Compositions comprising a combination of an Anti-pd-1 antibody and another antibody
a technology of anti-pd-1 antibody and antibody, which is applied in the field of compositions containing a combination of anti-pd-1 antibody and another antibody, can solve the problems of multiple intravenous injections at different times, and the difficulty of single formulation identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
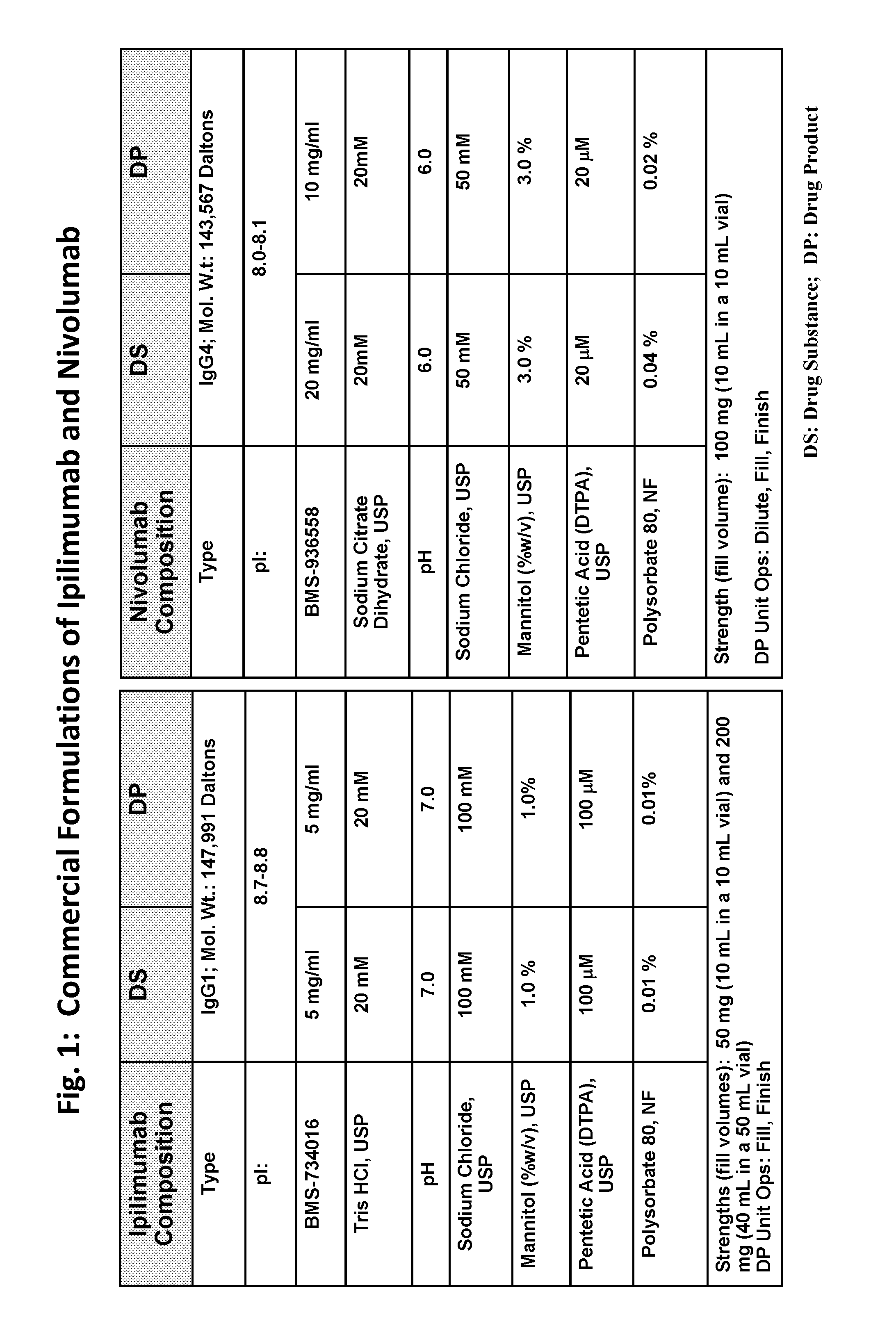
[0203]A feasibility study was performed to evaluate the stability of ipilimumab and nivolumab in a single fixed dose ratio combination (FDRC) formulation created by mixing the individual formulations of ipilimumab and nivolumab (FIG. 1) to a final ratio of ipilimumab to nivolumab of 1:1.
[0204]Ipilimumab (BMS-734016) DP contains 5 mg / mL ipilimumab in 20 mM Tris-HCl, 100 mM NaCl, 1.0% (w / v) Mannitol, 100 μM pentetic acid (DTPA), and 0.01% polysorbate 80 (PS80), at pH 7.0, and it is available as 40 mL in a 50 mL bottle and 10 ml in a 10 ml vial (FIG. 1). Nivolumab (BMS-936558) DP contains 10 mg / mL nivolumab in 20 mM citrate buffer (sodium citrate dihydrate), 50 mM NaCl, 3.0% (w / v) Mannitol, 20 μM DTPA, and 0.02% PS80, at pH 6.0, and it is available as 10 mL in a 10 ml vial (FIG. 1).
[0205]To achieve a 1:1 ratio of ipilimumab to nivolumab, 80 mL of ipilimumab DP (2 bottles) was mixed with 40 mL of nivolumab DP (4 vials), yielding a combined product having 3.3 mg / mL ipilimumab and 3.3 mg / ...
example 2
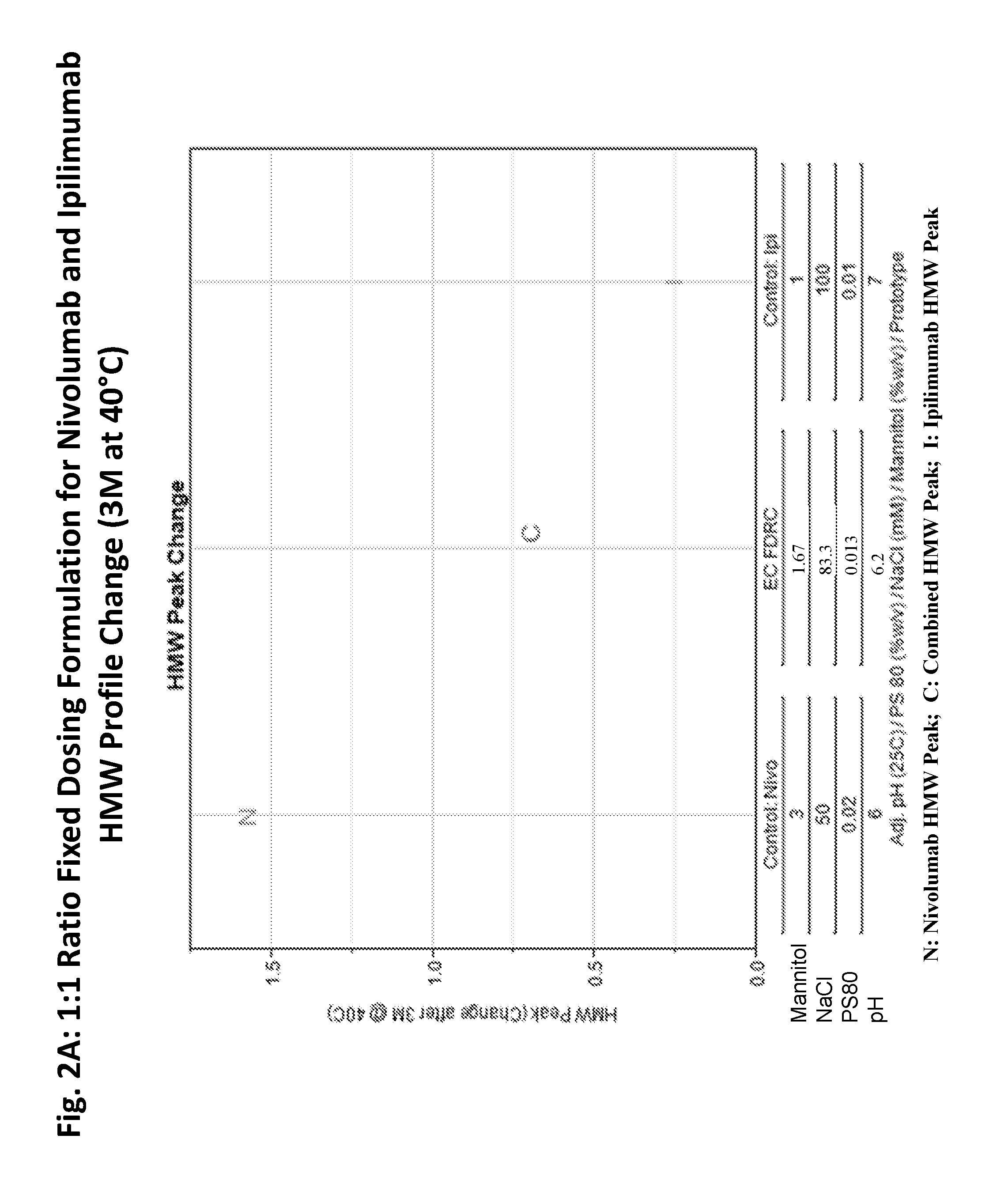
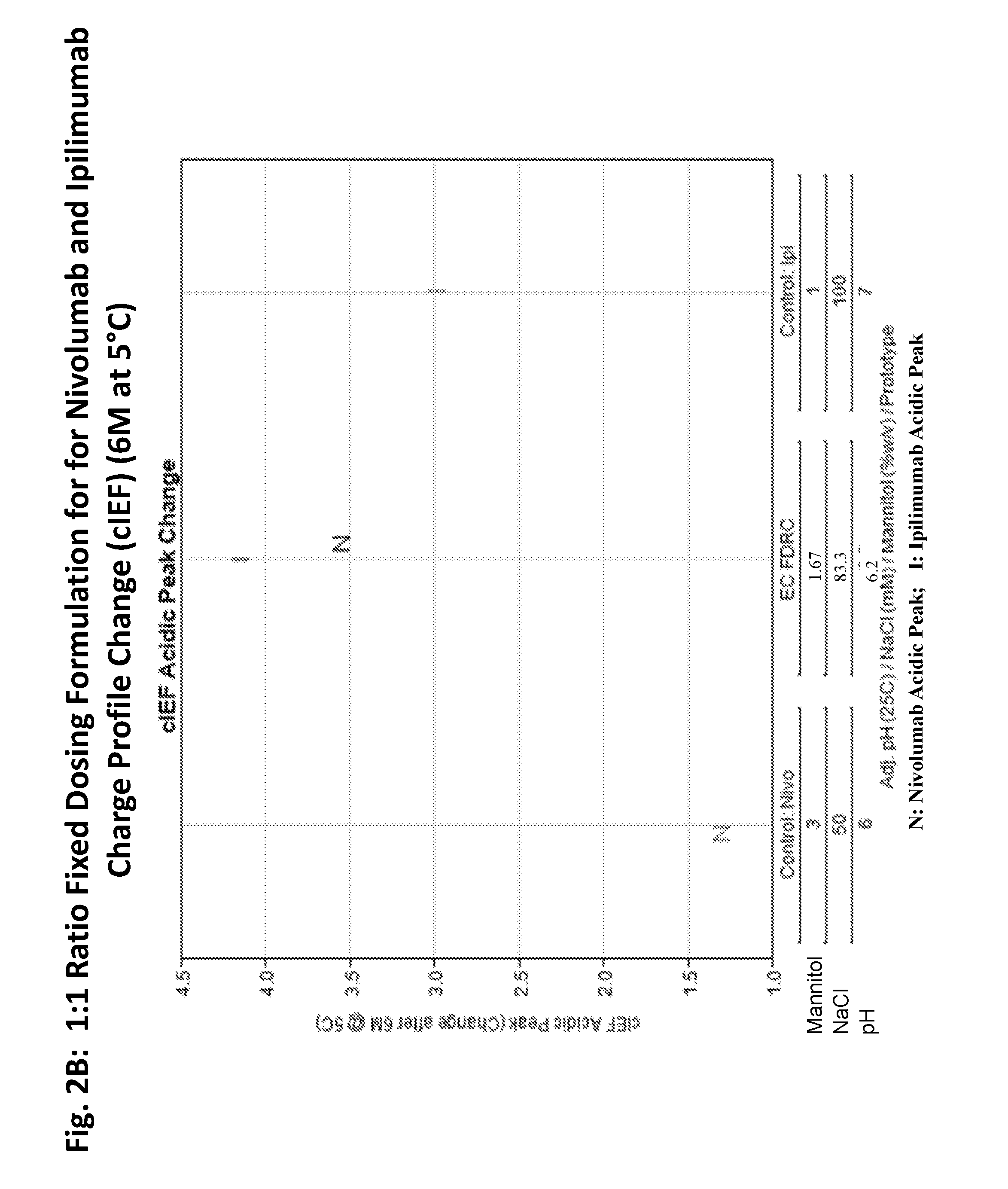
[0213]A feasibility study was performed to evaluate the stability of an ipilimumab / nivolumab FDRC created by mixing the individual formulations of ipilimumab and nivolumab to final ratios of 3:1, 1:1, and 1:3 (Table 3). The FDRC formulations were generated by mixing the ipilimumab DS at 5 mg / mL and nivolumab DS at 20 mg / mL to achieve 3:1, 1:1, and 1:3 protein ratios (Table 3). Each combined solution was further mixed with a stir bar at room temperature for 30 min, transferred to vials, and stored for stability over time. The vials were stored at 5° C., 25° C., and 40° C. for up to 12 months.
TABLE 3EC FDRC (3:1; 1:1; 1:3)—Combinations of Formulations of Ipilimumab DP andNivolumab DPFinal Conc'n in Vial:(mg / mL)MannitolNaClDTPAPS 80PrototypeRatioIpiNivopH% w / vmMμM% w / vEC: pH 6.63:14.621.546.61.1596.1593.850.012EC: pH 6.01:32.868.576.01.8678.5765.710.023EC: pH 6.21:14.004.006.21.6783.3373.330.013Prototype EC: pH 6.6, having a 3:1 ratio of ipilimumab to nivolumab, contained 4.62 mg / mL ip...
example 3
[0219]A design of experiments (DoE) study was performed to identify new candidate ipilimumab / nivolumab formulations. Prototype ipilimumab / nivolumab FDRC (3:1) formulations were made in selected histidine or citrate formulations, as shown in Table 4. All DoE FDRC prototypes were prepared to a final concentration of ipilimumab / nivolumab of 10 mg / mL at a ratio of 3:1 (Table 4). FDRC prototype “Combo 4” contained 20 mM citrate, 50 mM NaCl, 50 μM DTPA, 6% w / v sucrose, and 0.05% w / v PS80, at a theoretical pH of 6. FDRC prototype “Combo 5” contained 20 mM histidine, 50 mM NaCl, 50 μM DTPA, 6% w / v sucrose, and 0.05% w / v PS80, at a theoretical pH of 6.0. FDRC prototype “Combo 6” contained 20 mM histidine, 50 mM NaCl, 50 μM DTPA, 6% w / v sucrose, and 0.05% w / v PS80, at a theoretical pH of 7. FDRC prototype “Combo New” contained 20 mM histidine, 50 μM DTPA, 8.5% w / v sucrose, and 0.05% w / v PS80, at a theoretical pH of 6. FDRC prototype “Combo 8,” which was similar to the current nivolumab DP for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com