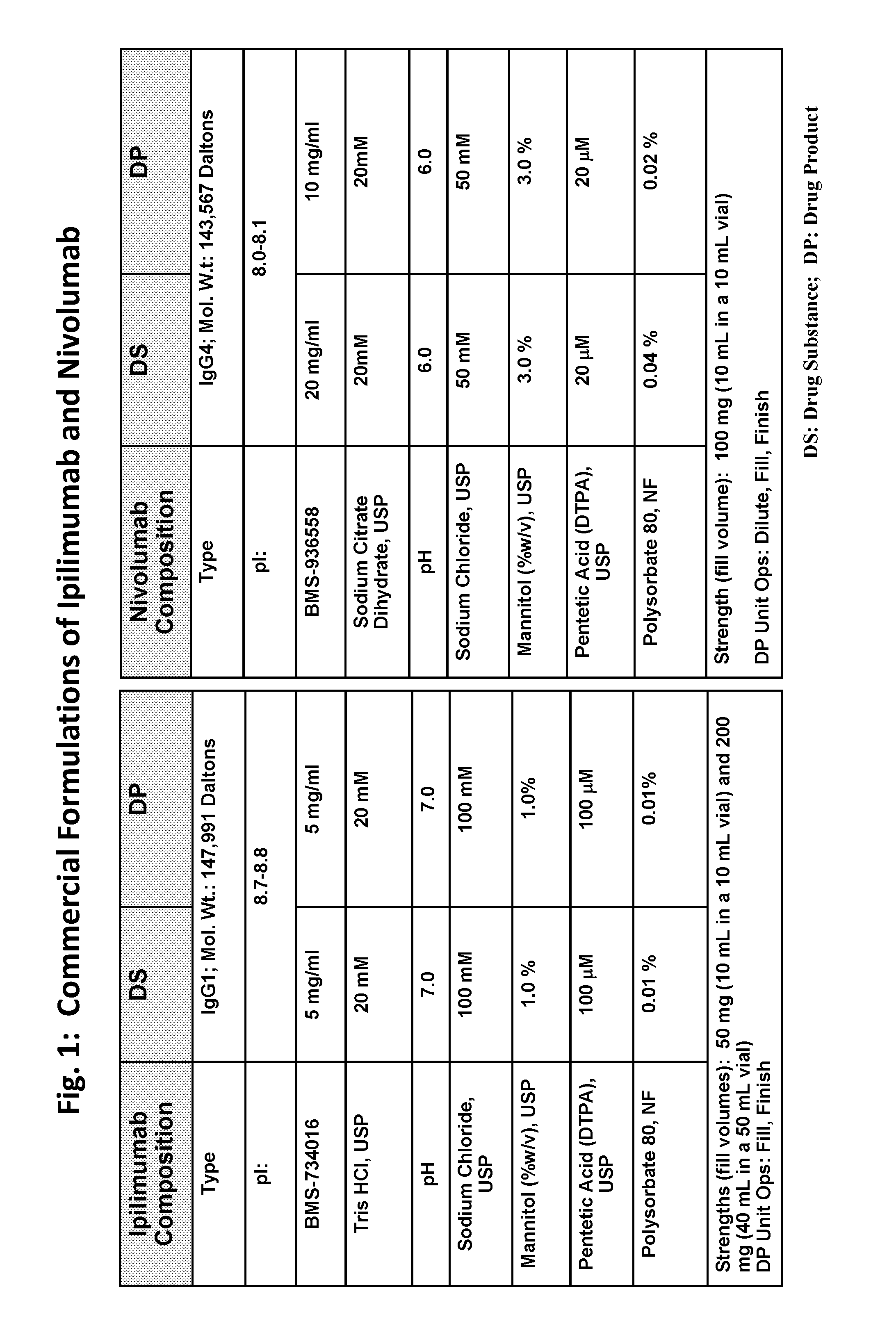
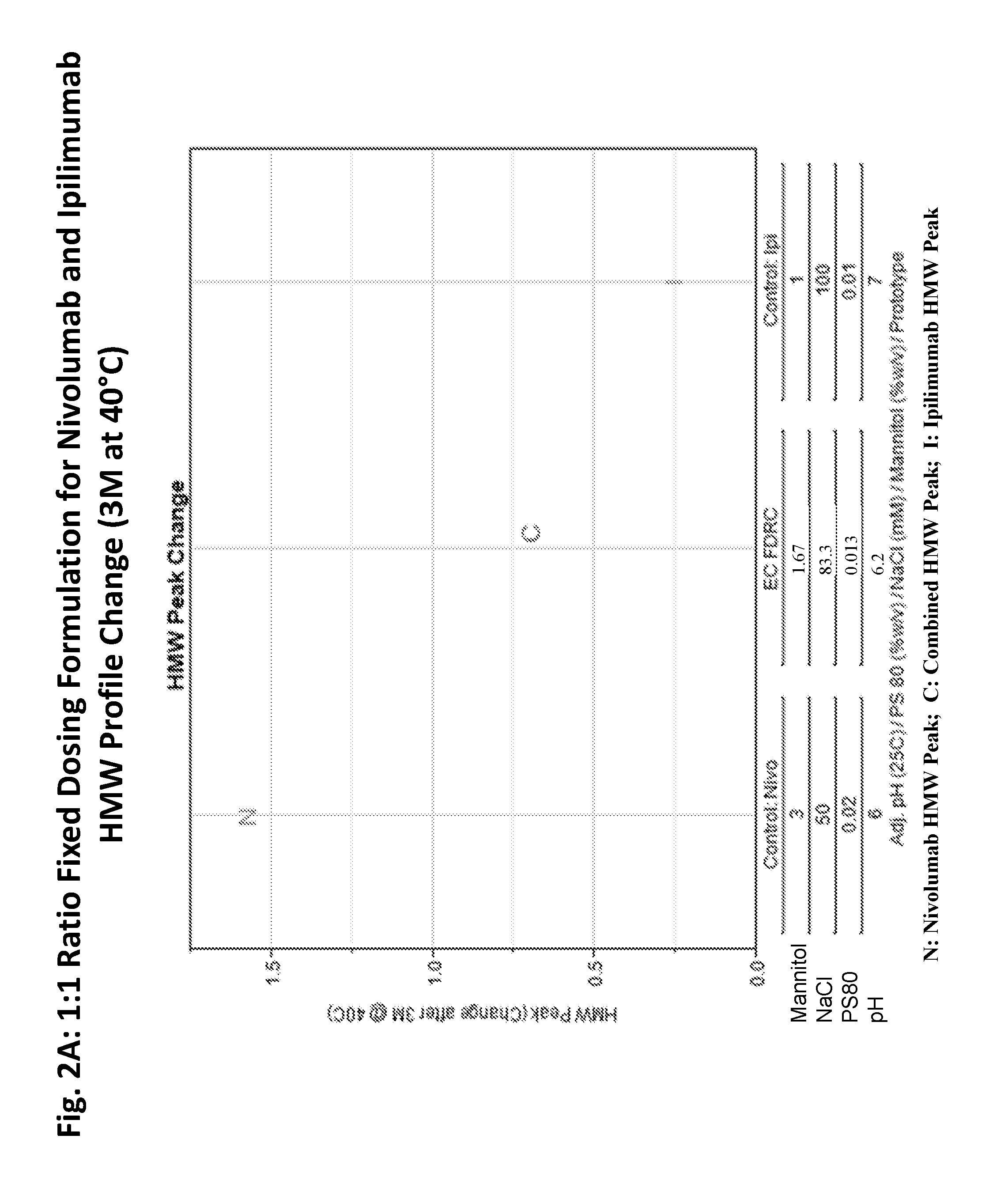
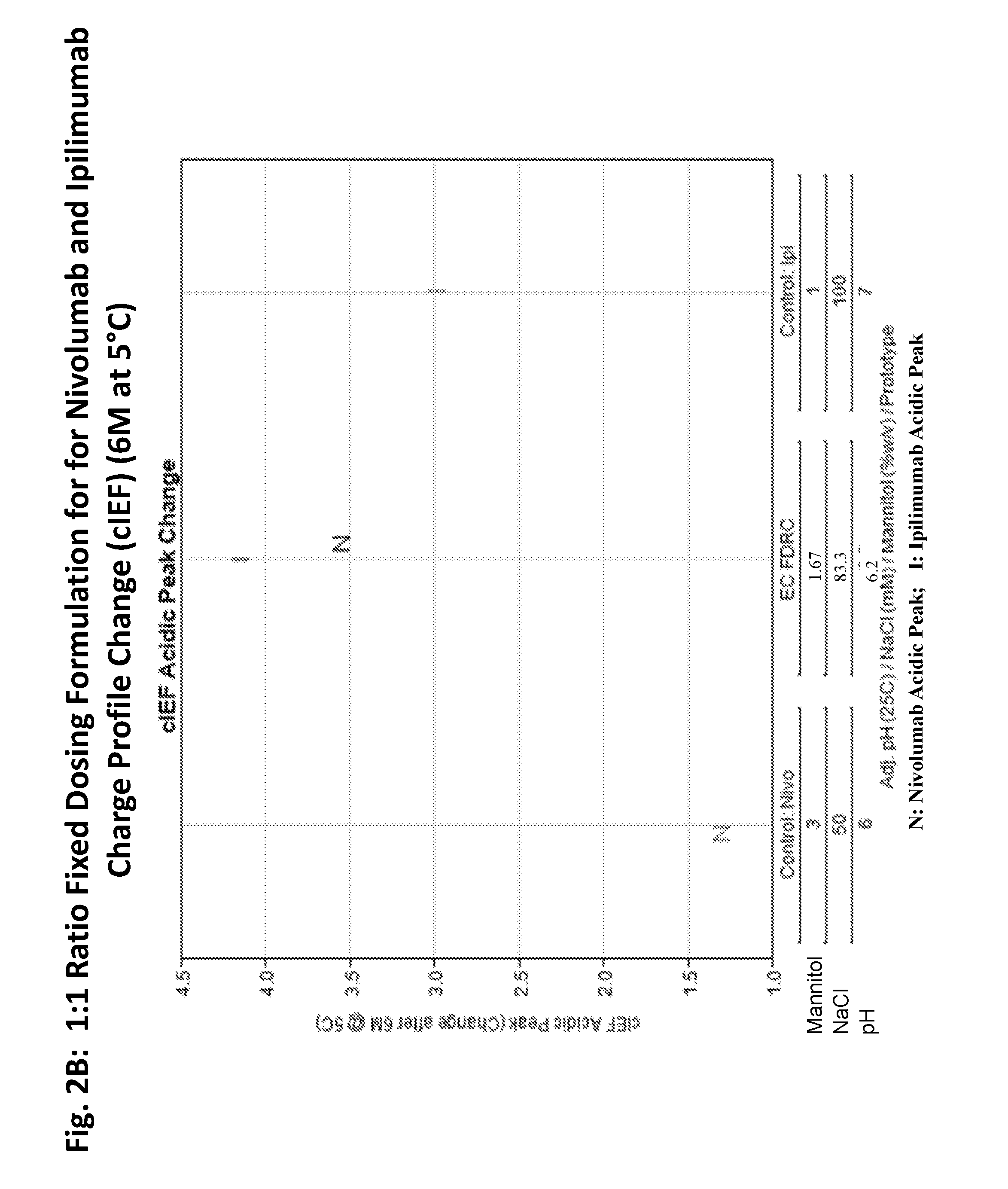
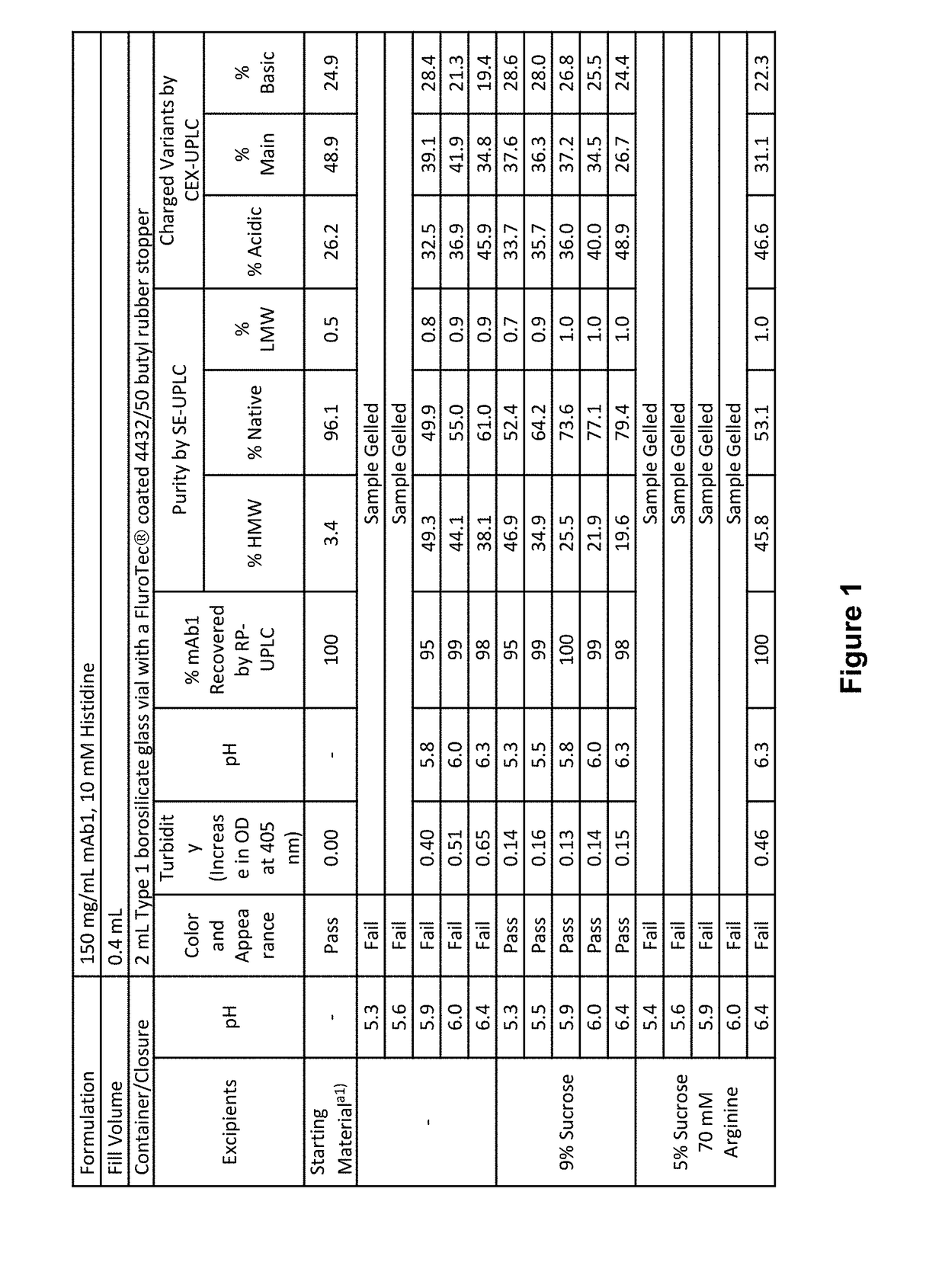
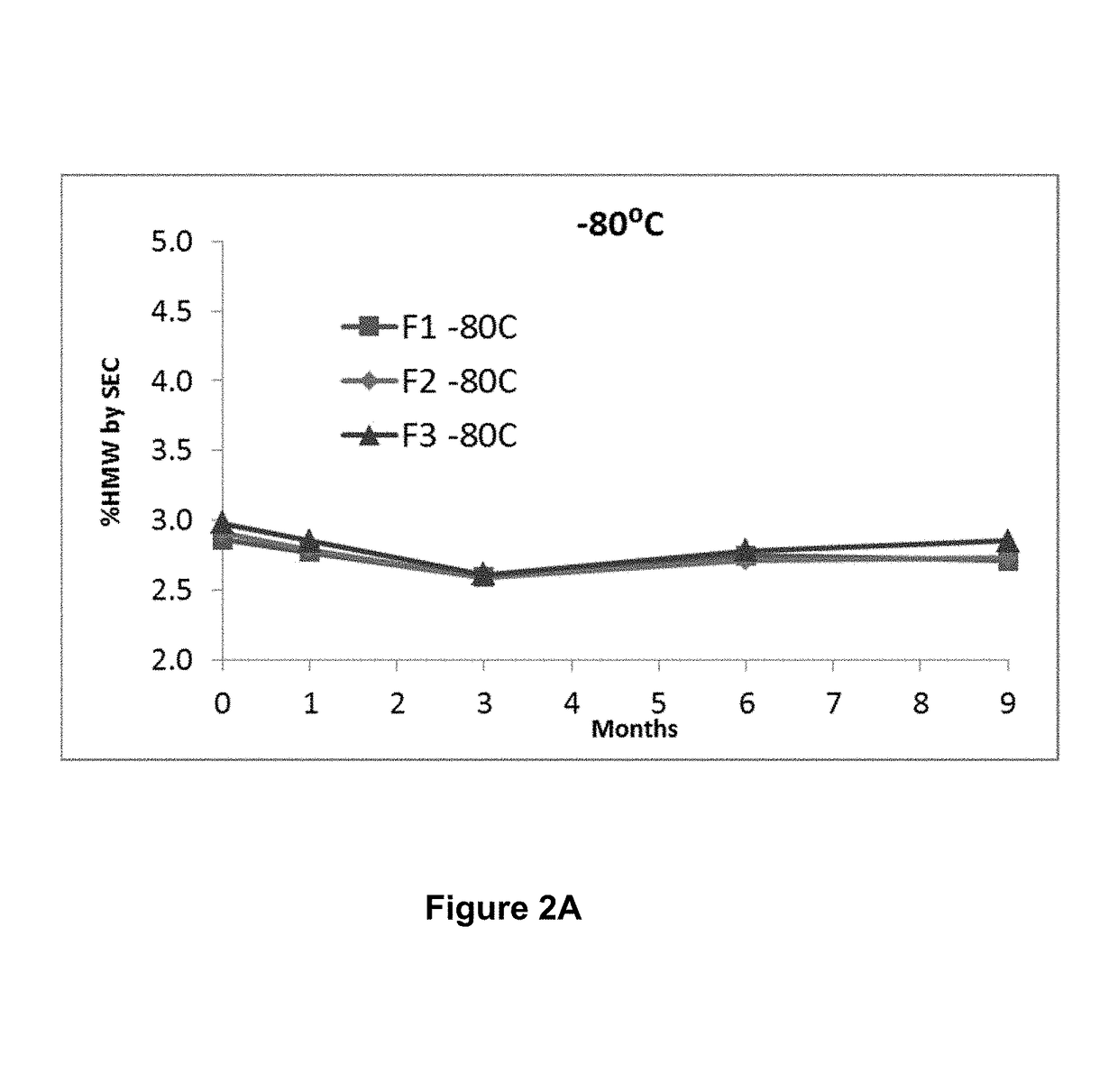
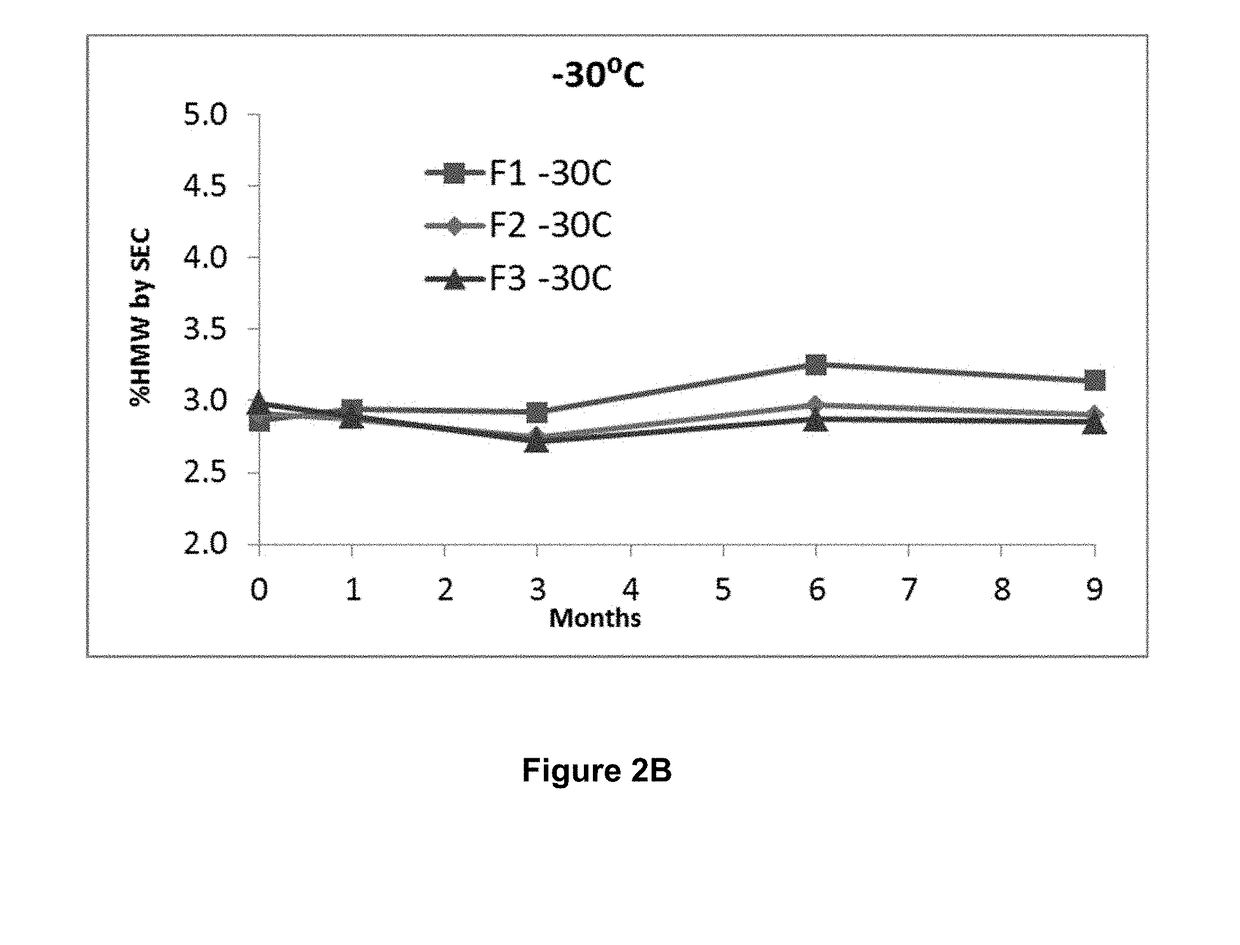
ActiveUS20190040137A1Pharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathPharmaceutical formulation
The present invention provides stable pharmaceutical formulations comprising a human antibody that specifically binds to human programmed death-1 protein (PD-1). In certain embodiments, the formulations contain, in addition to an anti-PD-1 antibody, a buffer, an amino acid, a non-ionic surfactant, and a sugar. The pharmaceutical formulations of the present invention exhibit a substantial degree of antibody stability upon stress and storage.
Owner:REGENERON PHARM INC
Antibodies directed against programmed death-1 (PD-1)
The invention relates to an isolated immunoglobulin heavy chain polypeptide and an isolated immunoglobulin light chain polypeptide that bind to a programmed death-1 (PD-1) protein. The invention provides a PD-1-binding agent that comprises the aforementioned immunoglobulin heavy chain polypeptide and immunoglobulin light chain polypeptide. The invention also provides related vectors, compositions, and methods of using the PD-1-binding agent to treat a cancer or an infectious disease.
Owner:ANAPTYSBIO INC
Monoclonal antibody capable of antagonizing and inhibiting bonding of programmed death-1 receptor (PD-1) and ligand thereof as well as encoding sequence and use of monoclonal antibody
ActiveCN104558177AAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsProtein detectionHeavy chain
Disclosed in the present invention are a mouse monoclonal antibody for antagonizing and inhibiting the binding of a programmed death-1 (PD-1) to the ligand thereof, and the heavy chain variable region and light chain variable region amino acid sequences thereof. Also disclosed in the present invention is a DNA molecule nucleotide sequence encoding the heavy chain variable region and light chain variable region of the antibody. Also disclosed in the present invention are a method for preparing a human-mouse chimeric antibody of the antibody and derivatives thereof, and the use thereof in PD-1 protein detection.
Owner:ACROIMMUNE BIOTECH CO LTD
Treating cancer with a combination of a pd-1 antagonist and dinaciclib
ActiveUS20160193334A1Improve anti-tumor efficacyOrganic active ingredientsAntibody ingredientsPD-L1Dinaciclib
The present disclosure describes combination therapies comprising an antagonist of Programmed Death 1 receptor (PD-1) and the CDK inhibitor dincaciclib, and the use of the combination therapies for the treatment of cancer, and in particular for treating cancers that express PD-L1.
Owner:MERCK SHARP & DOHME LLC
Methods of inducing tolerance
InactiveUS20120269806A1Overcome CD T cell resistancePromote graft acceptanceDigestive systemMedical devicesTolerabilityGraft acceptance
Methods of inducing tolerance, and promoting graft acceptance, are described herein. The methods include administering to a recipient hematopoietic stem cells and an agonist of Programmed Death 1 (PD-1).
Owner:THE GENERAL HOSPITAL CORP
Checkpoint Blockade and Microsatellite Instability
InactiveUS20170267760A1High mutational burdenMicrobiological testing/measurementDigestive systemProgrammed deathImmune tolerance
Blockade of immune checkpoints such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) shows promise in patients with cancer. Inhibitory antibodies directed at these receptors have been shown to break immune tolerance and promote anti-tumor immunity. These agents work particularly well in patients with a certain category of tumor. Such tumors may be particularly susceptible to treatment because of the multitude of neoantigens which they produce.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
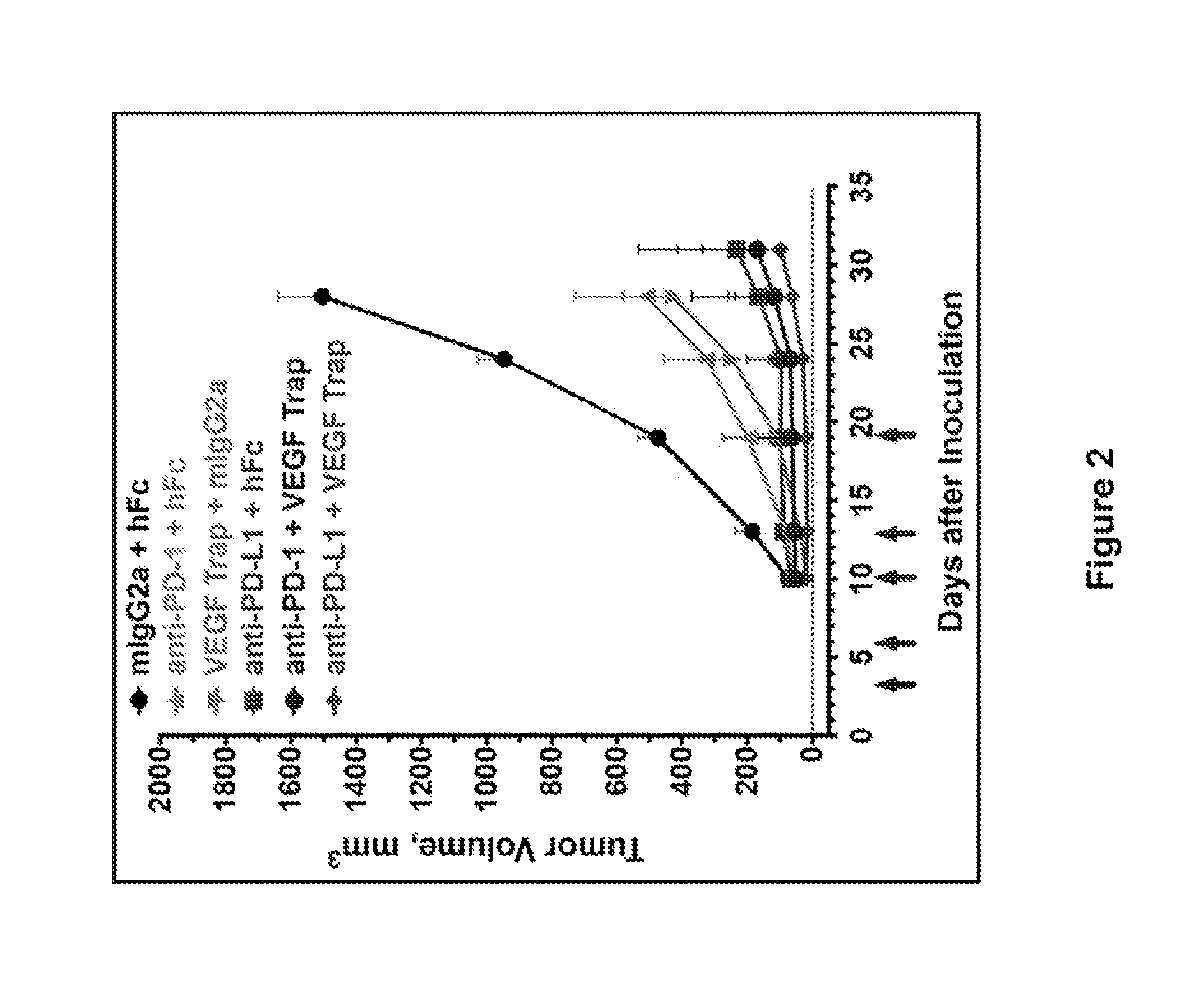
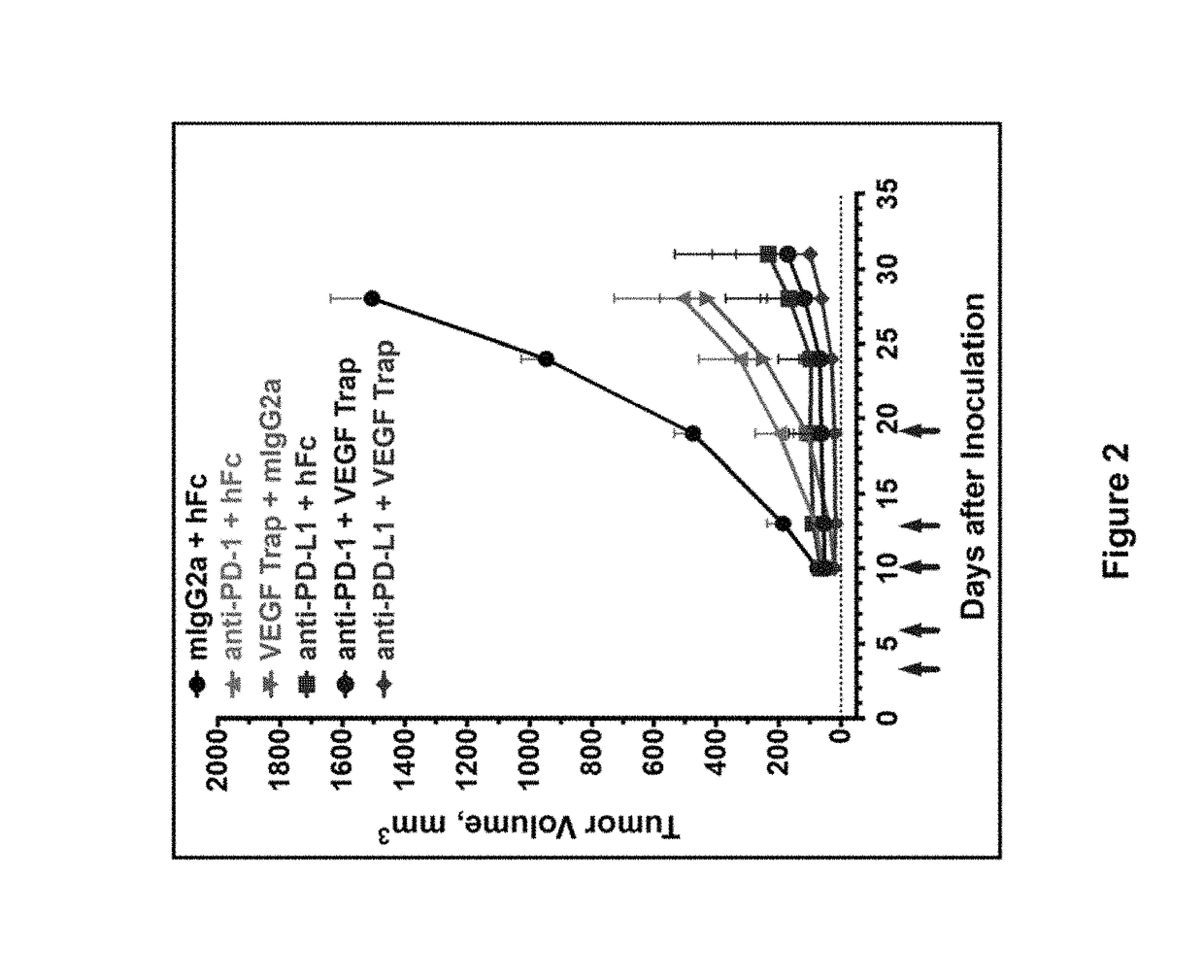
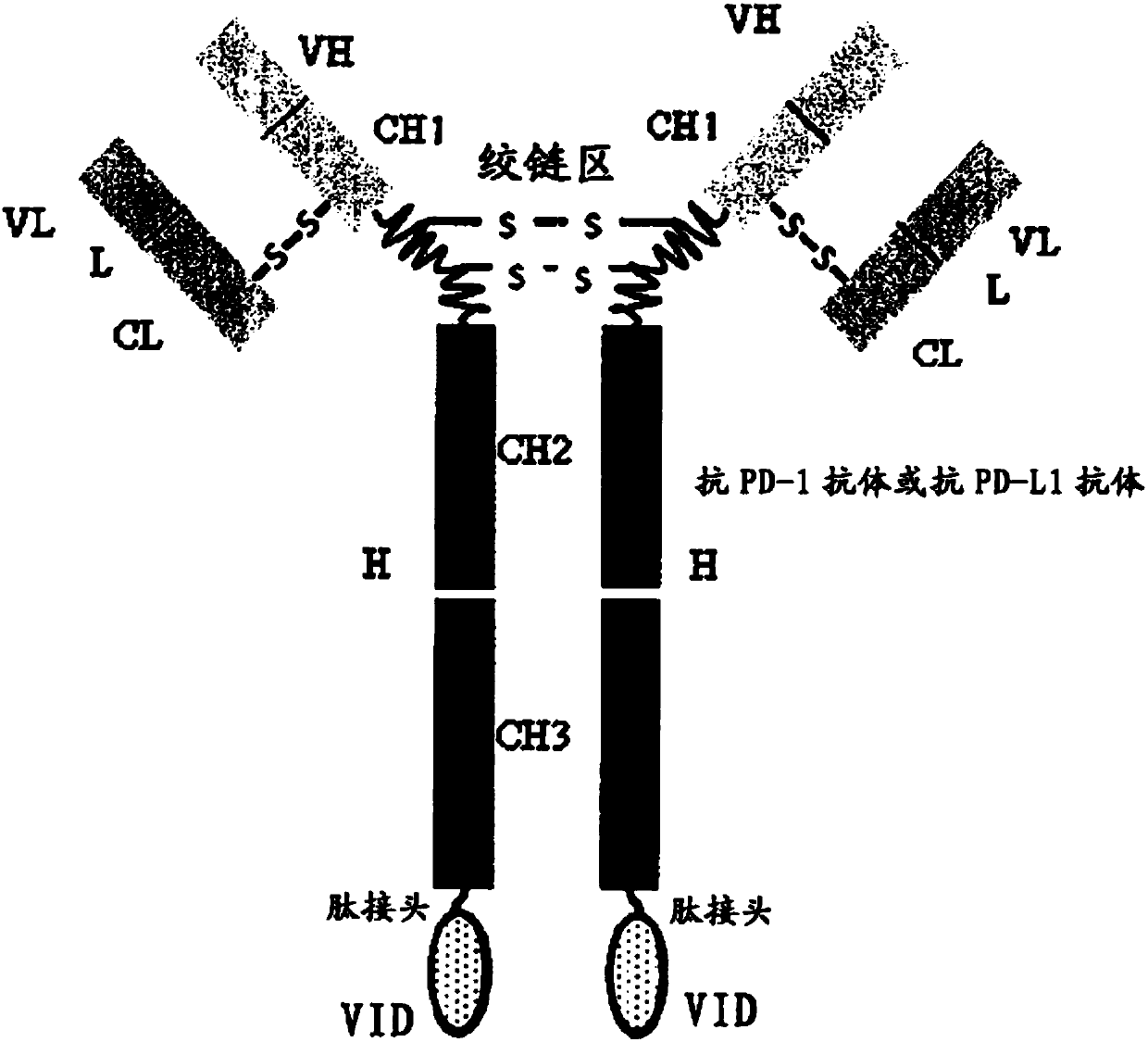
Double-targeted fusion protein of target programmed death-1 (PD-1) or target programmed death-1 ligand (PD-L1) and a target vascular endothelial cell growth factor (VEGF) family, and application of double-targeted fusion protein
The invention provides a double-targeted fusion protein of target programmed death-1 (PD-1) or target programmed death-1 ligand (PD-L1) and a target vascular endothelial cell growth factor (VEGF) family. The double-targeted fusion protein comprises (i) an anti-PD-1 antibody or an anti-PD-L1 antibody and (ii) a VEGF family inhibiting domain (VID) effectively connected to the C end of each of two heavy chains of the anti-PD-1 antibody or the anti-PD-L1 antibody. The invention further provides polynucleotide coding the double-targeted fusion protein, a carrier containing the polynucleotide, a host cell containing the polynucleotide or the carrier, and application of the double-targeted fusion protein to therapy, prevention and / or diagnosis of diseases related to PD-1 activity, PD-L1 activityand VEGF family activity in an individual.
Owner:BEIJING BIYANG BIOTECH
Monoclonal antibodies for antagonizing and inhibiting combination of programmed death-1 (PD-1) and ligands of PD-1, hybridoma cell line secreting monoclonal antibodies and application of monoclonal antibodies
InactiveCN104560884ABinding block inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureProgrammed deathMouse monoclonal antibody
The invention discloses mice monoclonal antibodies for antagonizing and inhibiting combination of programmed death-1 (PD-1) and ligands of PD-1 and a hybridoma cell line secreting the monoclonal antibodies. The preservation number of the hybridoma cell line is CGMCC No. 8351. The invention further discloses an application of the monoclonal antibodies to detection of PD-1 protein.
Owner:SUZHOU STAINWEI BIOTECH INC
Combination of a pd-1 antagonist and an ido1 inhibitor for treating cancer
InactiveUS20170037125A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsPD-L1Dioxygenase
The present disclosure describes combination therapies comprising an antagonist of Programmed Death 1 receptor (PD-1) and a selective inhibitor of indoleamine 2, 3-dioxygenase 1 (IDO1), and the use of the combination therapies for the treatment of cancer, and in particular for treating cancers that express PD-L1.
Owner:MERCK SHARP & DOHME CORP +1
Treatment of lung cancer using a combination of an Anti-pd-1 antibody and another Anti-cancer agent
InactiveUS20180319892A1Reliable responseHeavy metal active ingredientsImmunoglobulins against growth factorsAnticarcinogenTyrosine-kinase inhibitor
Owner:BRISTOL MYERS SQUIBB CO
Checkpoint Blockade and Microsatellite Instability
InactiveUS20170313775A1Microbiological testing/measurementDigestive systemProgrammed deathImmune tolerance
Blockade of immune checkpoints such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) shows promise in patients with cancer. Inhibitory antibodies directed at these receptors have been shown to break immune tolerance and promote anti-tumor immunity. These agents work particularly well in patients with a certain category of tumor. Such tumors may be particularly susceptible to treatment because of the multitude of neoantigens which they produce.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Use of interferon in treatment of tumor, and related product and method
ActiveCN103536917AImprove efficiencyGood treatment effectPeptide/protein ingredientsAntibody ingredientsMedicineTreatment field
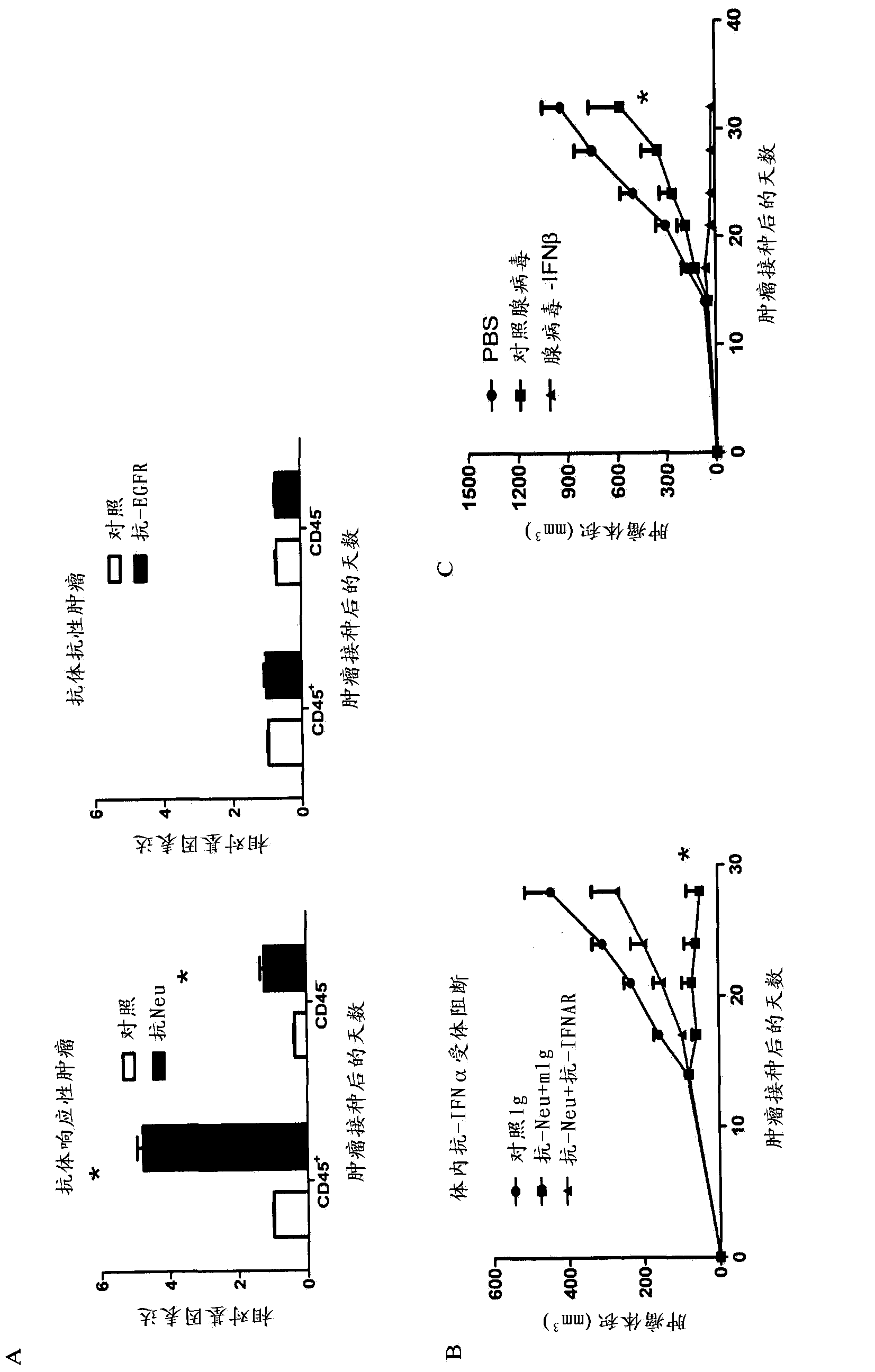
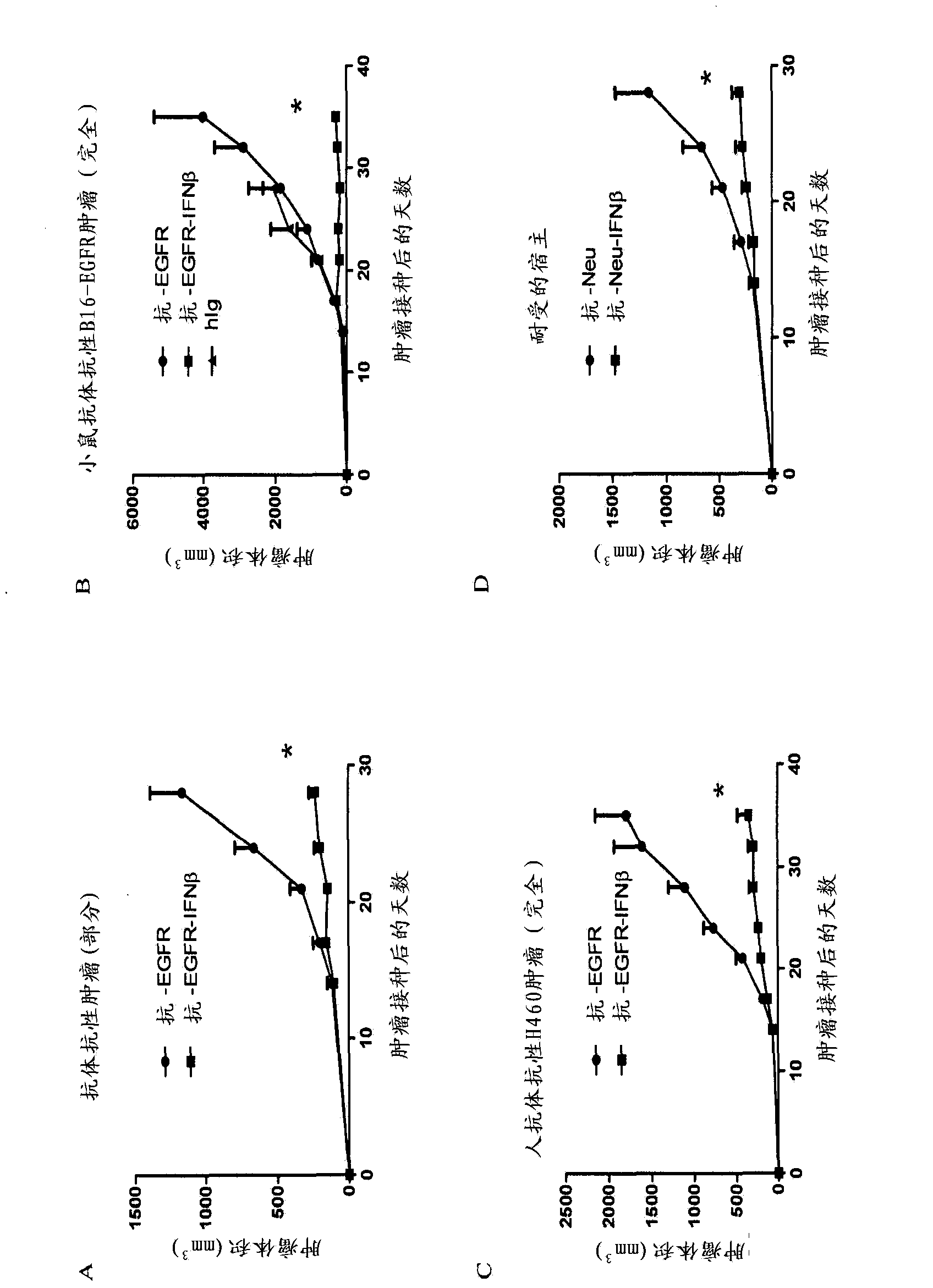
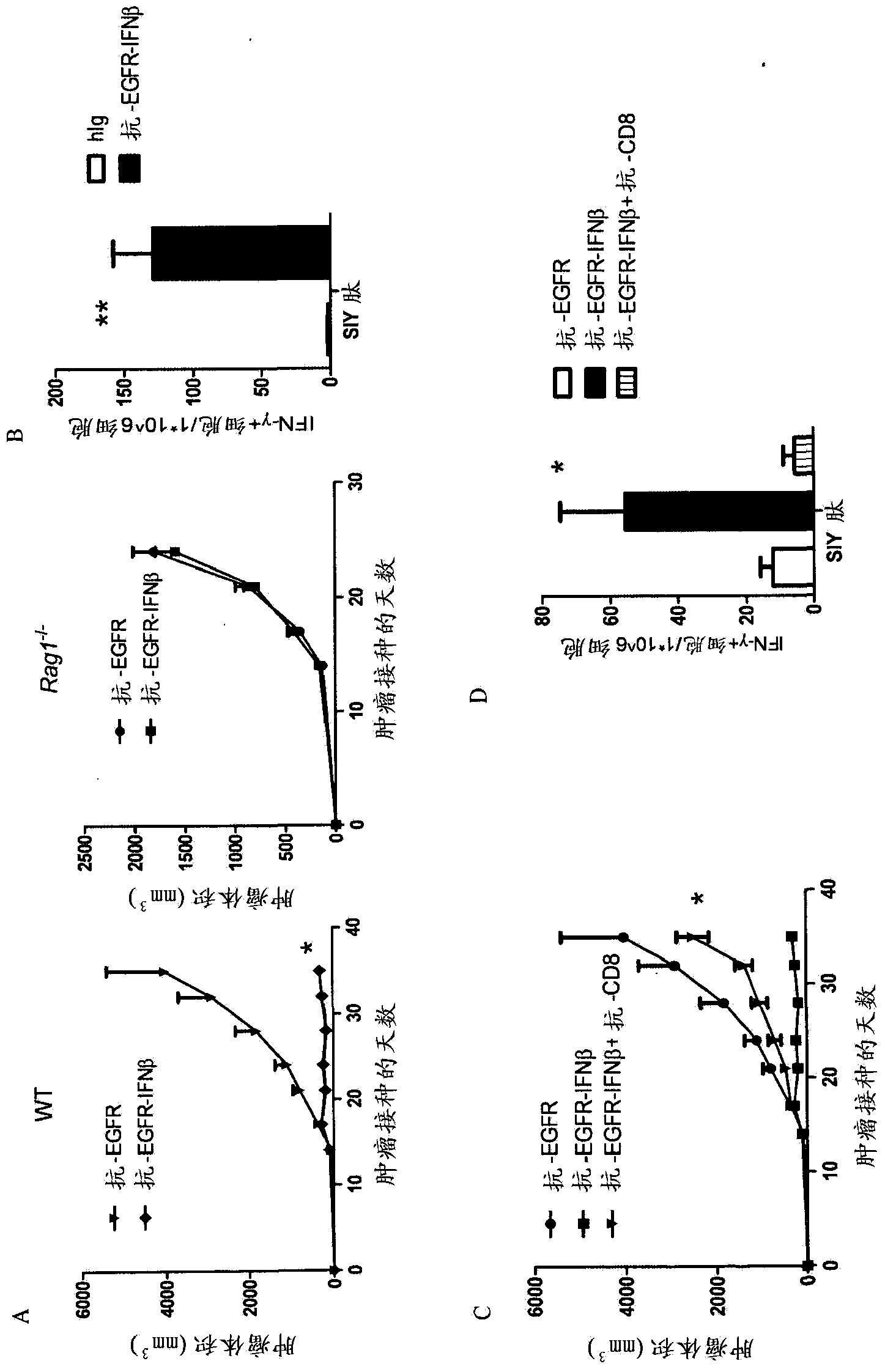
The invention relates to use of interferon in treatment of a tumor, and related products and a method, particularly relates to the field of tumor treatment, and especially aims at overcoming the resistance of the tumor on an antibody therapy. Particularly, the invention relates to application, and related product and method for inhibiting growth and recurrence of the tumor and causing tumor regression by combined utilization of interferon, especially I-type interferon, and a compound for blocking up a programmed death 1 or programmed death ligand (PD-1 / PDL) passage.
Owner:DINGFU BIOTARGET
Preparation method of high-efficiency killer cell preparation adopting immunodetection point dual-block CTL (cytotoxic lymphocyte)
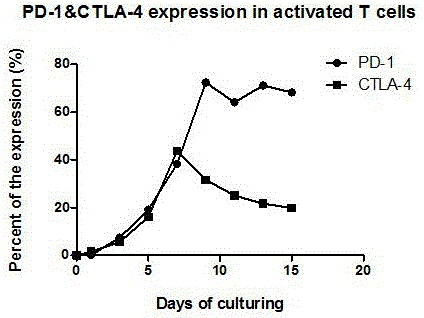
ActiveCN106177931AReduce exhaustEnhance tumor killing effectCancer antigen ingredientsBlood/immune system cellsTumor specificInterleukin 4
The invention discloses a preparation method of a high-efficiency killer cell preparation adopting an immunodetection point dual-block CTL (cytotoxic lymphocyte). The preparation method comprises steps as follows: step 1), collecting mononuclear cells in peripheral blood; step 2), performing DC (dendritic cell) separation and T cell separation on the separated mononuclear cells with an adherence method; step 3), adding adhering DCs to a serum-free culture medium containing GM-CSF (granulocyte-macrophage colony-stimulating factor) and IL-4 (interleukin-4) for preparation of a DC vaccine; step 4), adding suspending T cells to CD3 and IL-2 for T cell culture and amplification; step 5), adding antigen-supported DCs to the amplified T cells for preparation of the CTL, and adding PD-1 (Programmed death 1) and CTLA-4 (cytotoxic t lymphocyte associated antigen-4) antibodies for preparation of the cell preparation with the dual-block CTL effect. According to the novel technology and the novel method provided by the invention, an immune brake effect is effectively regulated and T cell depletion is retarded by blocking inhibitory molecules on the tumor specific CTL surfaces, so that the tumor cytotoxicity of CTL effector cells is substantially improved.
Owner:浓孚雨医药河北有限公司
Human monoclonal anti-PD-L1 antibodies and methods of use
ActiveUS9828434B2Hybrid immunoglobulinsImmunoglobulins against cytokines/lymphokines/interferonsProgrammed deathPD-L1
The present invention comprises human monoclonal antibodies that bind to PD-L1 (also known as programmed death ligand 1 or B7H1). Binding of the invented antibody to PD-L1 inhibits binding to its receptor, PD1 (programmed death 1), and ligand-mediated activities and can be used to treat cancer and chronic viral infections.
Owner:DANA FARBER CANCER INST INC
Soluble PD-1 (programmed death-1) molecule with high affinity
InactiveCN107987153AHigh affinityCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsWild typeLymphocyte
The invention provides a soluble PD-1 (programmed death-1) molecule with high affinity, and particularly provides a PD-1 molecule. The PD-1 molecule is mutated on the basis of a wild PD-1 molecule, and affinity of the PD-1 molecule and a PDL-1 (Programmed Death Ligand-1) molecule is at least twice that of the wild PD-1 molecule and a wild PDL-1 molecule. Meanwhile, the PD-1 molecule can effectively improve the killing capacity of lymphocytes. Besides, the invention provides nucleic acid encoding the PD-1 molecule and a PD-1 molecule complex. The PD-1 molecule can be used alone or in combination with other molecules.
Owner:GUANGDONG XIANGXUE LIFE SCI LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com