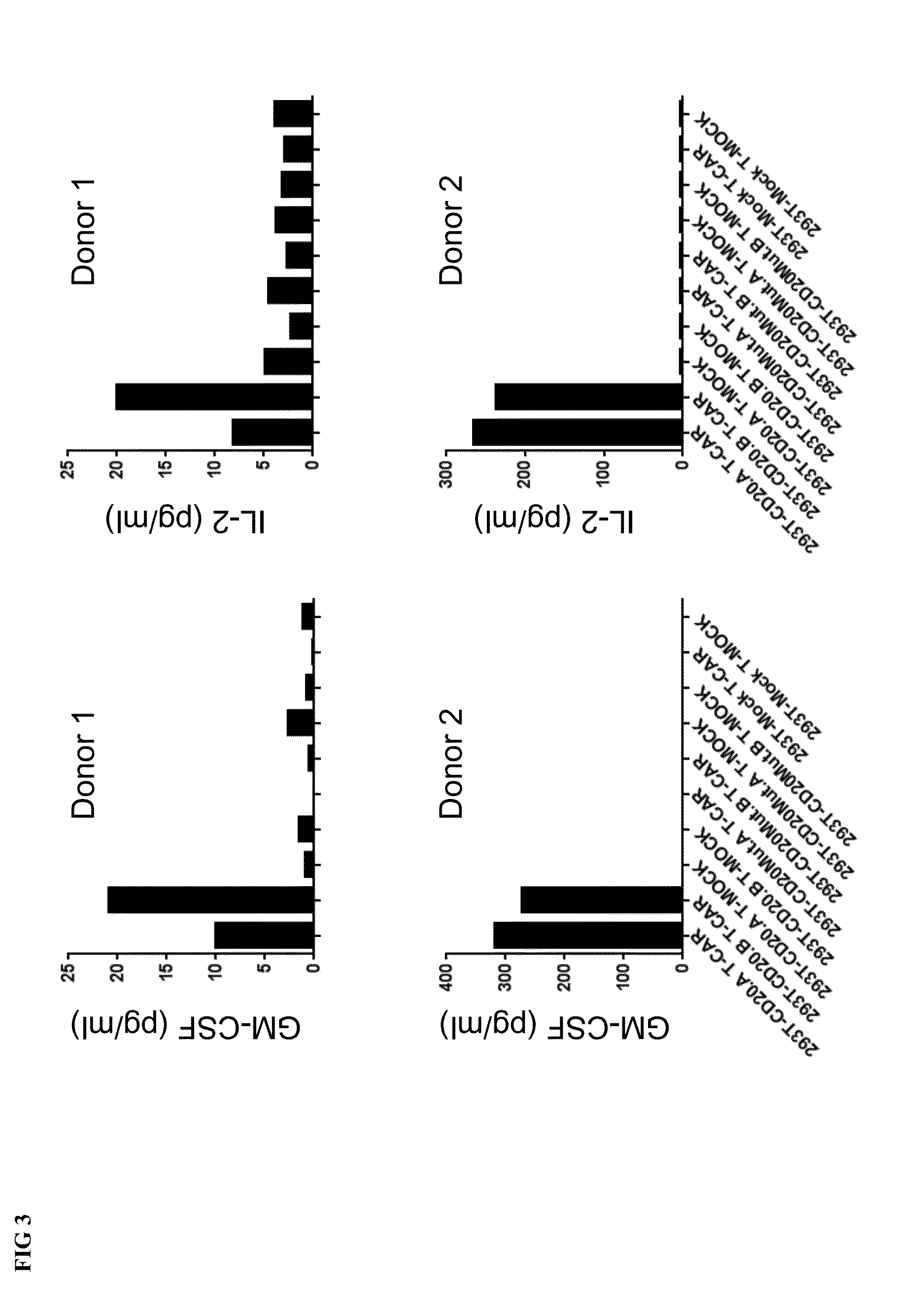
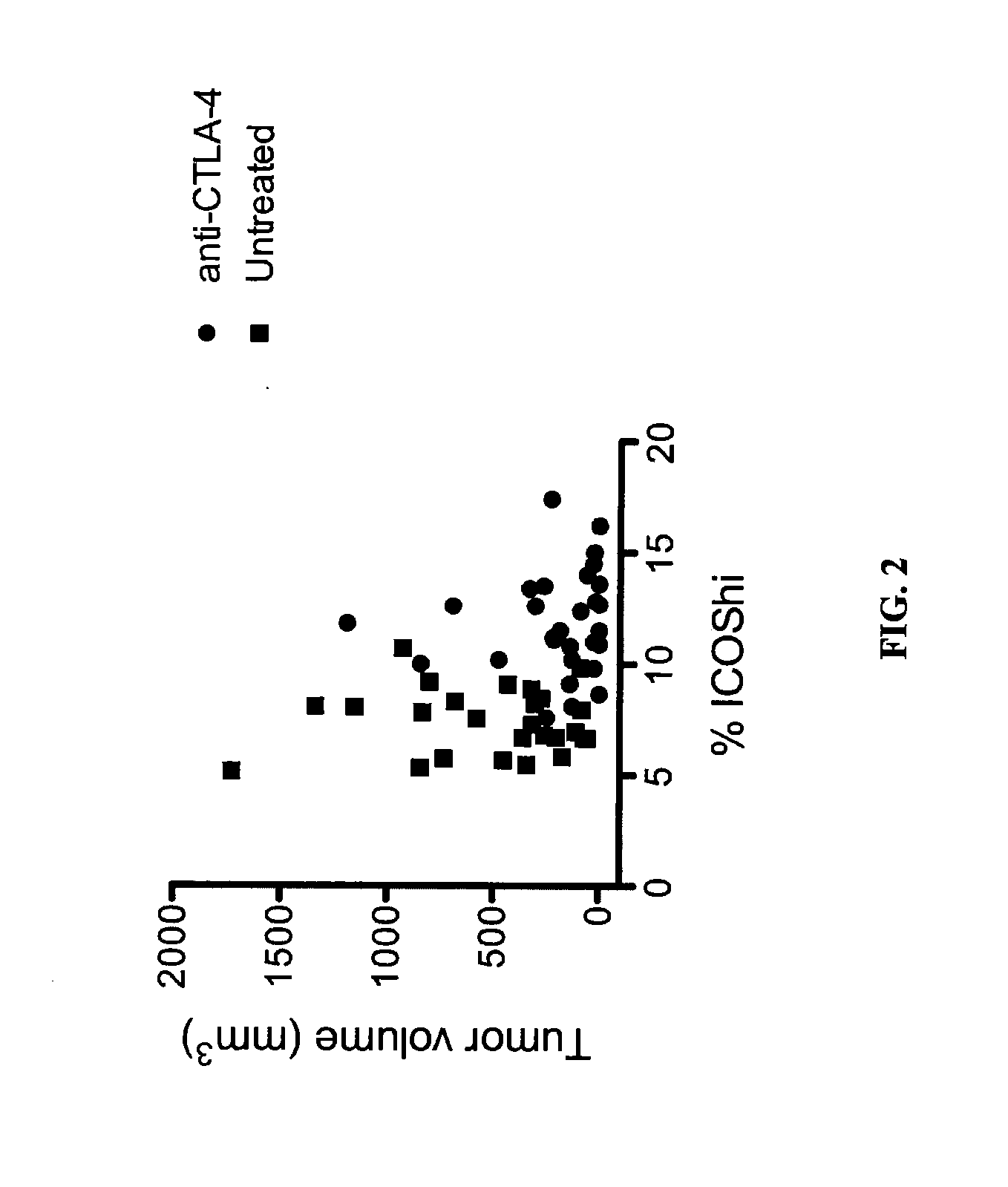
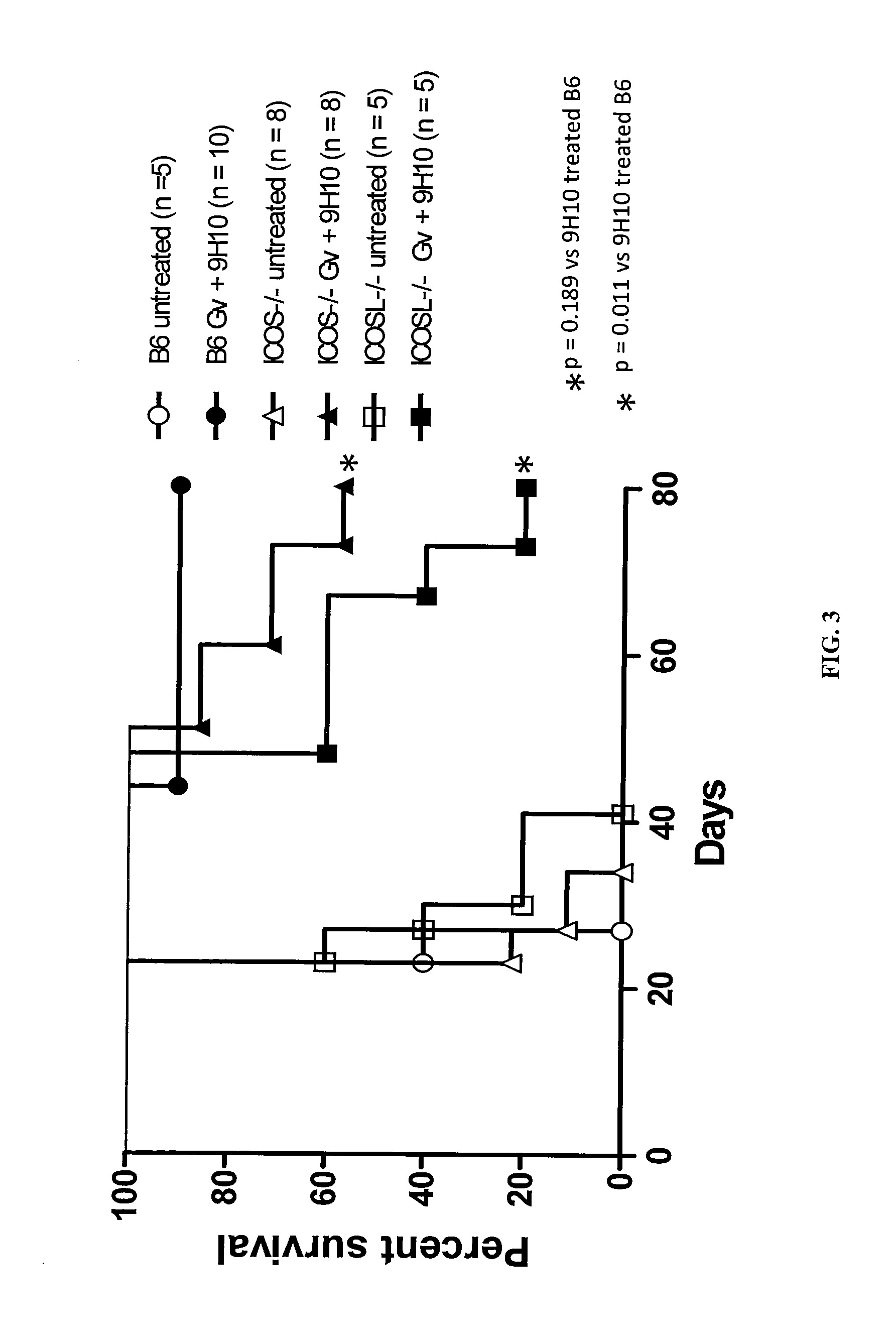
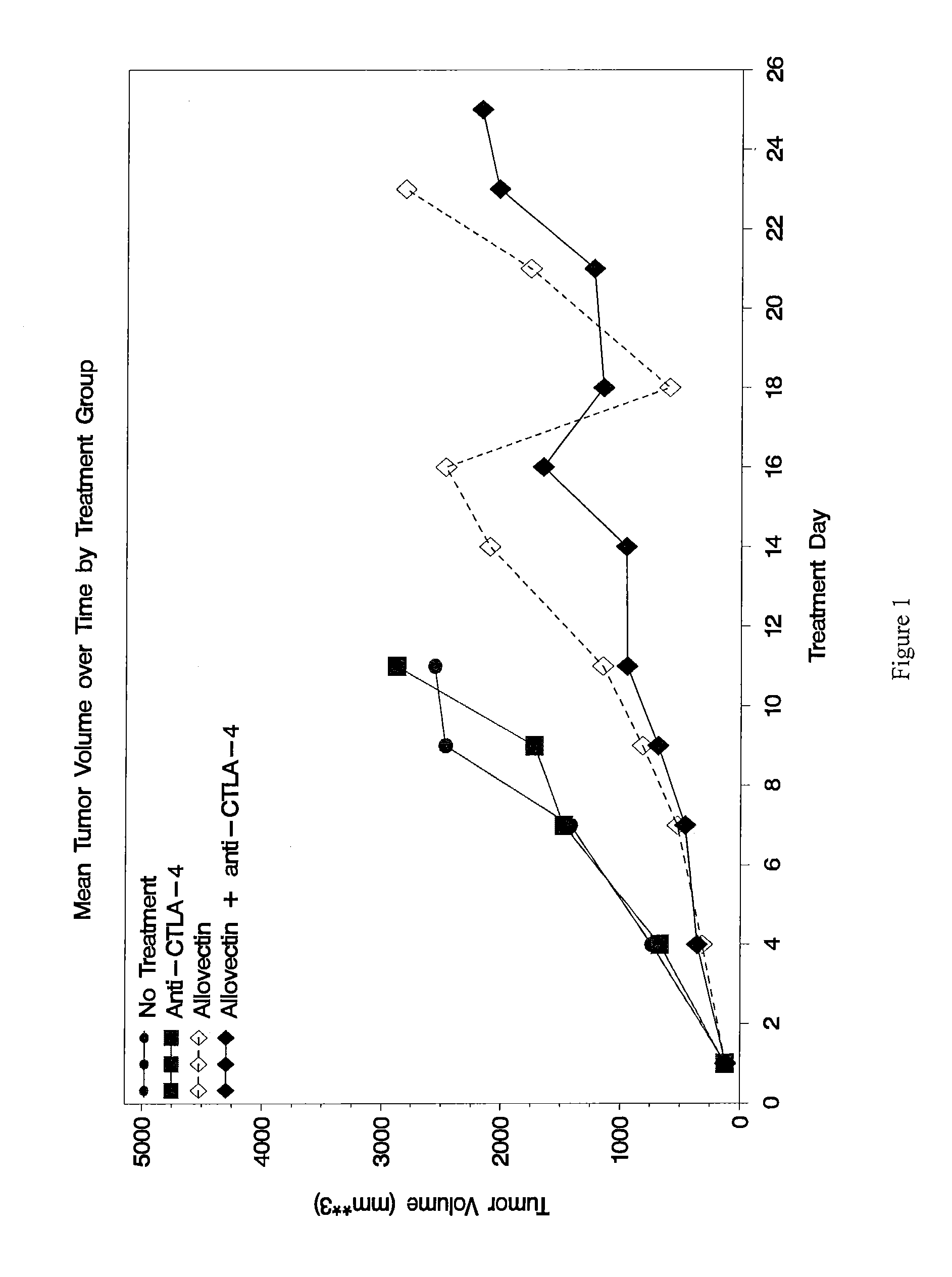
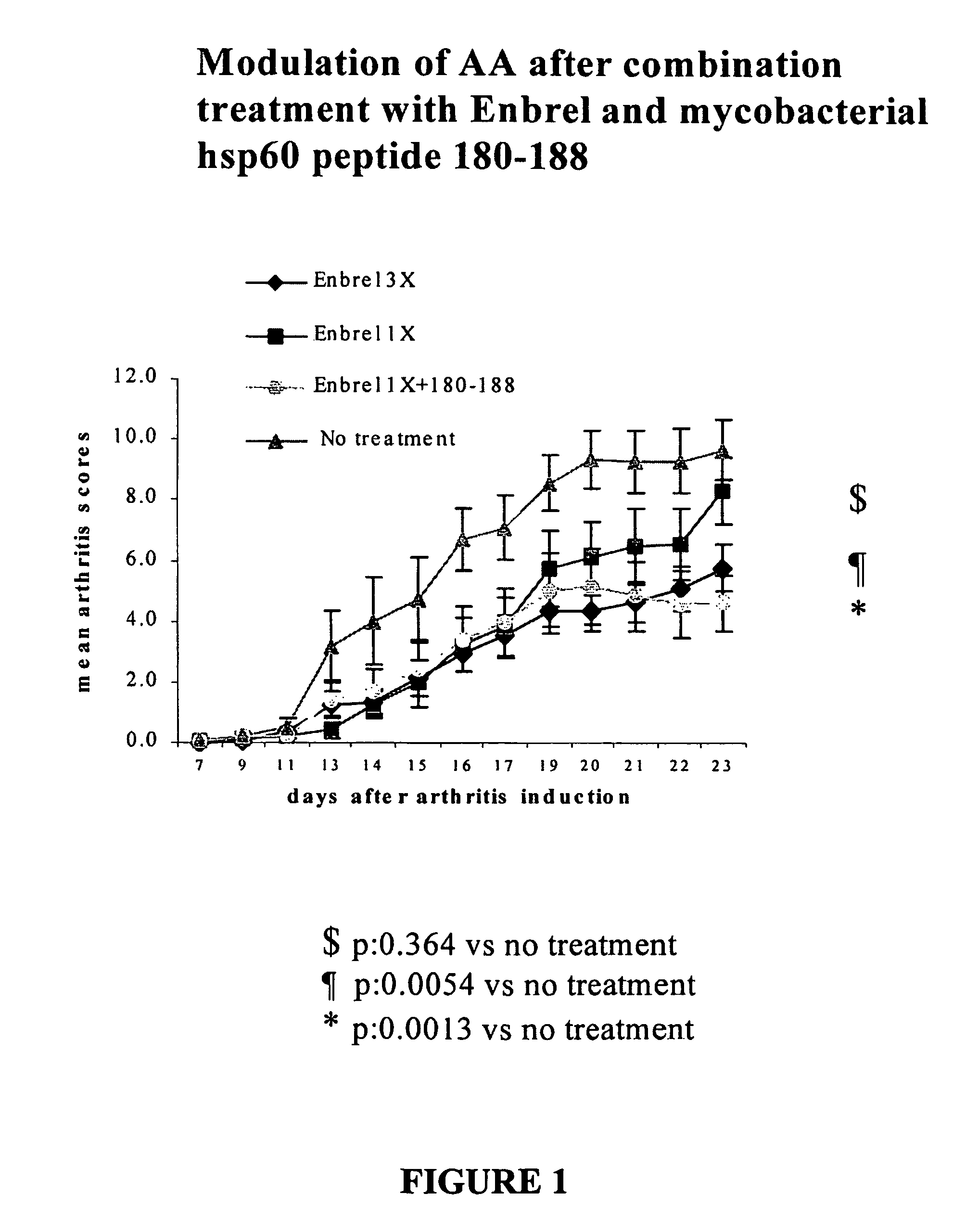
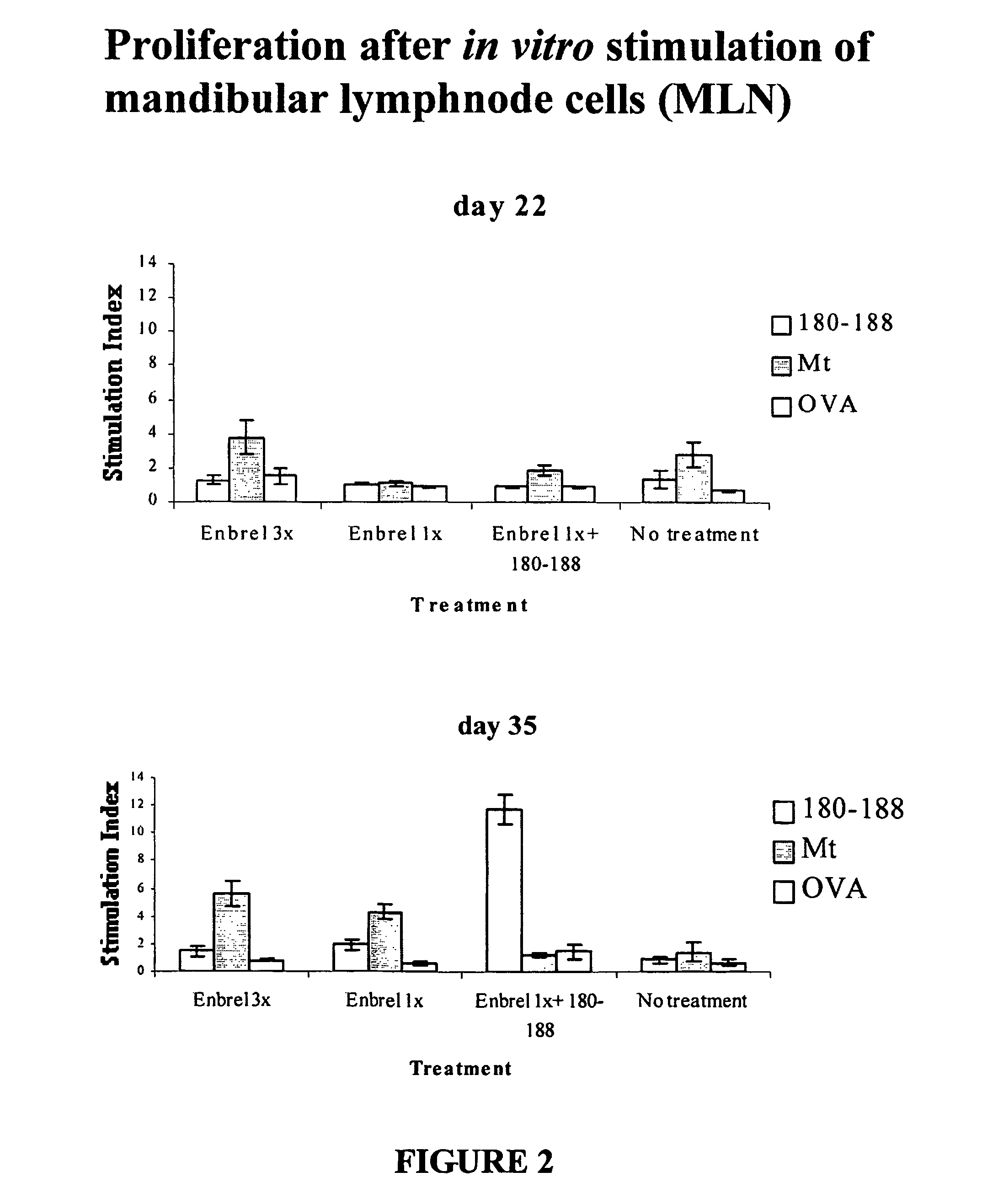
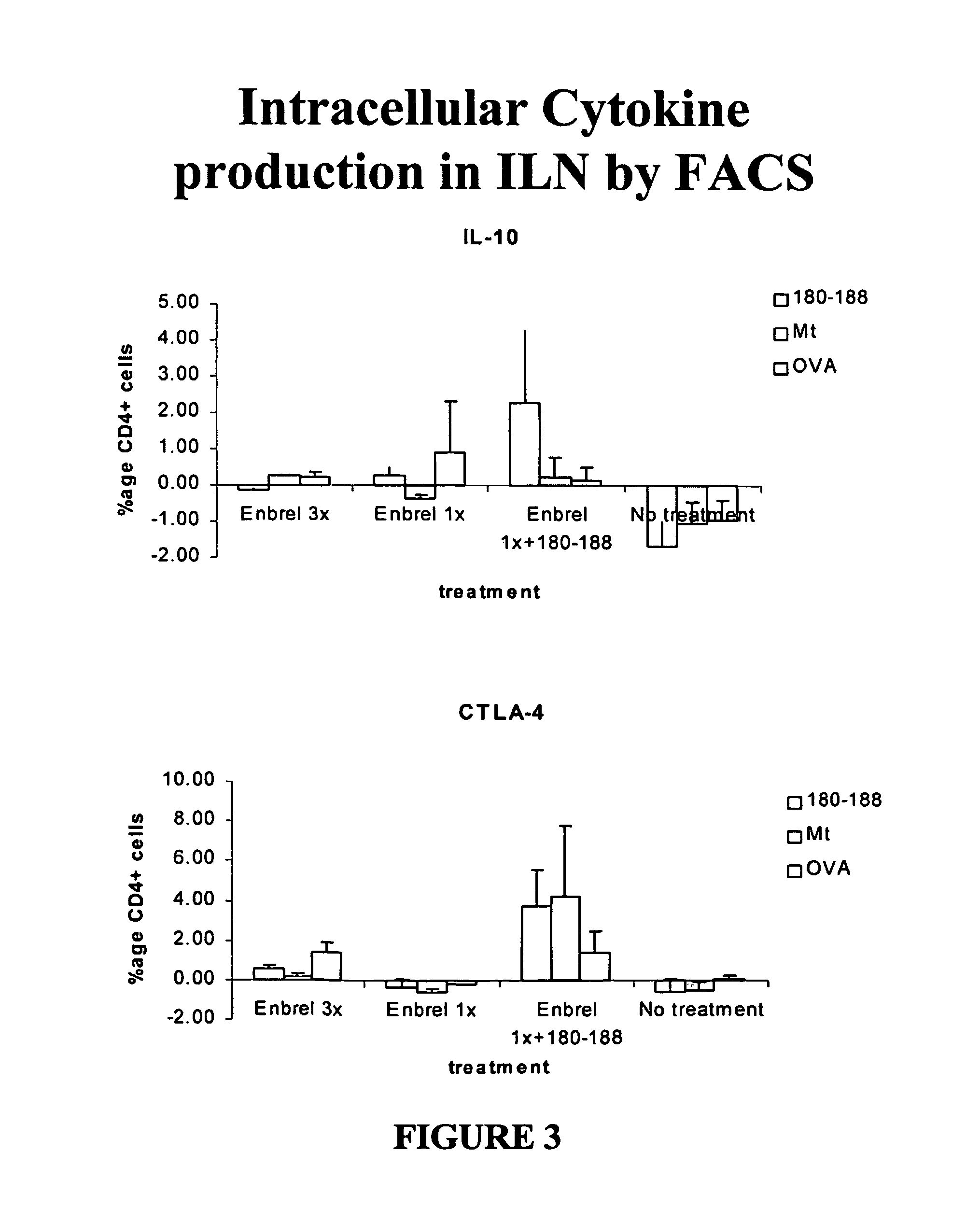
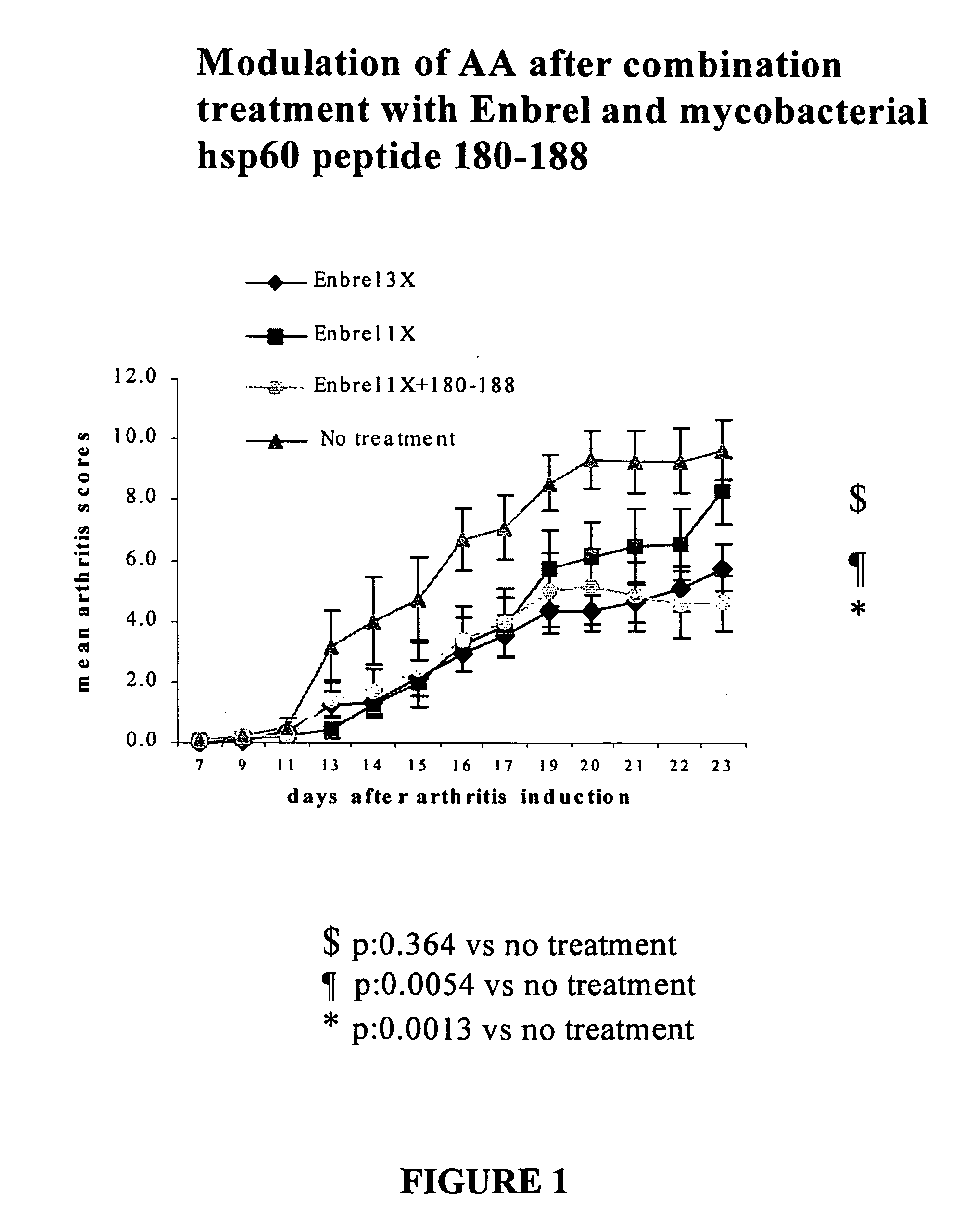
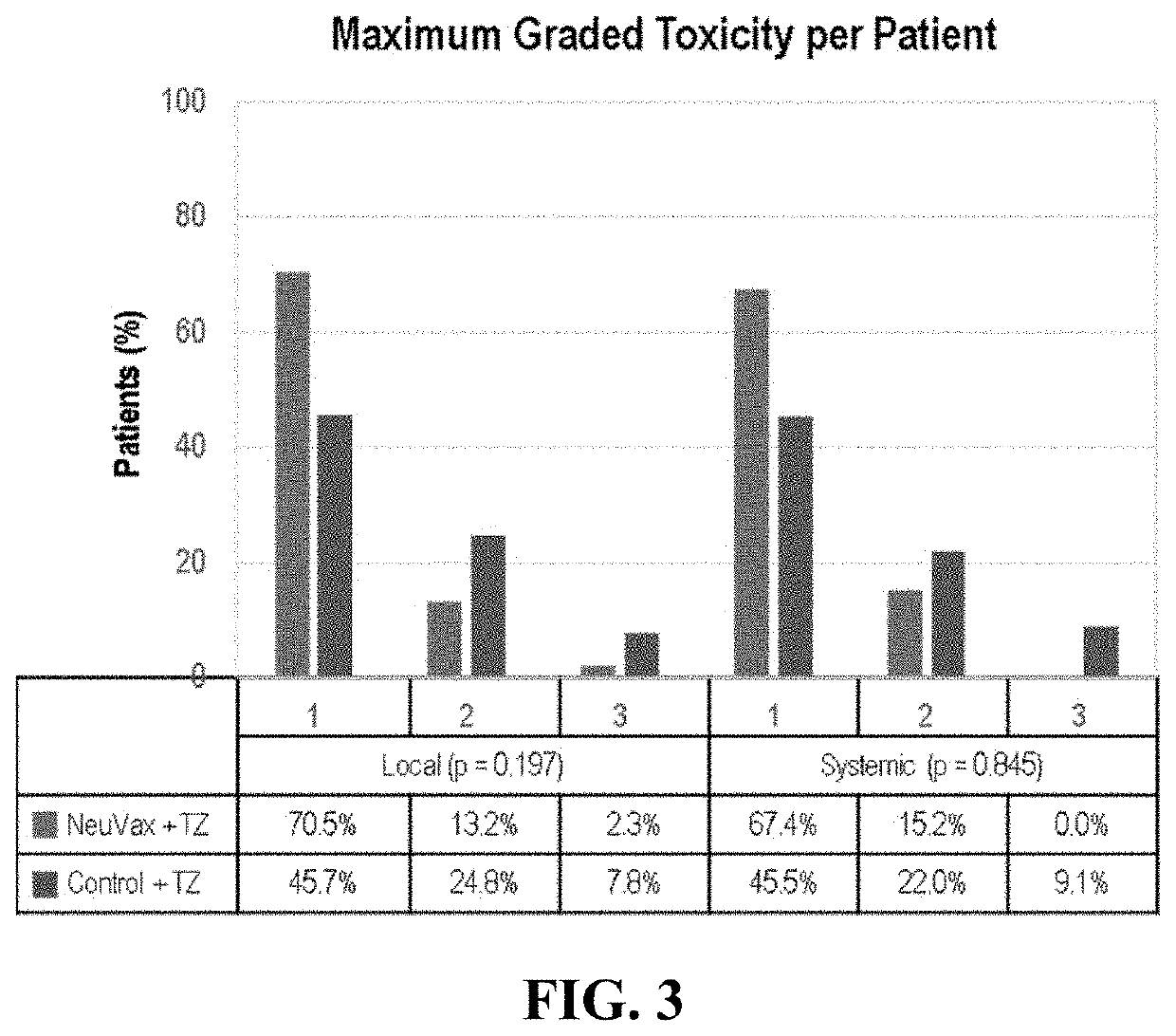
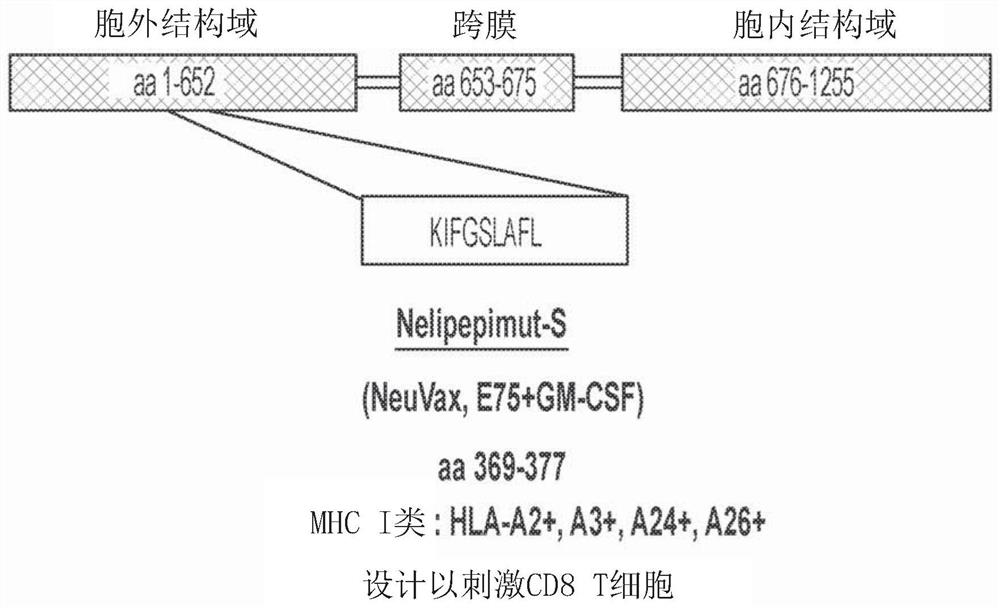
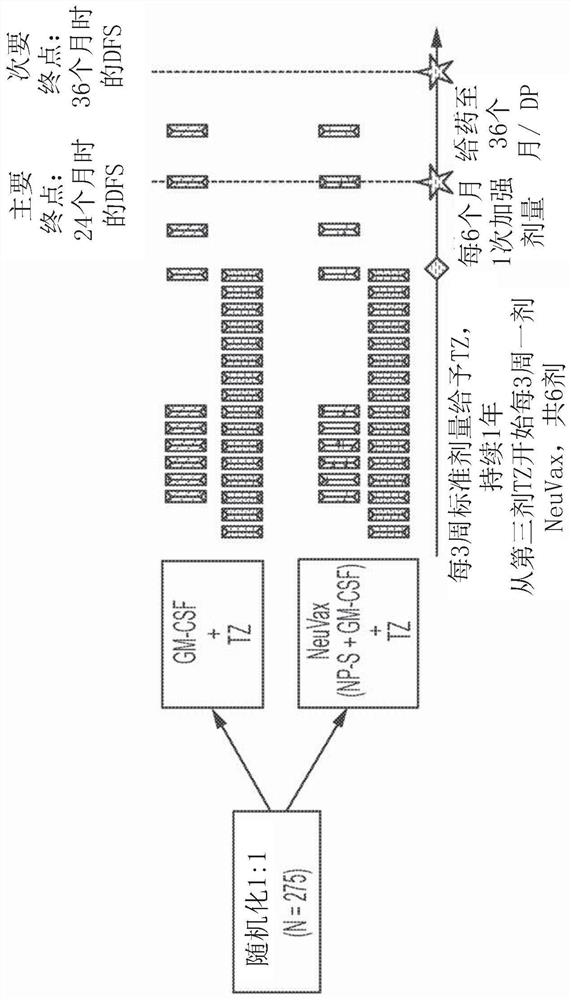
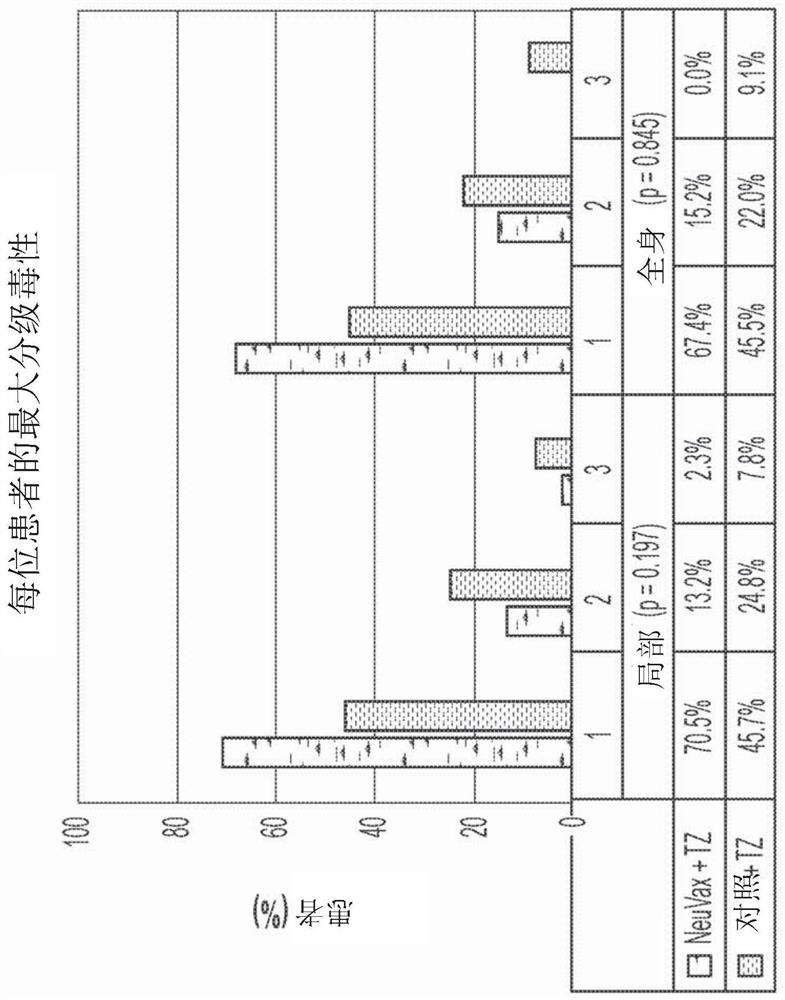
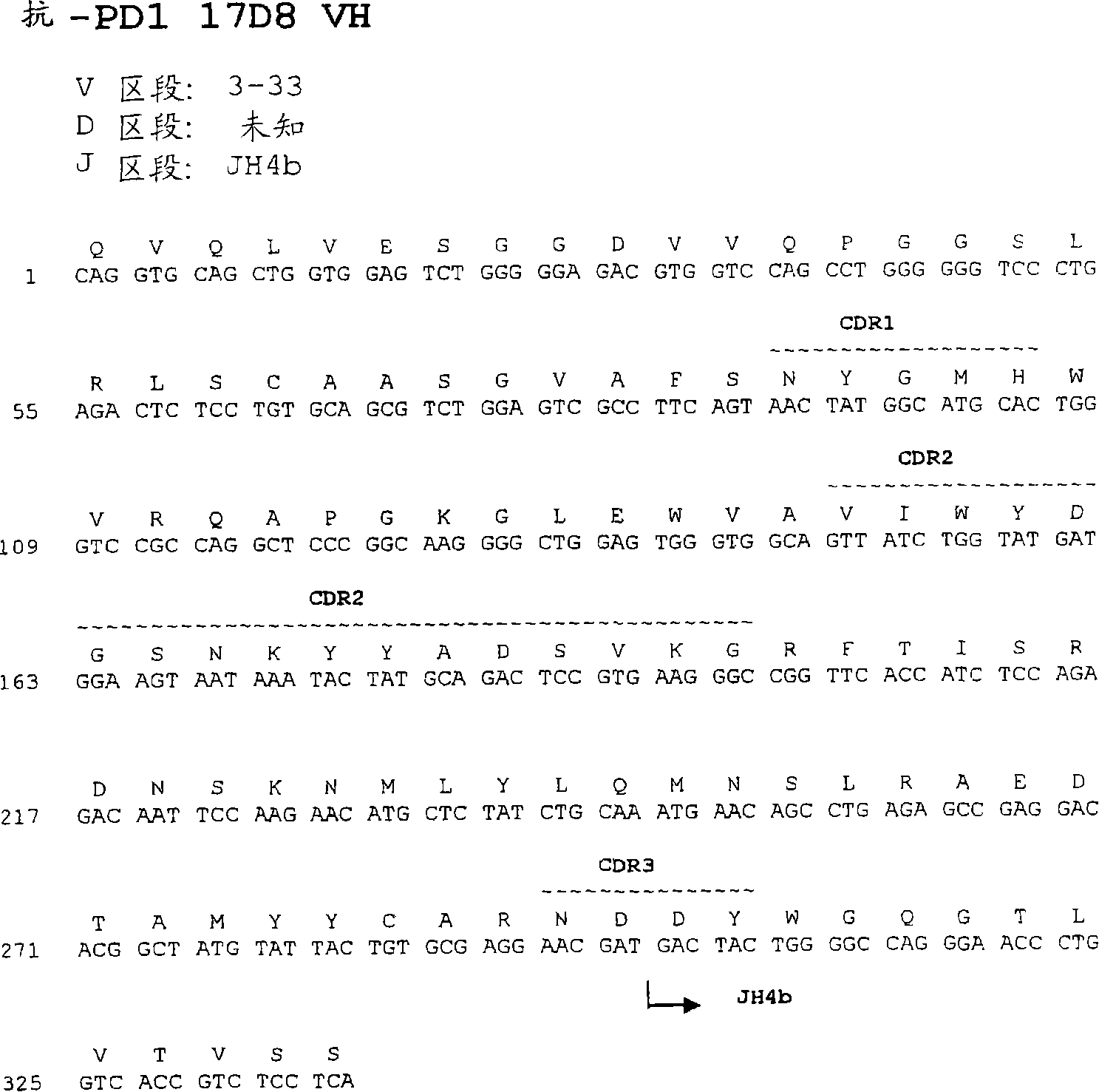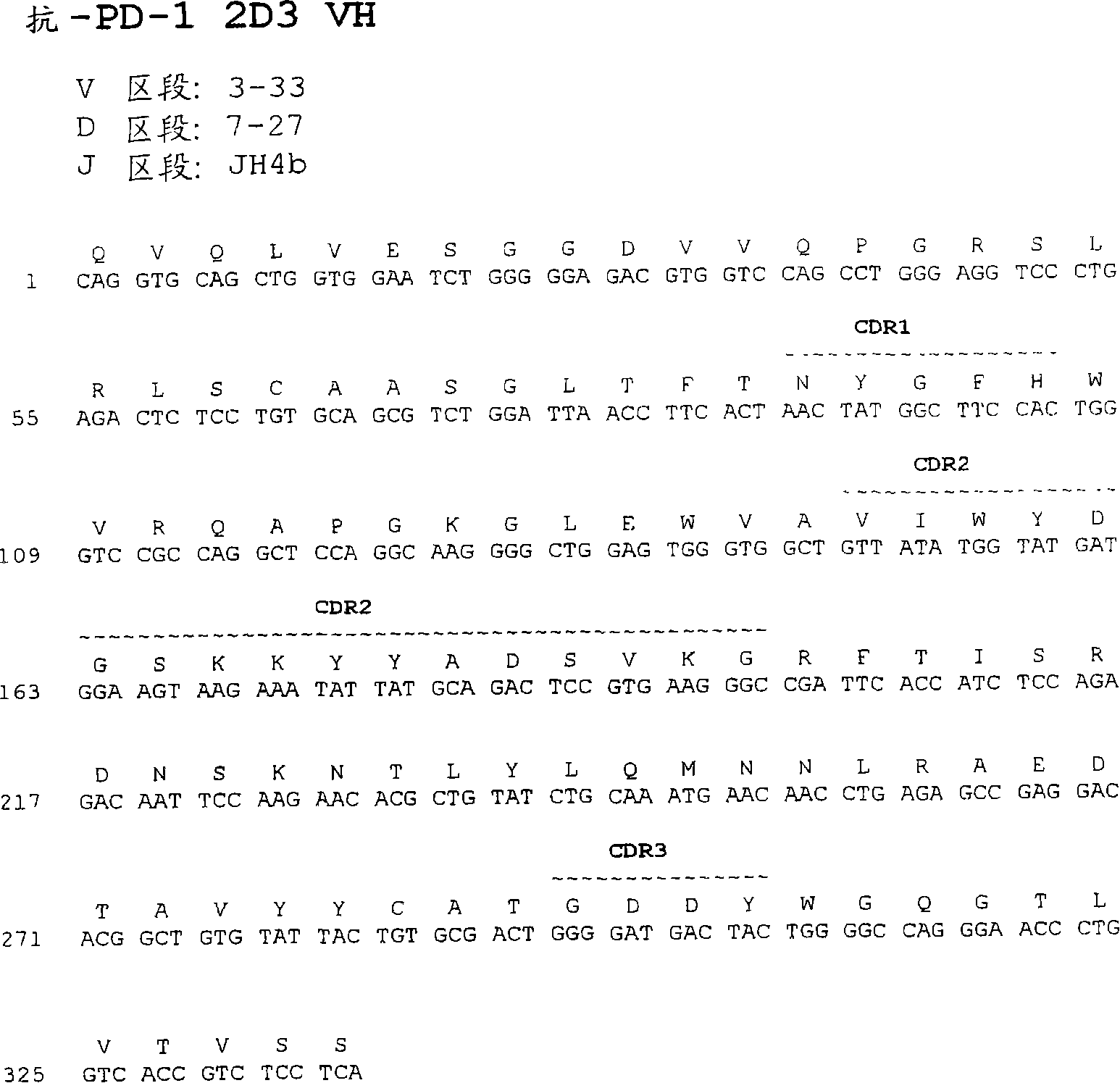Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Combination immunotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Combination immunotherapy promises to deliver long-term survival benefits that may be unavailable with current approaches. The Second Annual Combination Immunotherapy meeting will explore the most effective combinations of immunotherapy with conventional cancer therapy, with other immunotherapy or with targeted therapy.
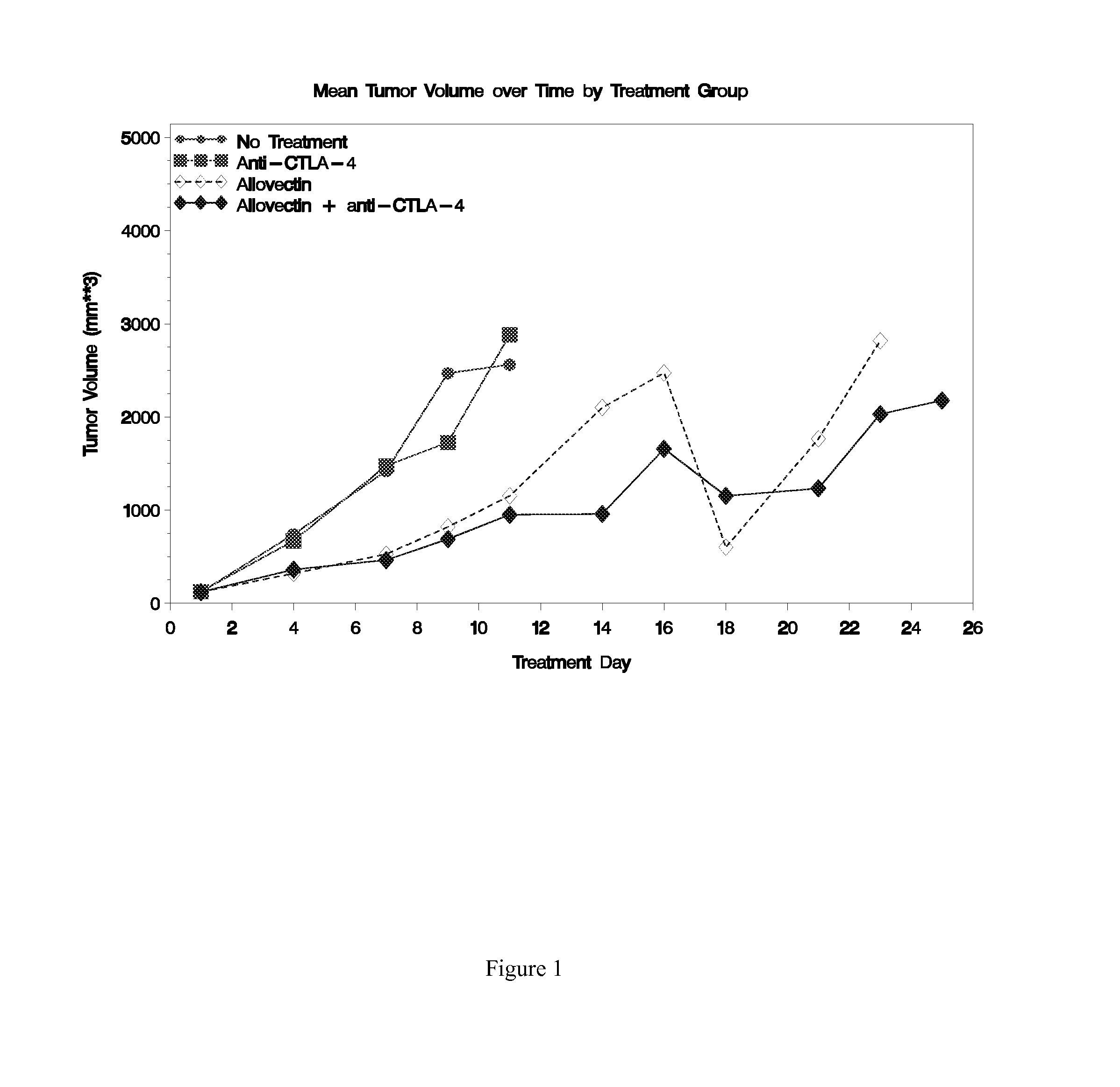
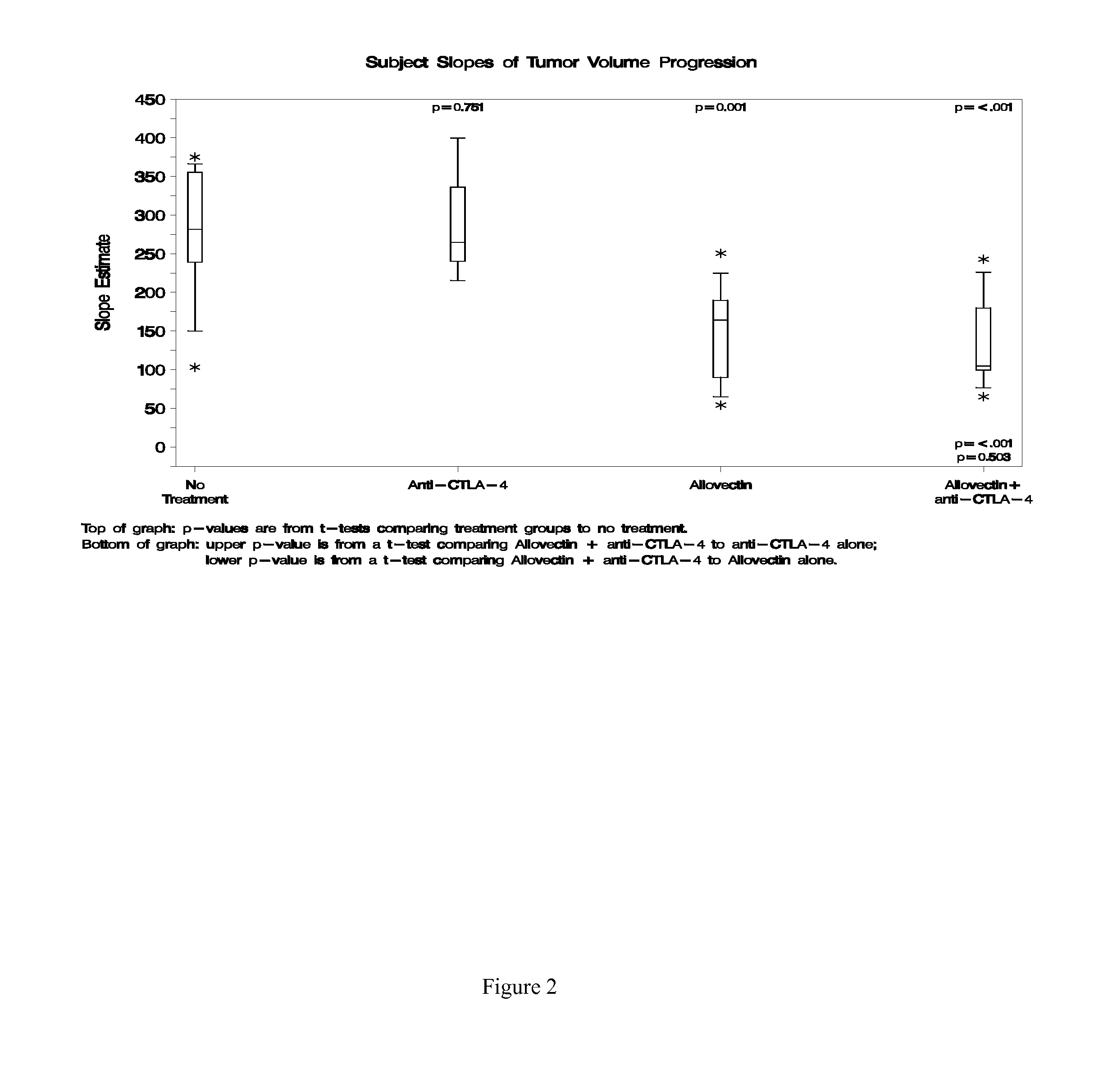
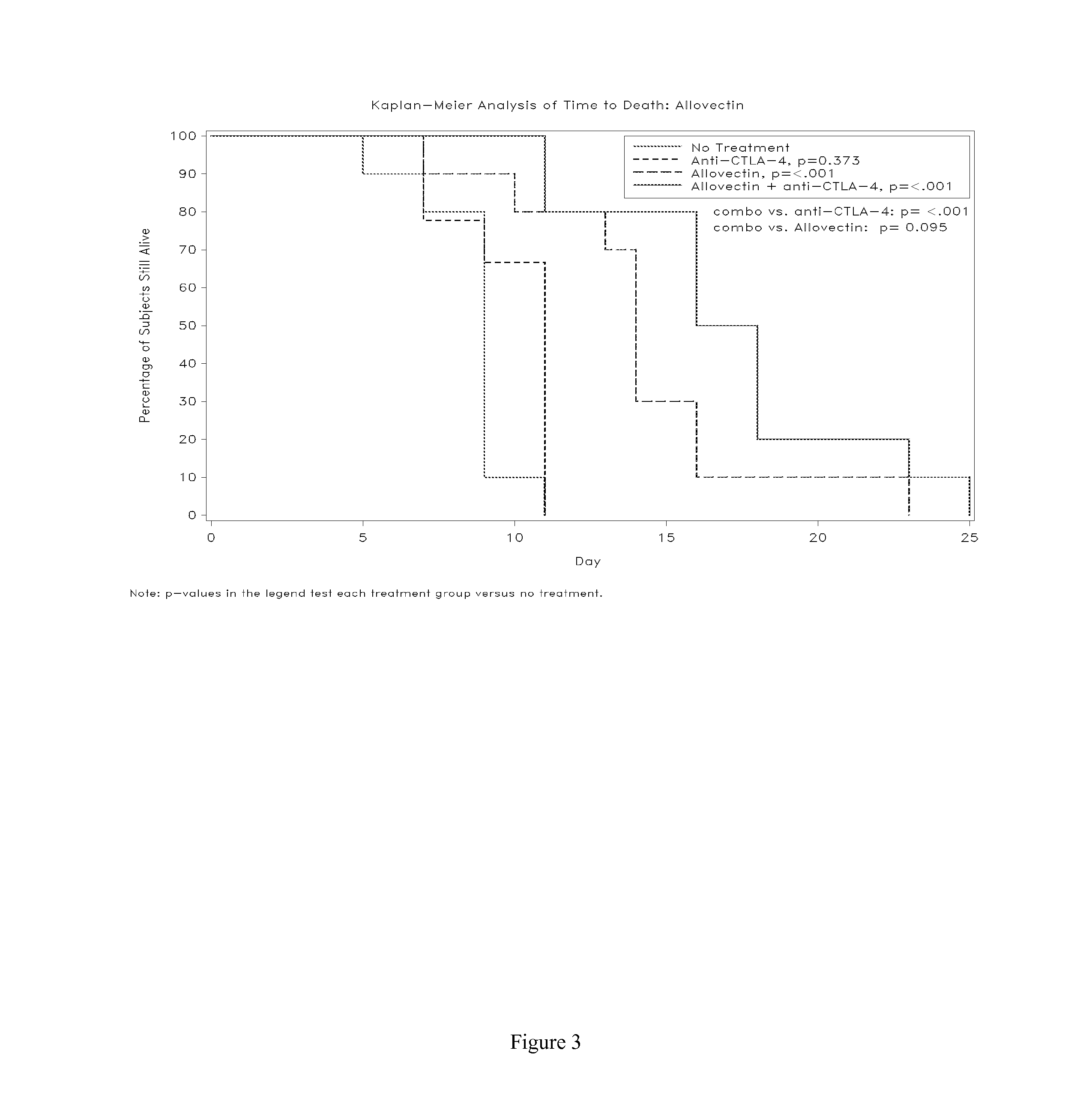
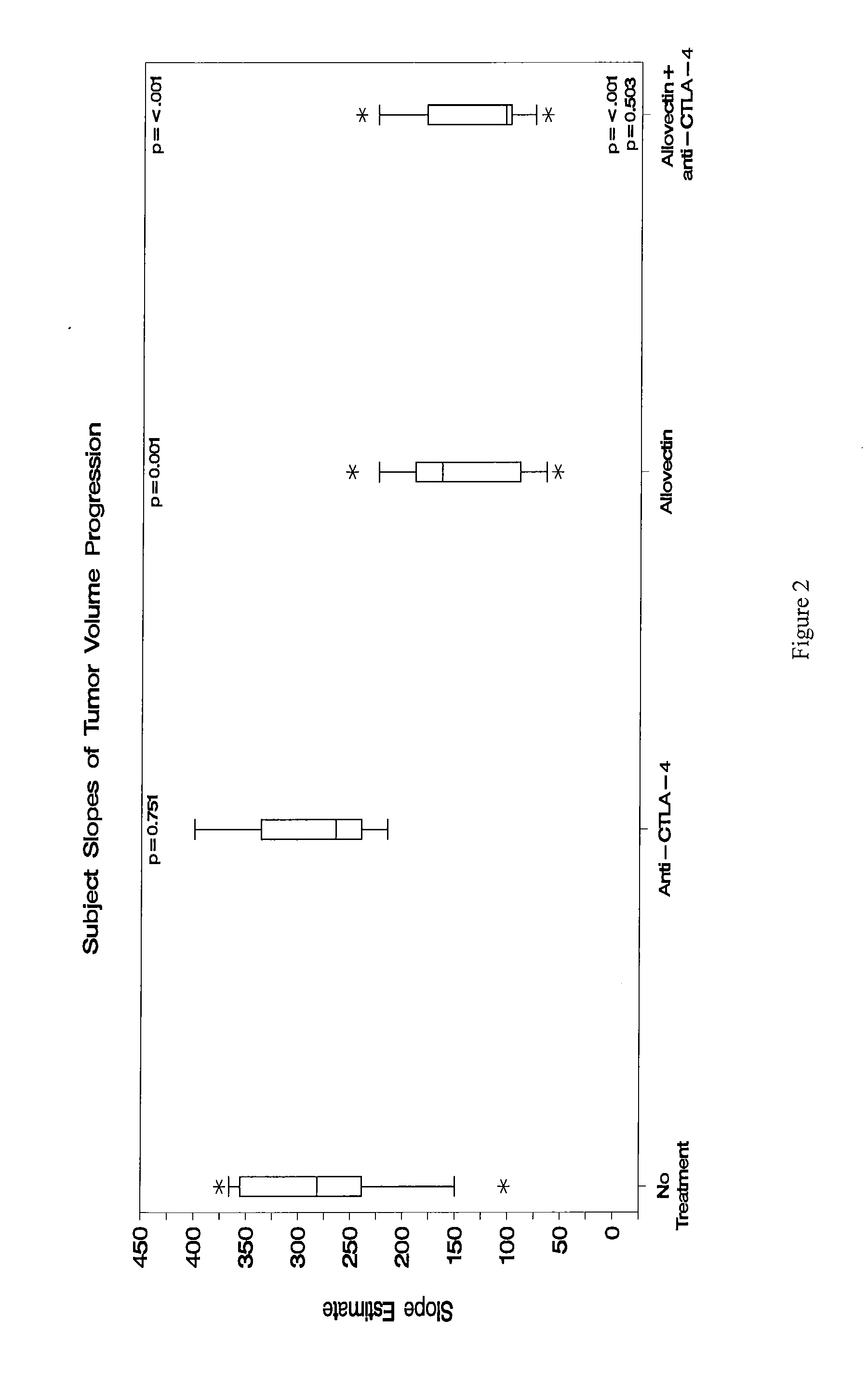
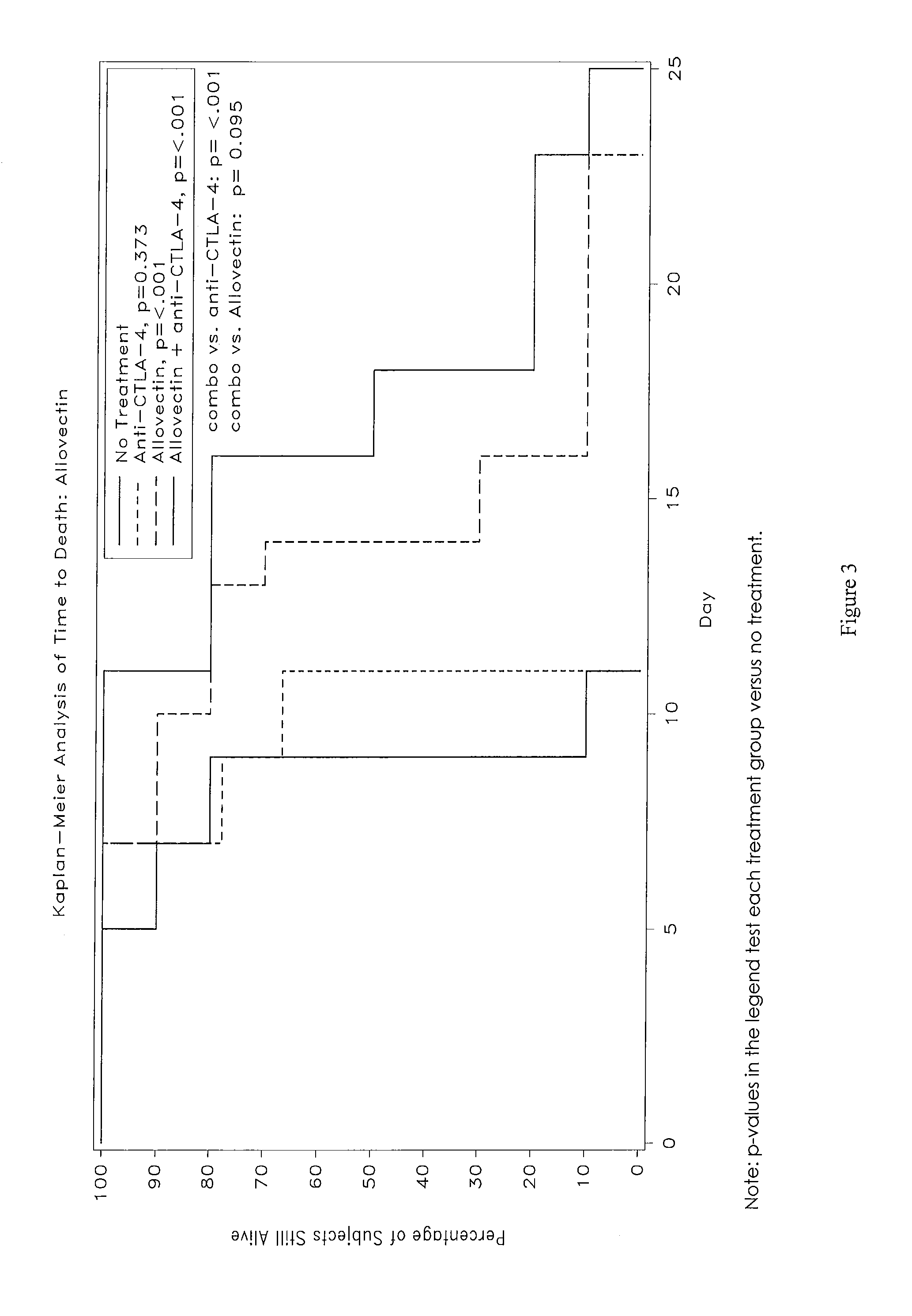
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130071403A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentIrritation
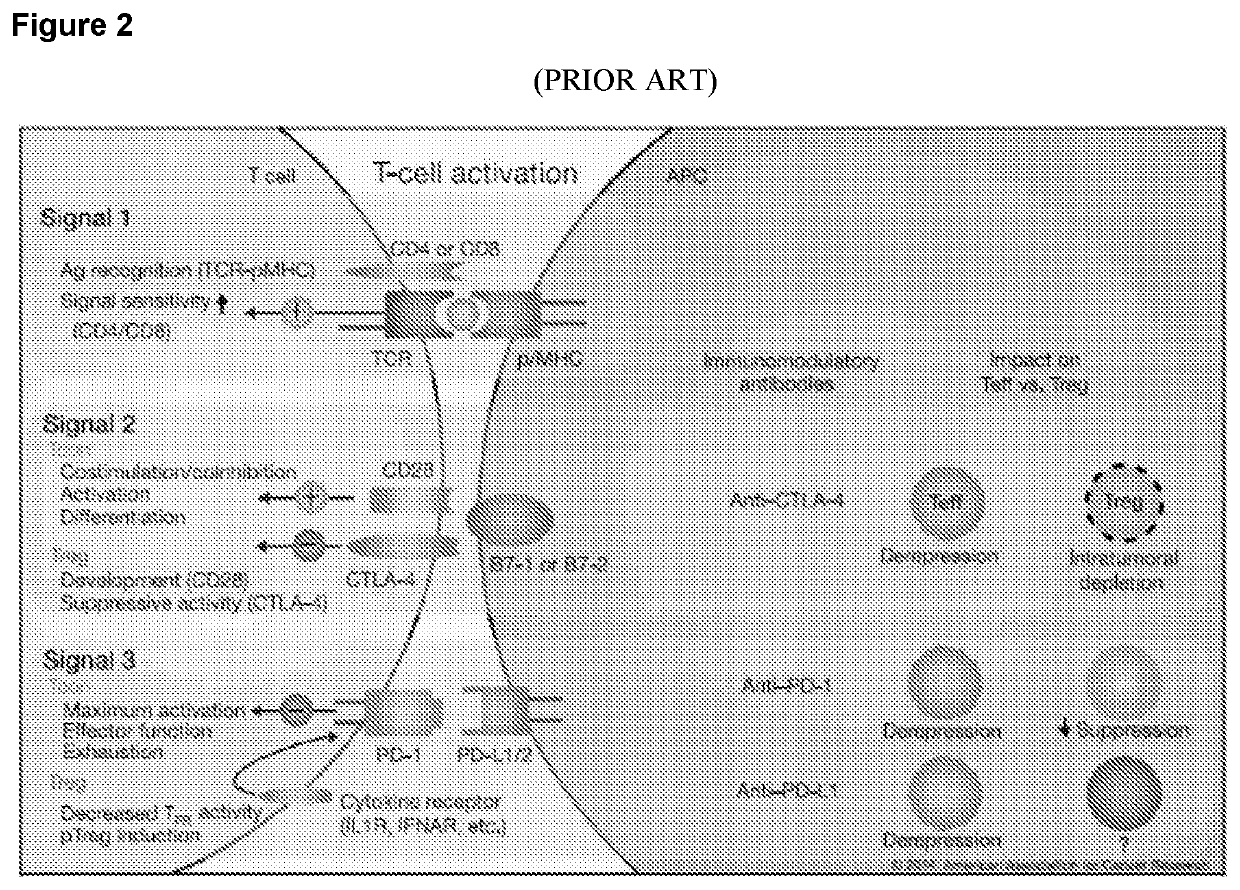
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
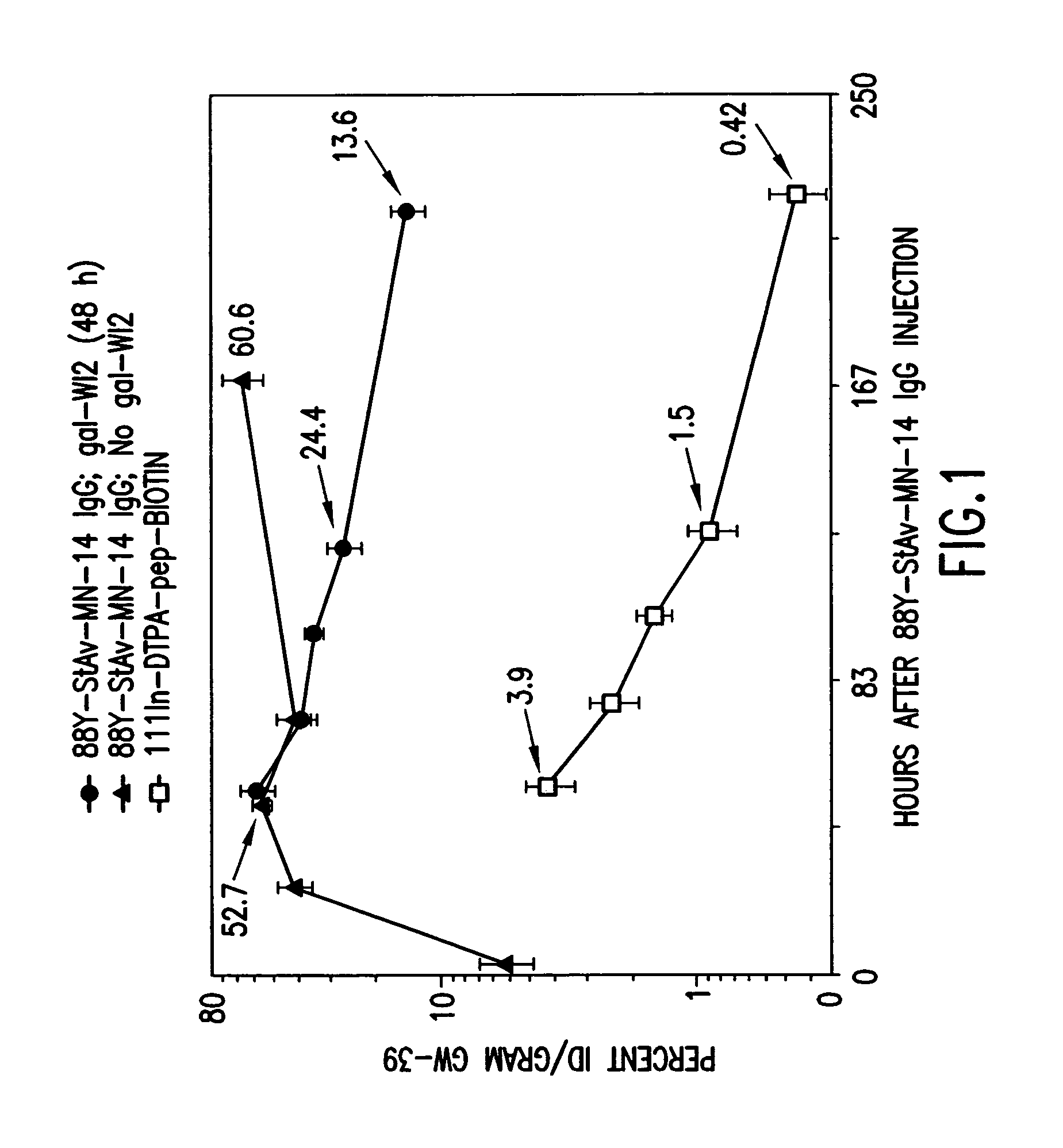
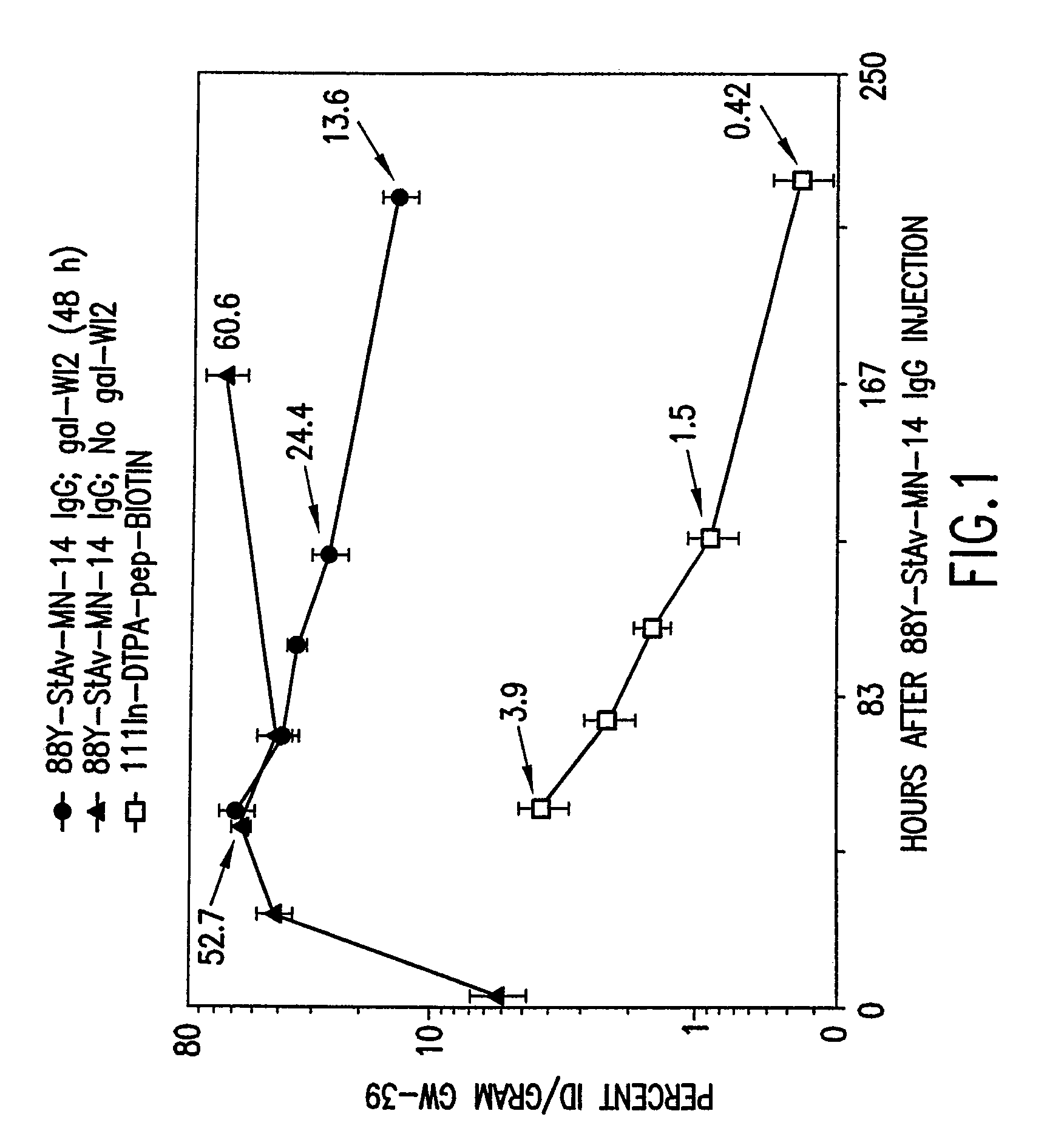
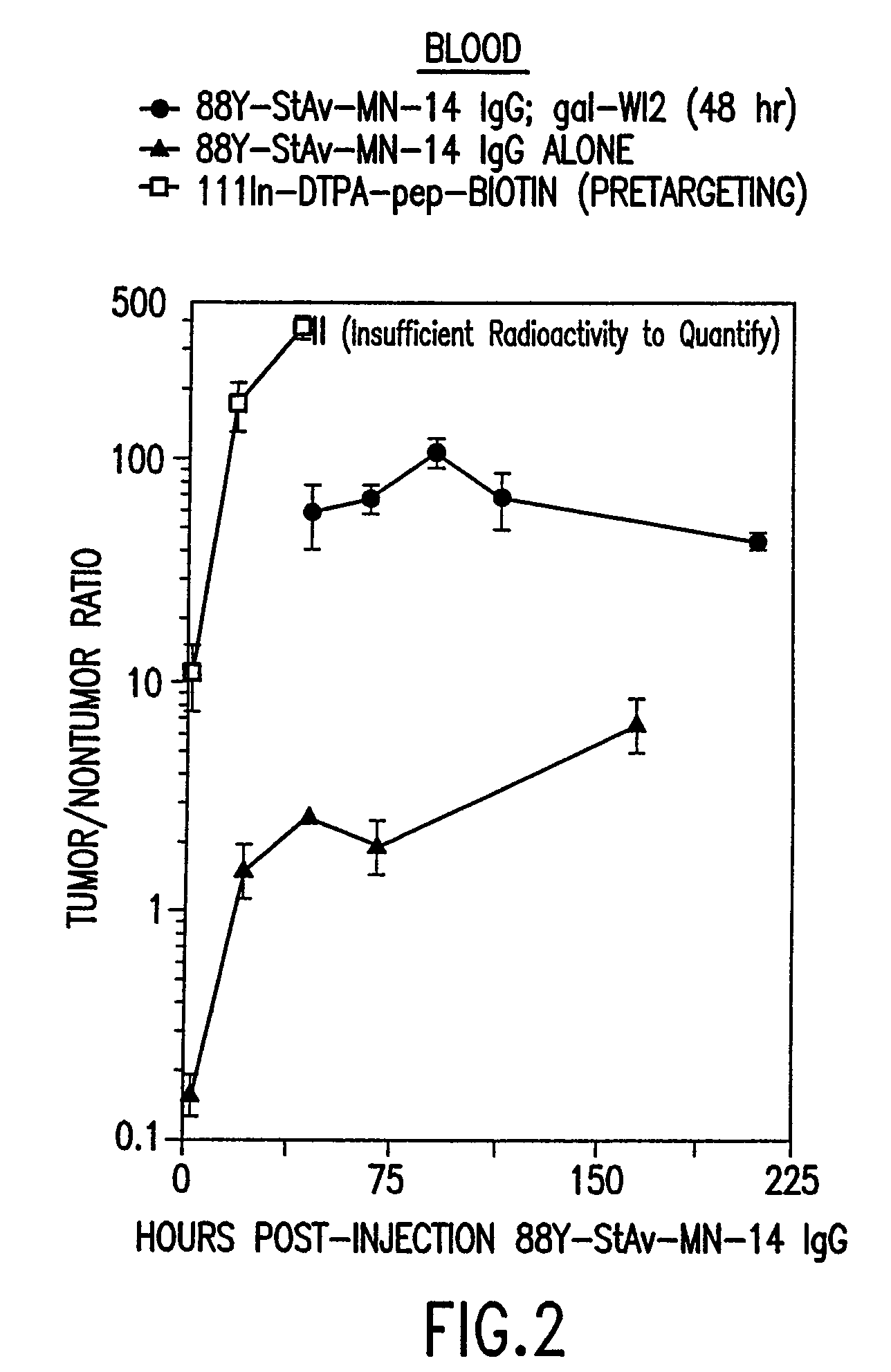
Targeted combination immunotherapy of cancer and infectious diseases
InactiveUS7011812B1Efficient deliveryMinimize ToxicityHeavy metal active ingredientsOrganic active ingredientsCancer therapyWilms' tumor
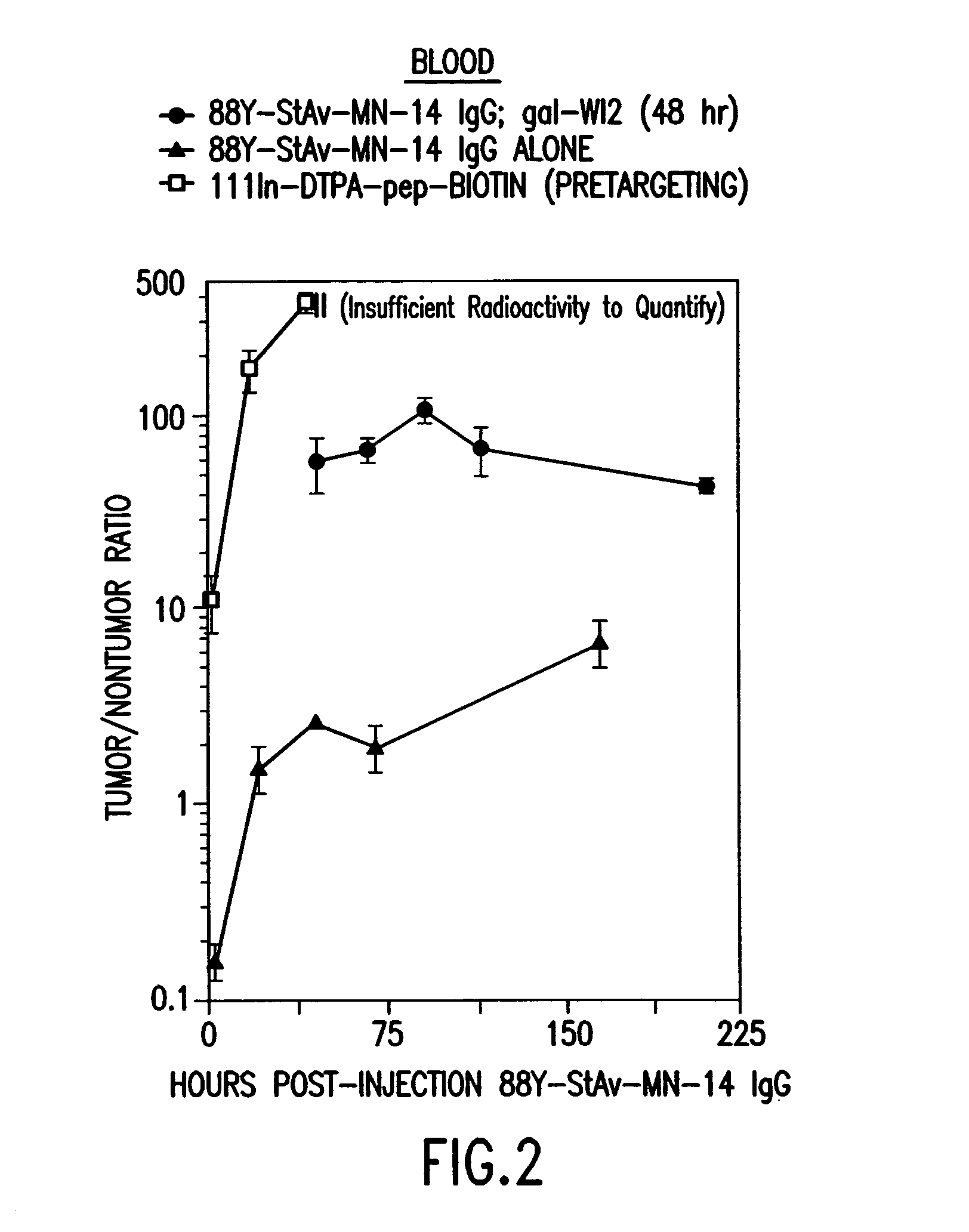
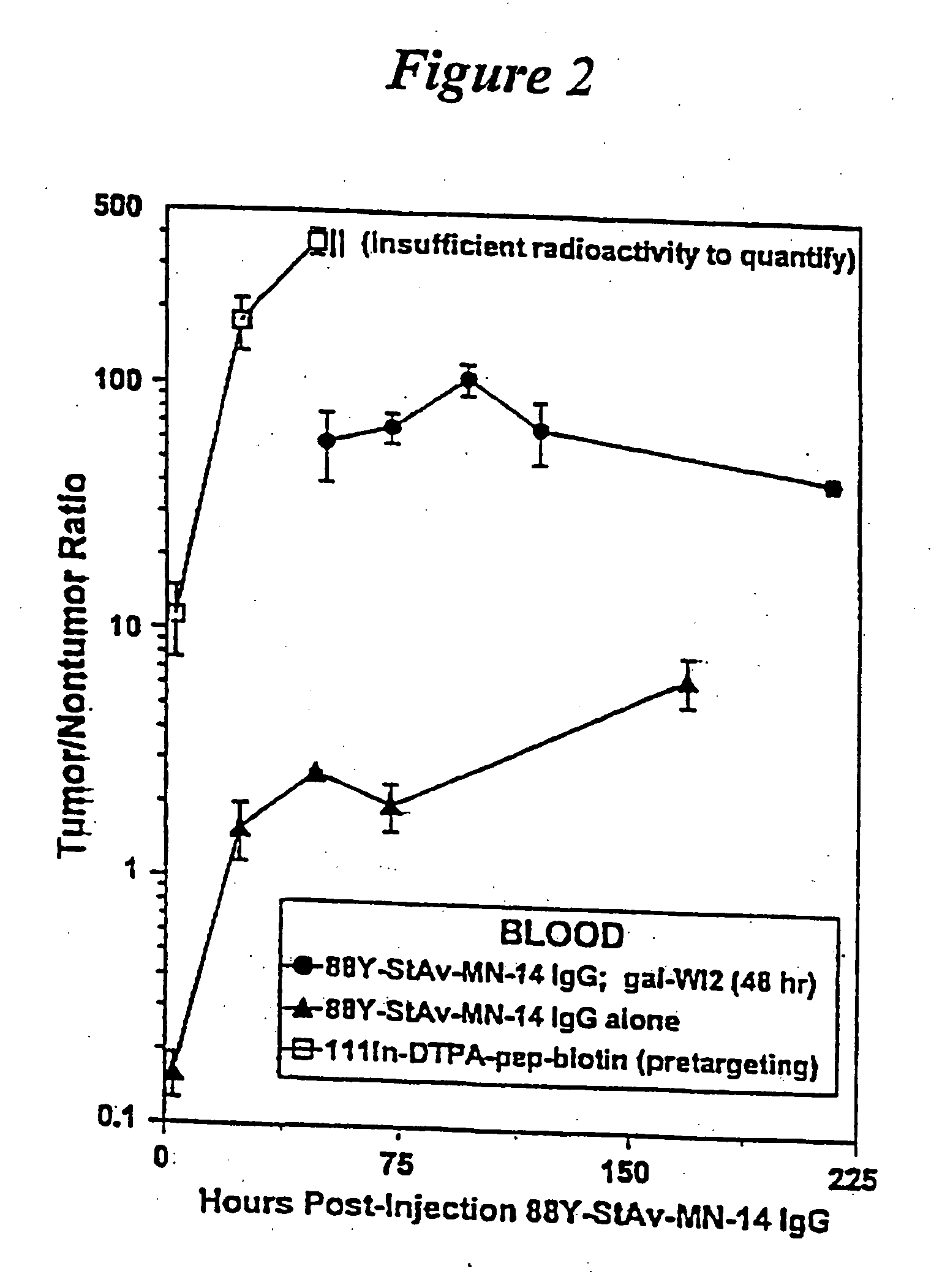
The present invention is directed to methods for treating cancer wherein more than one therapeutic agent is used, with each of the therapeutic agents having different tumor-killing capabilities, and wherein the therapeutic agents are delivered to the tumor sites using combined targeting and pre-targeting methods. The methods of the present invention achieve good tumor to non-tumor ratios of the therapeutic agents, and are effective for cancer therapy.
Owner:IMMUNOMEDICS INC
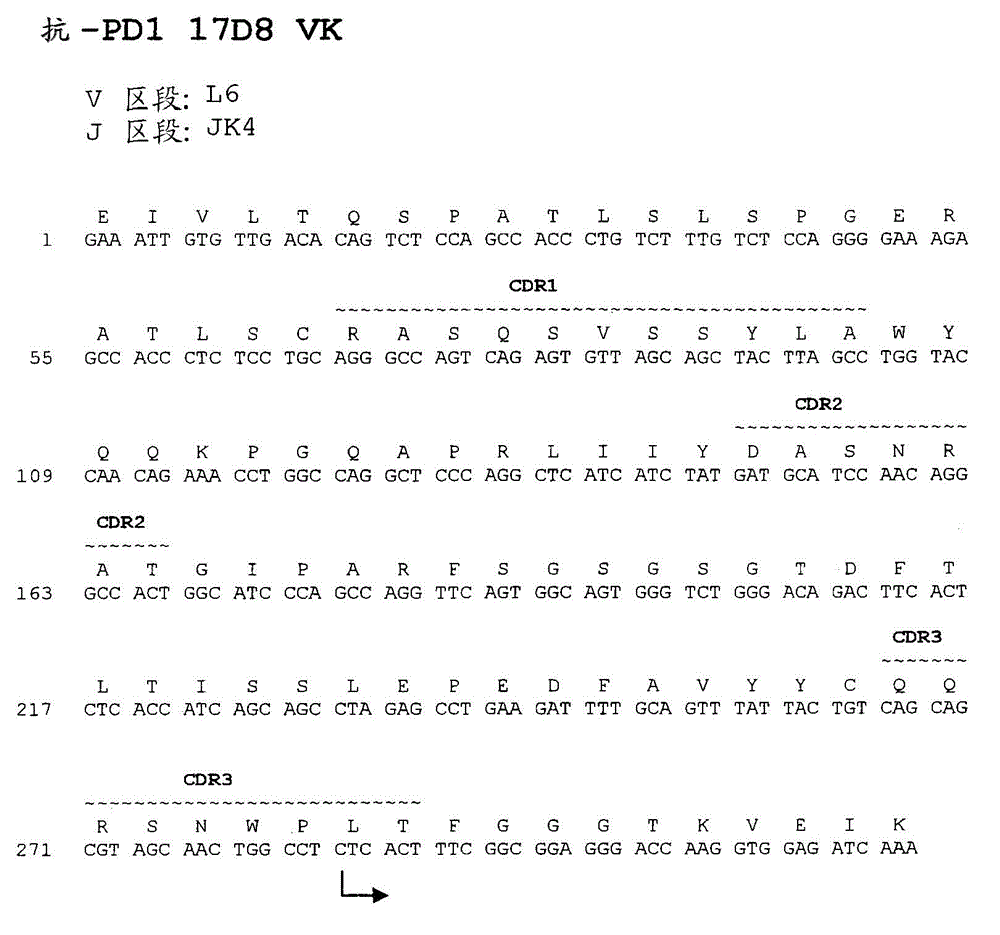
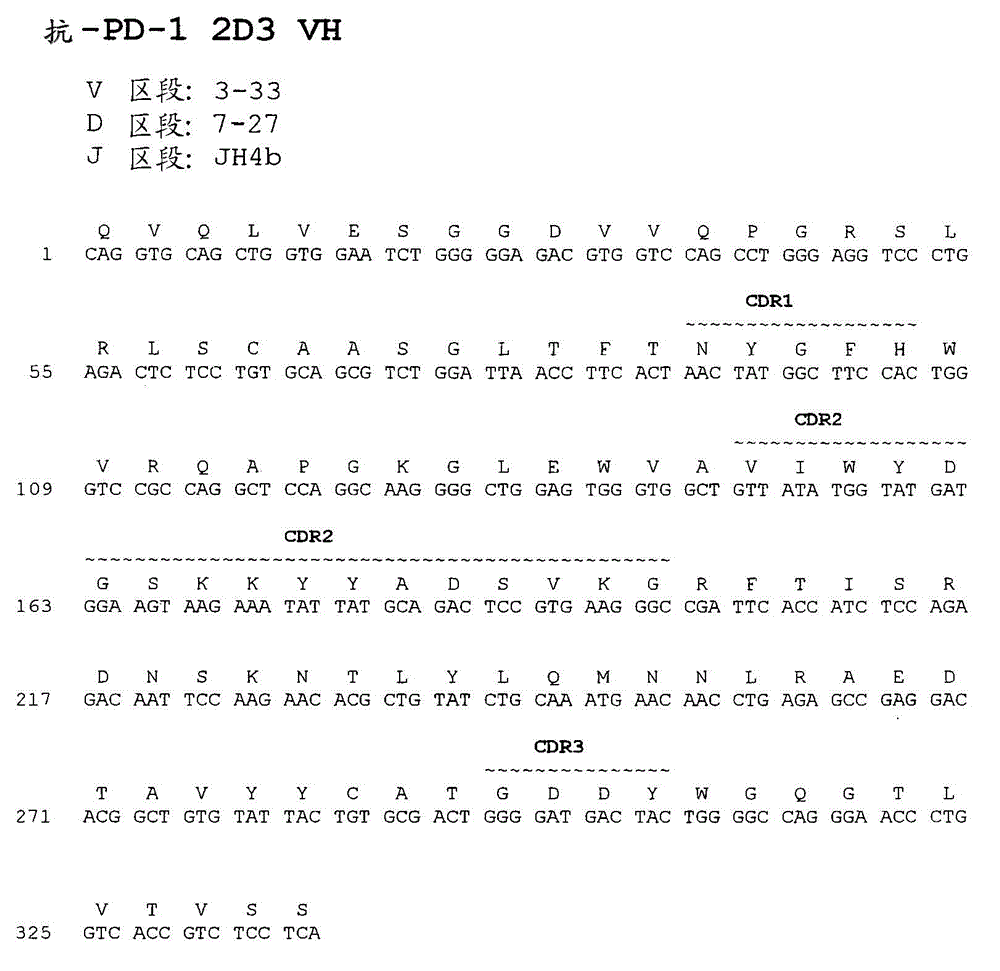
Human monoclonal antibodies to programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN101213297AMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsImmunotherapeutic agentProgrammed death
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
Combination immunotherapy for the treatment of cancer
ActiveUS8709417B2Improve anti-tumor effectPeptide/protein ingredientsAntibody ingredientsAgonistInhibitory receptors
Owner:MEMORIAL SLOAN KETTERING CANCER CENT +1
Combination immunotherapy compositions against cancer and methods
ActiveUS20120107347A1Reduces and prevents infectionInhibit tumor growthAntibacterial agentsAntimycoticsDiseaseCombination immunotherapy
Owner:GLOBE IMMUNE INC +1
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130280265A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentEfficacy
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
Human monoclonal antibodies to programmed death 1 (pd-1) and methods for treating cancer using anti-pd-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN103059138ARadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesInfectious Disorder
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
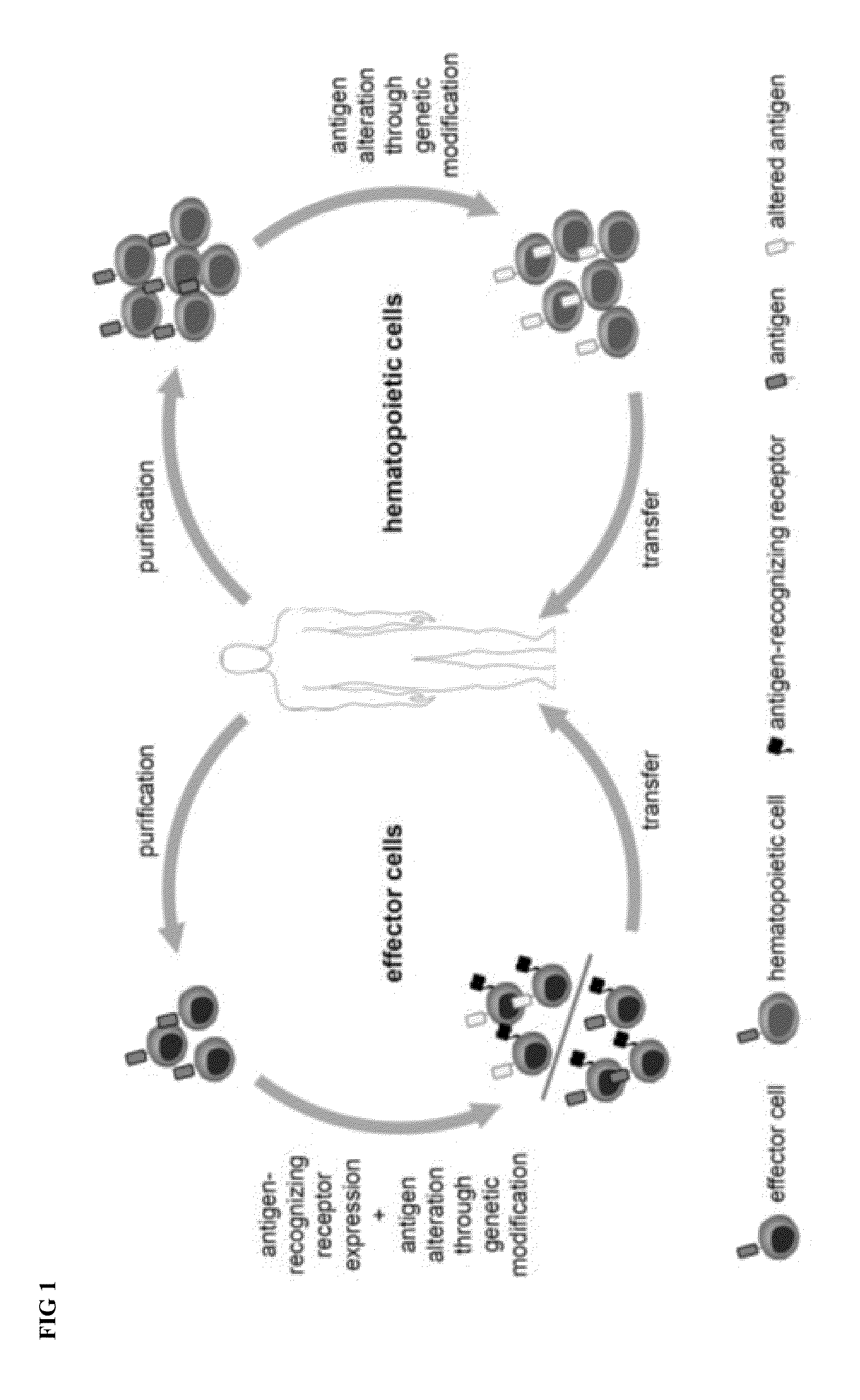
Combination immunotherapy of antigen-recognizing receptors and hematopoietic cells for the treatment of diseases
The invention provides a system that comprises pharmaceutical agents for use in immunotherapy for reducing the side-effects of an antigen-recognizing receptor against antigen-expressing non-target cells in an individual. The system includes an antigen-recognizing receptor that specifically recognizes an antigen on target cells and at least on one hematopoietic cell type in the individual. The antigen-recognizing receptor is exemplified by chimeric antigen receptors (CAR) be expressed on the surface of an immune effector cells. The system also includes hematopoietic cells resistant to recognition of the same antigen by the antigen-recognizing receptor.
Owner:MILTENYI BIOTEC B V & CO KG
Combination Immunotherapy for the Treatment of Cancer
ActiveUS20120251556A1Improve anti-tumor effectPeptide/protein ingredientsAntibody ingredientsAgonistInhibitory receptors
Owner:MEMORIAL SLOAN KETTERING CANCER CENT +1
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130273078A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentPD-L1
Owner:VICAL INC
Combination Immunotherapy
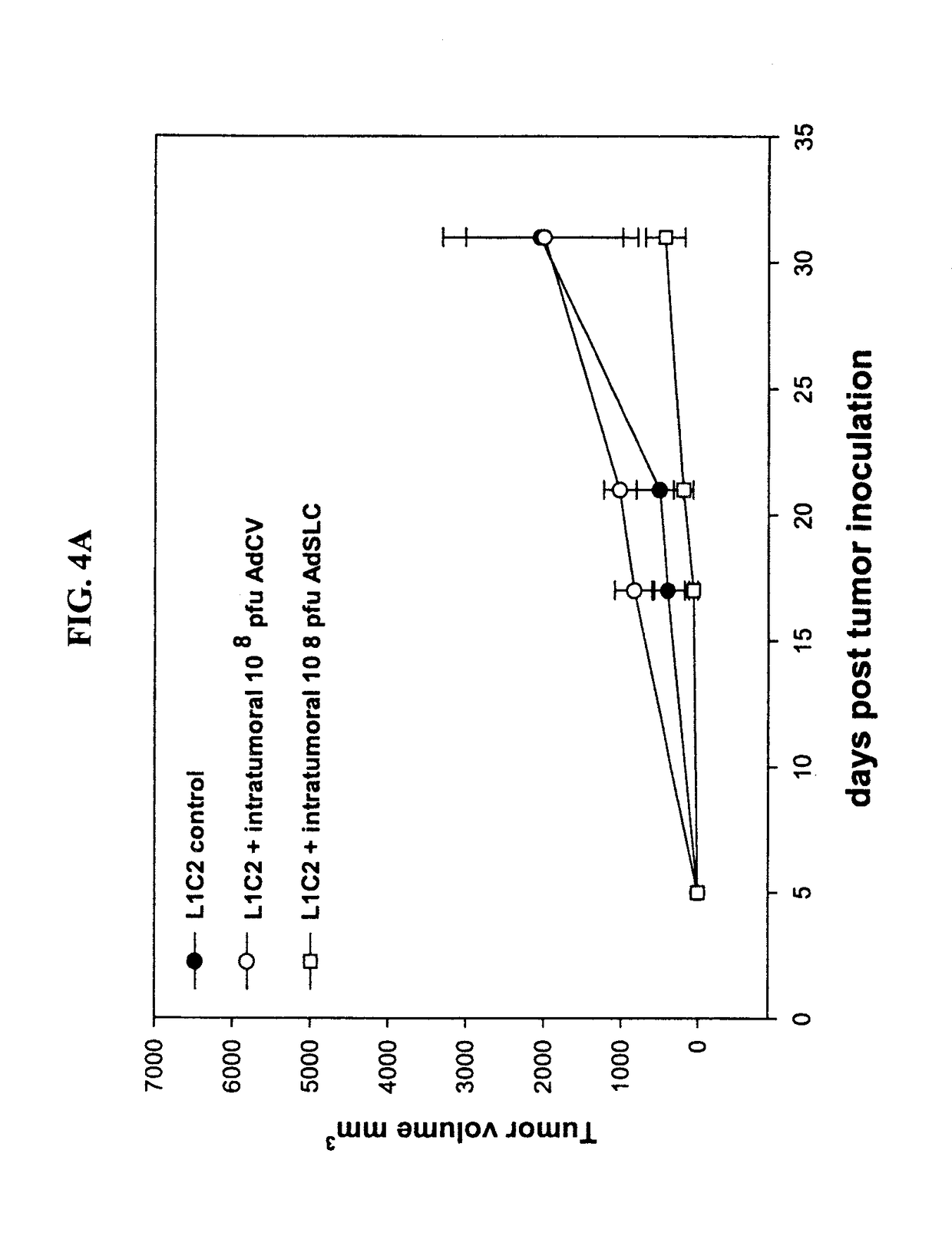
ActiveUS20180194825A1Lower Level RequirementsReduce the burden onChemokinesPeptide/protein ingredientsAntiendomysial antibodiesOncology
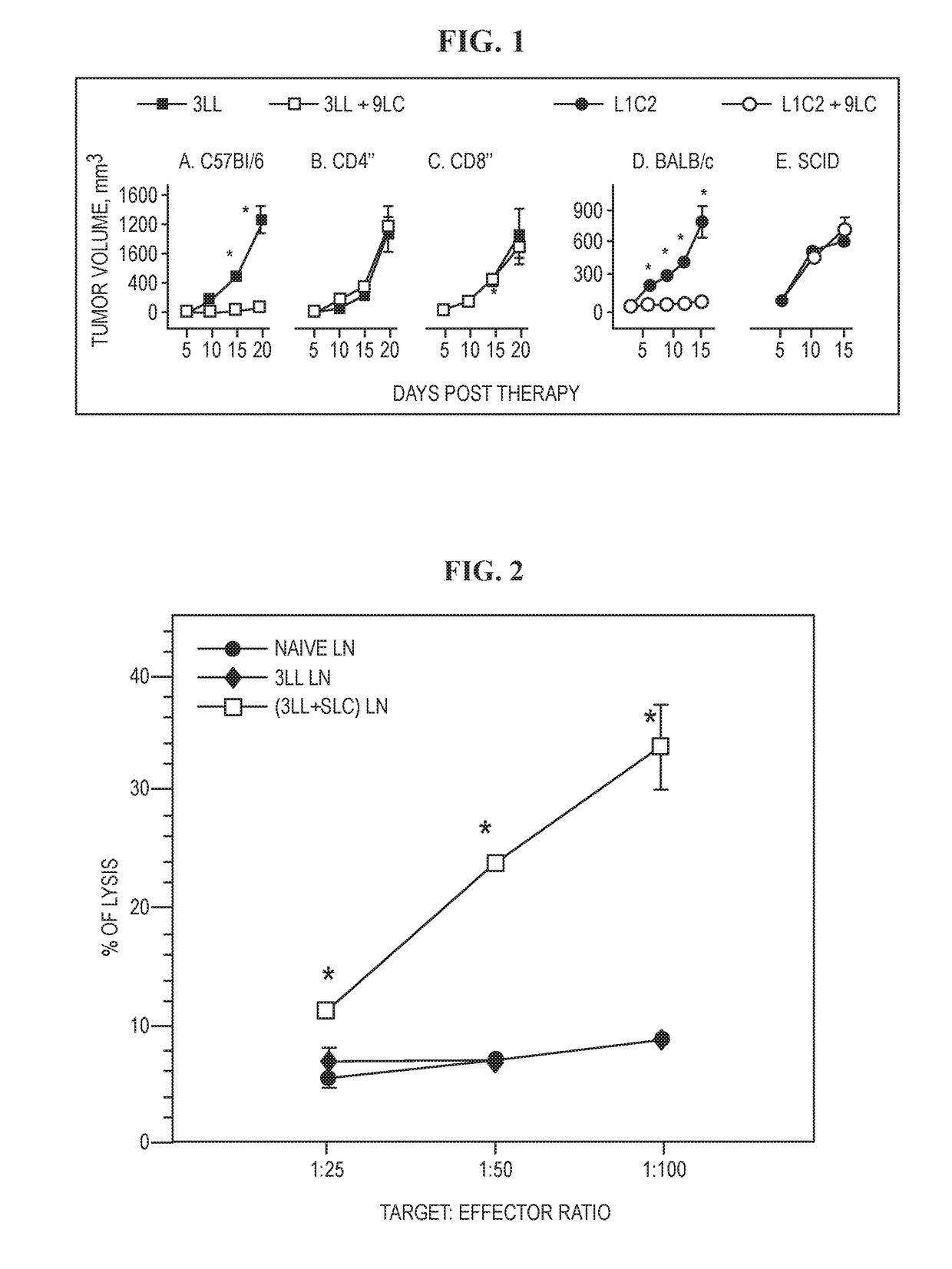
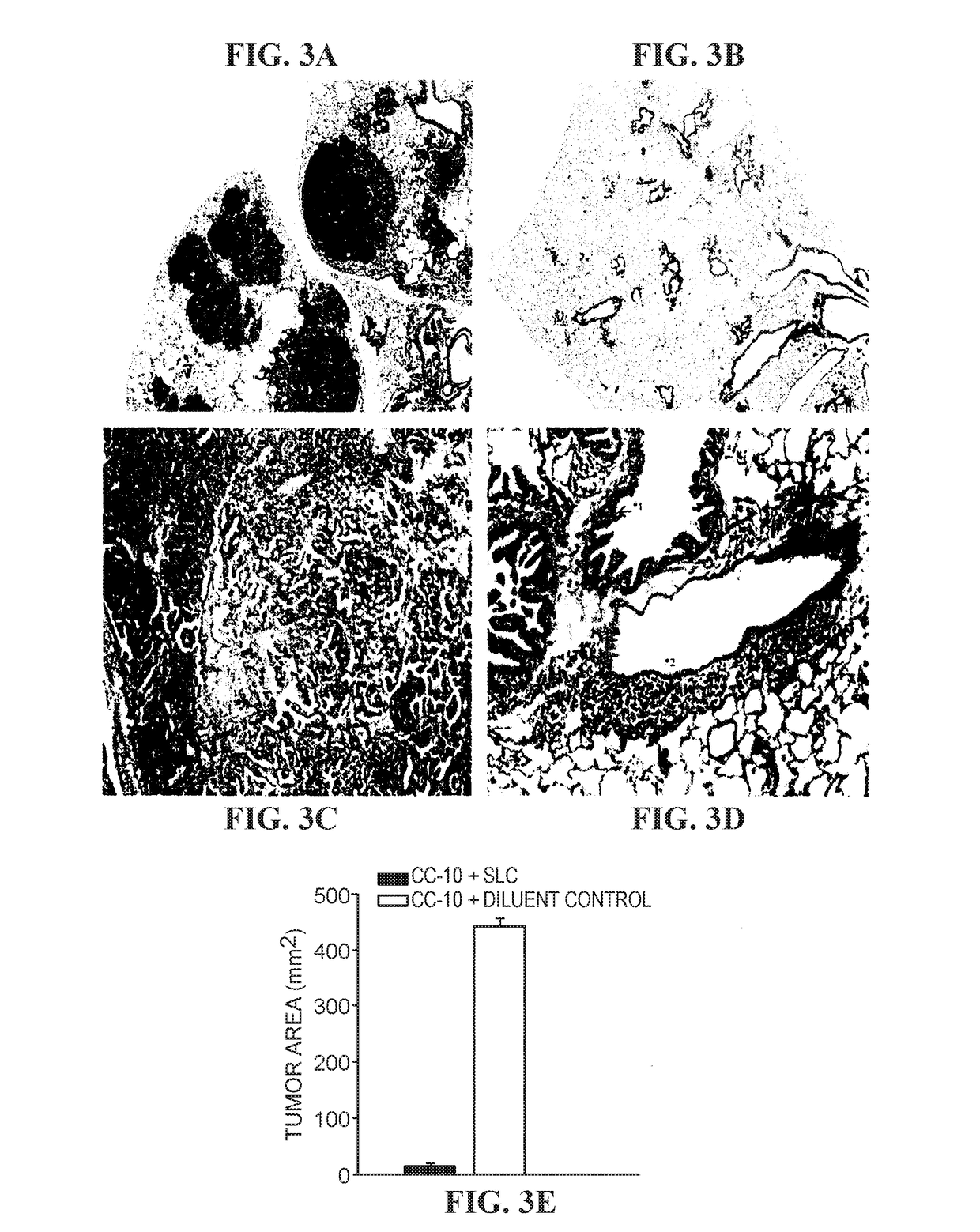
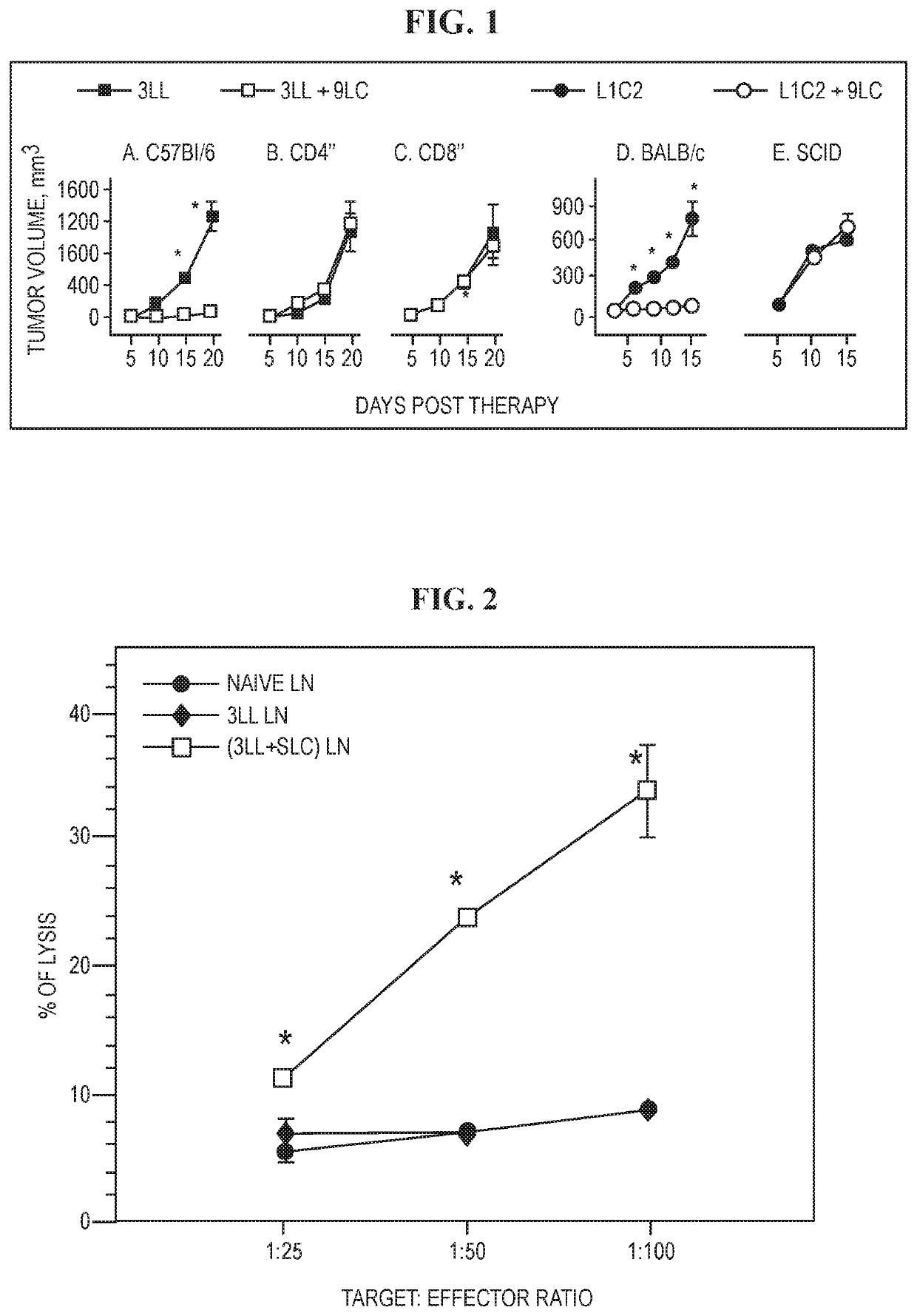
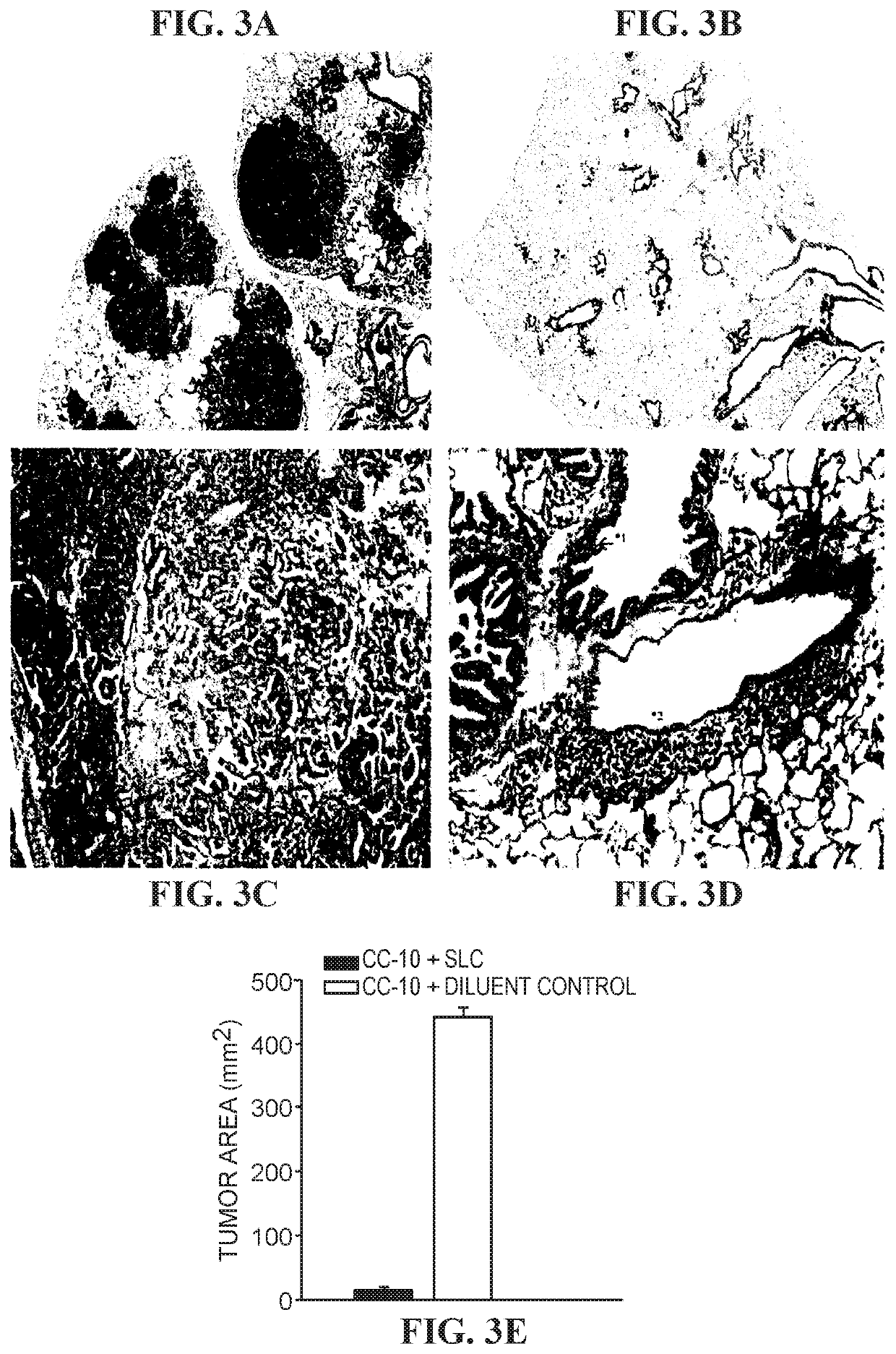
The invention is based on the disclosure provided herein that secondary lymphoid organ chemokine (SLC) inhibits the growth of syngeneic tumors in vivo. Thus, the invention provides a method of treating cancer in a mammal subject by administering a therapeutically effective amount of an SLC to the mammal in combination with a checkpoint inhibitor, including monoclonal antibodies and small molecule inhibitors. Exemplary checkpoint molecules include CTLA-4, a CTLA-4 receptor, PD-1, PD1-L1, PD1-L2, 4-1BB, OX40, LAG-3, TIM-3 or a combination thereof. SLCs useful in the methods of the invention include SLC polypeptides, variants and fragments and related nucleic acids.
Owner:RGT UNIV OF CALIFORNIA +1
Combination therapy and antibody panels
InactiveUS20070134248A1High sequence similarityAntibody ingredientsImmunoglobulinsSurface ImmunoglobulinImmunogenicity
The present invention provides combination immunotherapy for Non-Hodgkin's Lymphoma. In one embodiment, the combination immunotherapy first provides for the administration of a monoclonal antibody directed to a non-idotypic portion of a lymphoma cell surface immunoglobulin (e.g. a framework region of a variable region). The combination immunotherapy next provides for the administration of an immunogenic composition comprising at least a portion of the same lymphoma cell surface immunoglobulin, whether an idiotypic portion or non-idiotypic portion.
Owner:GENITOPE CORP
Targeted combination immunotherapy of cancer and infectious diseases
InactiveUS7514066B2Efficient deliveryMinimize ToxicityPeptide/protein ingredientsNanomedicineOncologyCancer therapy
The present invention is directed to methods for treating cancer wherein more than one therapeutic agent is used, with each of the therapeutic agents having different tumor-killing capabilities, and wherein the therapeutic agents are delivered to the tumor sites using combined targeting and pre-targeting methods. The methods of the present invention achieve good tumor to non-tumor ratios of the therapeutic agents, and are effective for cancer therapy.
Owner:IMMUNOMEDICS INC
Combination tumor immunotherapy
ActiveUS10682365B2Easy to operateInhibited anti-tumor immune responsePeptide/protein ingredientsImmunoglobulins against virusesAntiendomysial antibodiesWhite blood cell
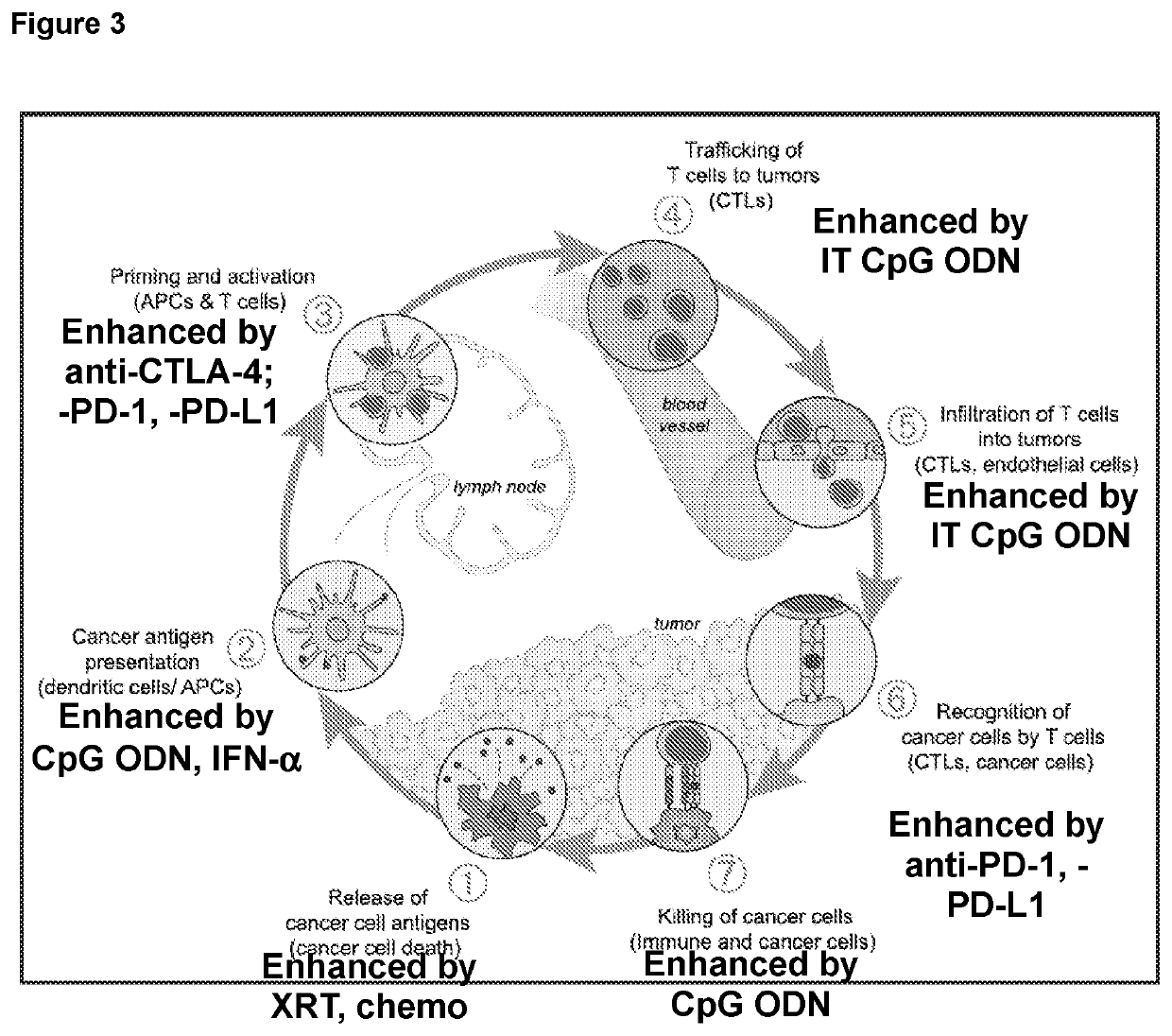
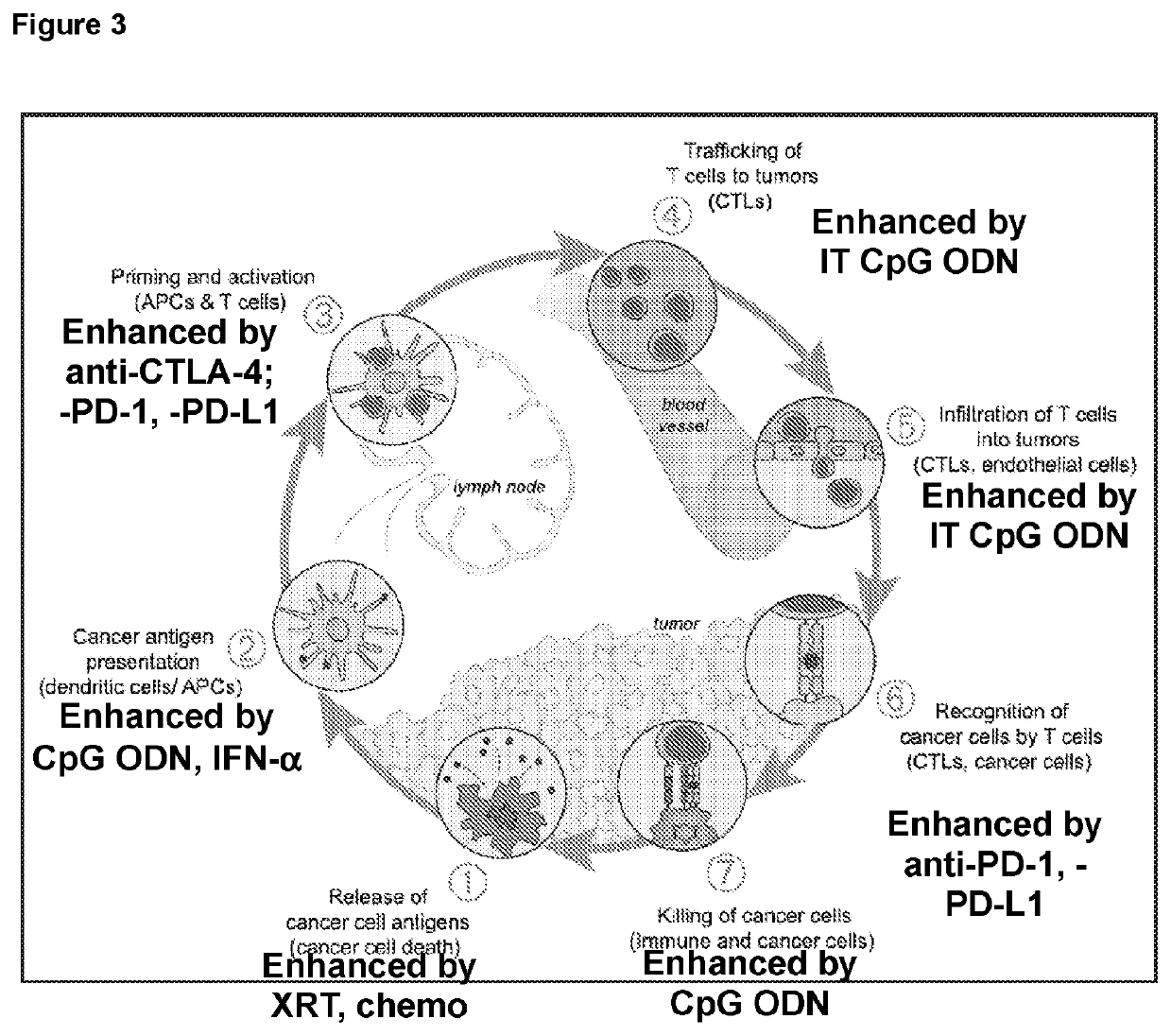
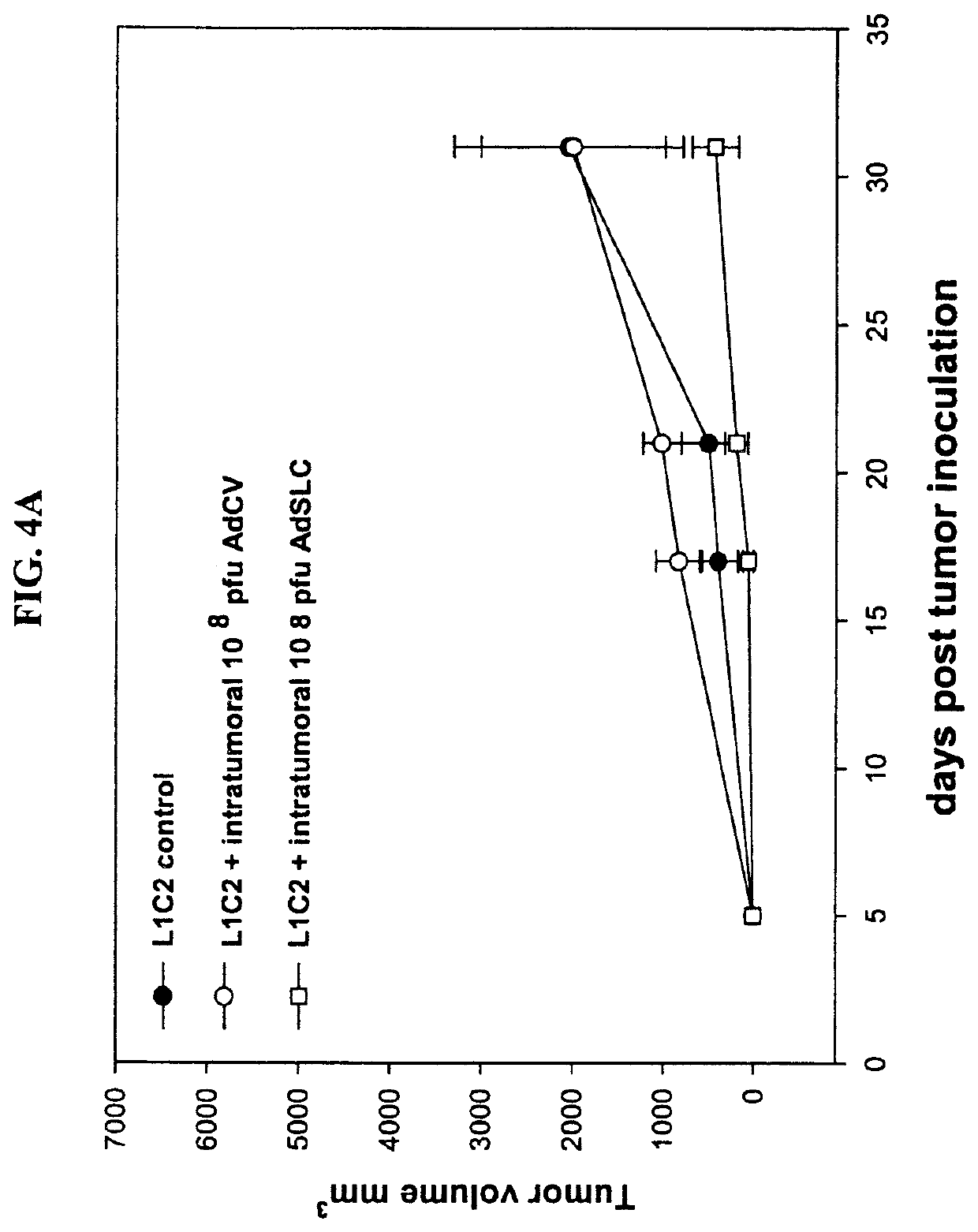
Provided are methods for treating cancer using local administration of certain CpG oligonucleotides (CpG ODN) and systemic administration of a checkpoint inhibitor such as an anti-PD-1 antibody, an anti-PD-L1 antibody, and / or an anti-CTLA-4 antibody. In preferred embodiments, the CpG ODN are selected based on their propensity to induce high amounts of interferon alpha (IFN-α) and T-cell activation relative to interleukin-10 (IL-10) and B-cell activation. In certain embodiments, the methods further include pretreatment with radiotherapy, to potentiate the combination immunotherapy.
Owner:CHECKMATE PHARM INC
Methods and compositions for combination immunotherapy
ActiveUS20180187211A1Permit immunizationOvercome obstaclesOrganic active ingredientsTumor rejection antigen precursorsVaccinationPreexisting immunity
Methods for generating immune responses using adenovirus vectors that allow multiple vaccinations with the same adenovirus vector and vaccinations in individuals with preexisting immunity to adenovirus are provided.
Owner:ETUBICS CORP
Combination immunotherapy compositions against cancer and methods
ActiveUS10383924B2Inhibit tumor growthReduce the burden onAntibacterial agentsAntimycoticsDiseaseCombination immunotherapy
Owner:GLOBE IMMUNE INC +1
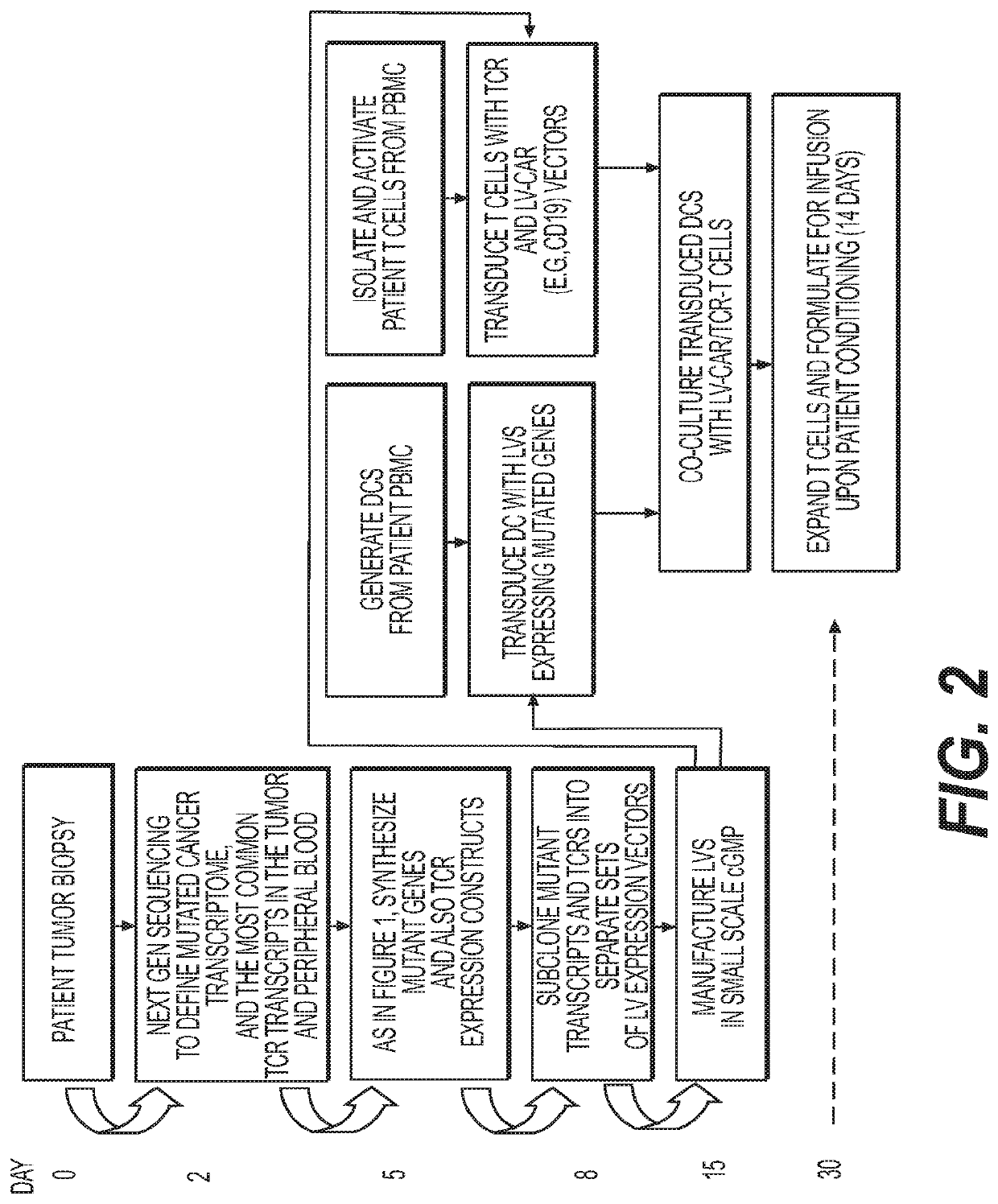
Method to treat cancer with engineered T-cells
ActiveUS10639329B2Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseT cell
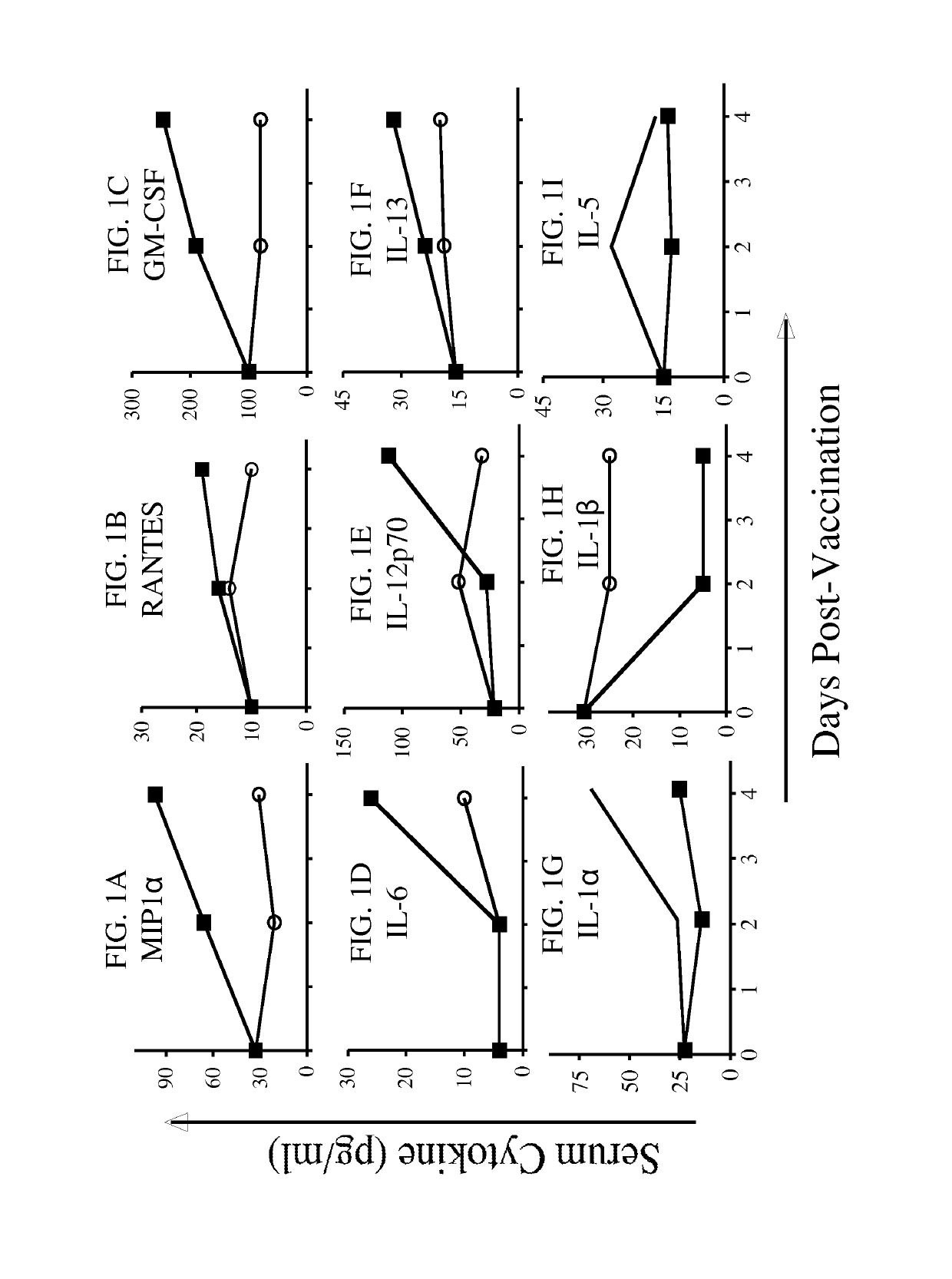
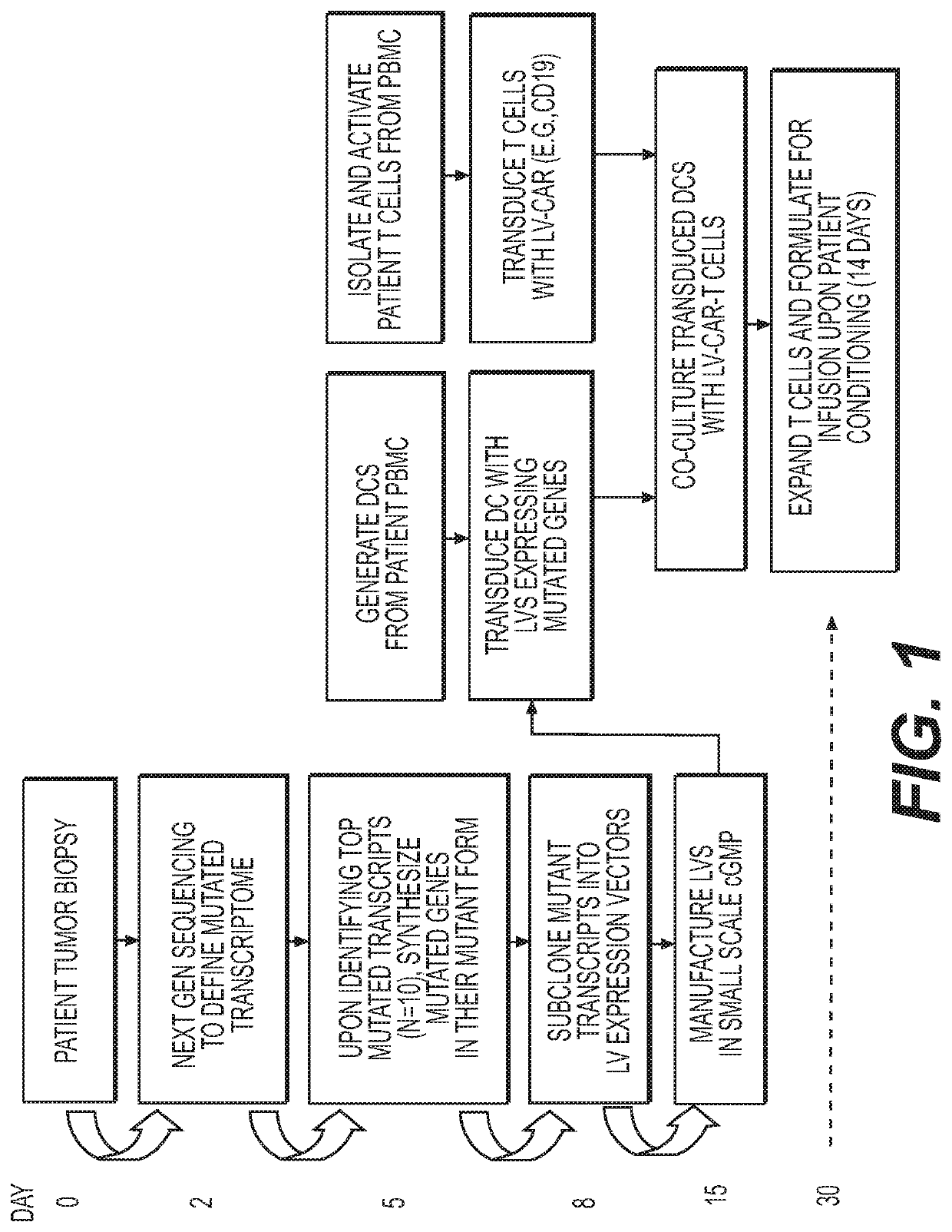
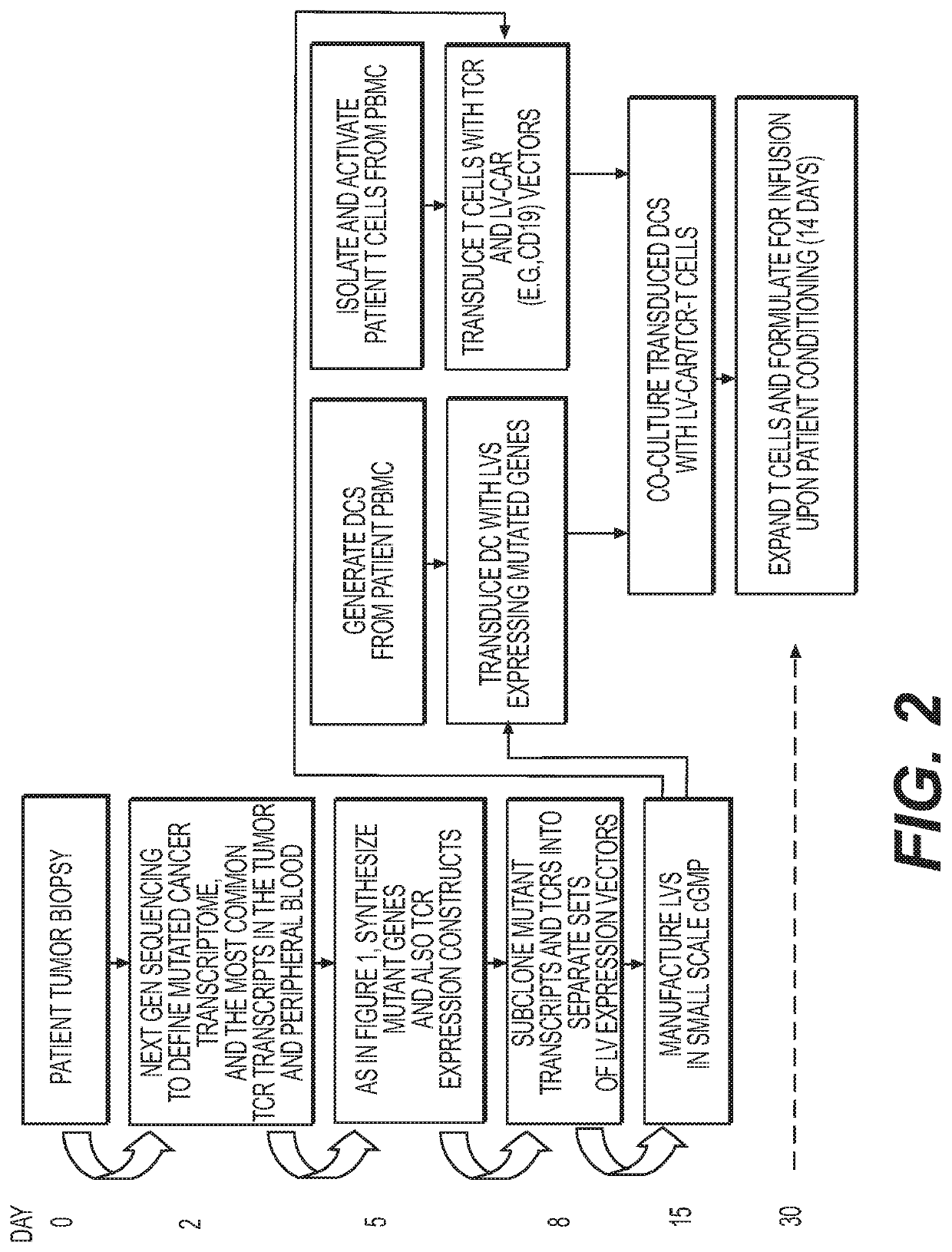
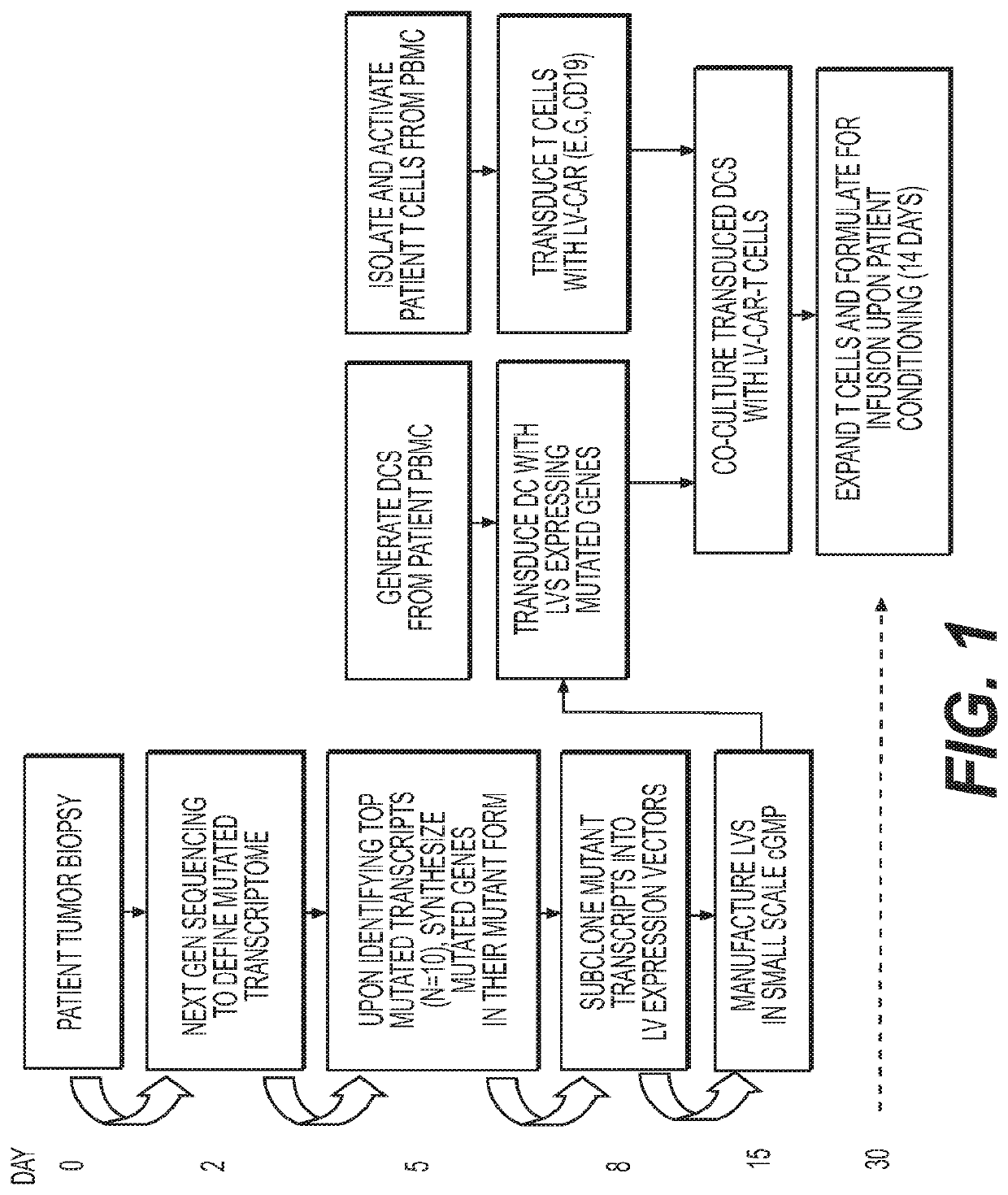
Novel adoptive immunotherapy compositions comprising co-cultured lentiviral vector-transduced autologous antigen presentation cells and T cells are provided herein as well as are methods of use of same in a patient-specific combination immunotherapy that can be used to treat cancers and other diseases and conditions.
Owner:LENTIGEN TECH INC
Targeted combination immunotherapy of cancer and infectious diseases
InactiveUS20060002855A1Minimizes patient toxicityLow toxicityHeavy metal active ingredientsBiocideAbnormal tissue growthInfectious Disorder
The present invention is directed to methods for treating cancer wherein more than one therapeutic agent is used, with each of the therapeutic agents having different tumor-killing capabilities, and wherein the therapeutic agents are delivered to the tumor sites using combined targeting and pre-targeting methods. The methods of the present invention achieve good tumor to non-tumor ratios of the therapeutic agents, and are effective for cancer therapy.
Owner:IMMUNOMEDICS INC
Methods for epitope-specific and cytokine/anticytokine combination immunotherapies
The current invention provides for methods of immunotherapy using a combination of epitope-specific and cytokine or anticytokine immunotherapy. The method provides for modulation of pathogenic immune responses and includes the identification of molecules comprising specific epitopes involved in a particular disease state of interest, administration of the epitope-specific molecule in conjunction with the cytokine or anticytokine, and downstream modification of the administration of the cytokine / anticytokine relative to the administration of the epitope-specific molecule.
Owner:RGT UNIV OF CALIFORNIA
Methods for epitope-specific and cytokine/anticytokine combination immunotherapies for modulation of pathogenic immune responses in immune mediated diseases
InactiveUS20060093574A1Reduce needImprove diseaseSenses disorderNervous disorderEpitope specificityPathogenicity
The current invention provides for methods of immunotherapy using a combination of epitope-specific and cytokine or anticytokine immunotherapy. The method provides for modulation of pathogenic immune responses and includes the identification of molecules comprising specific epitopes involved in a particular disease state of interest, administration of the epitope-specific molecule in conjunction with the cytokine or anticytokine, and downstream modification of the administration of the cytokine / anticytokine relative to the administration of the epitope-specific molecule.
Owner:RGT UNIV OF CALIFORNIA
Combination immunotherapies for treatment of cancer
ActiveUS20190298764A1High activityHigh expressionOrganic active ingredientsSsRNA viruses positive-senseDendritic cellTumor antigen
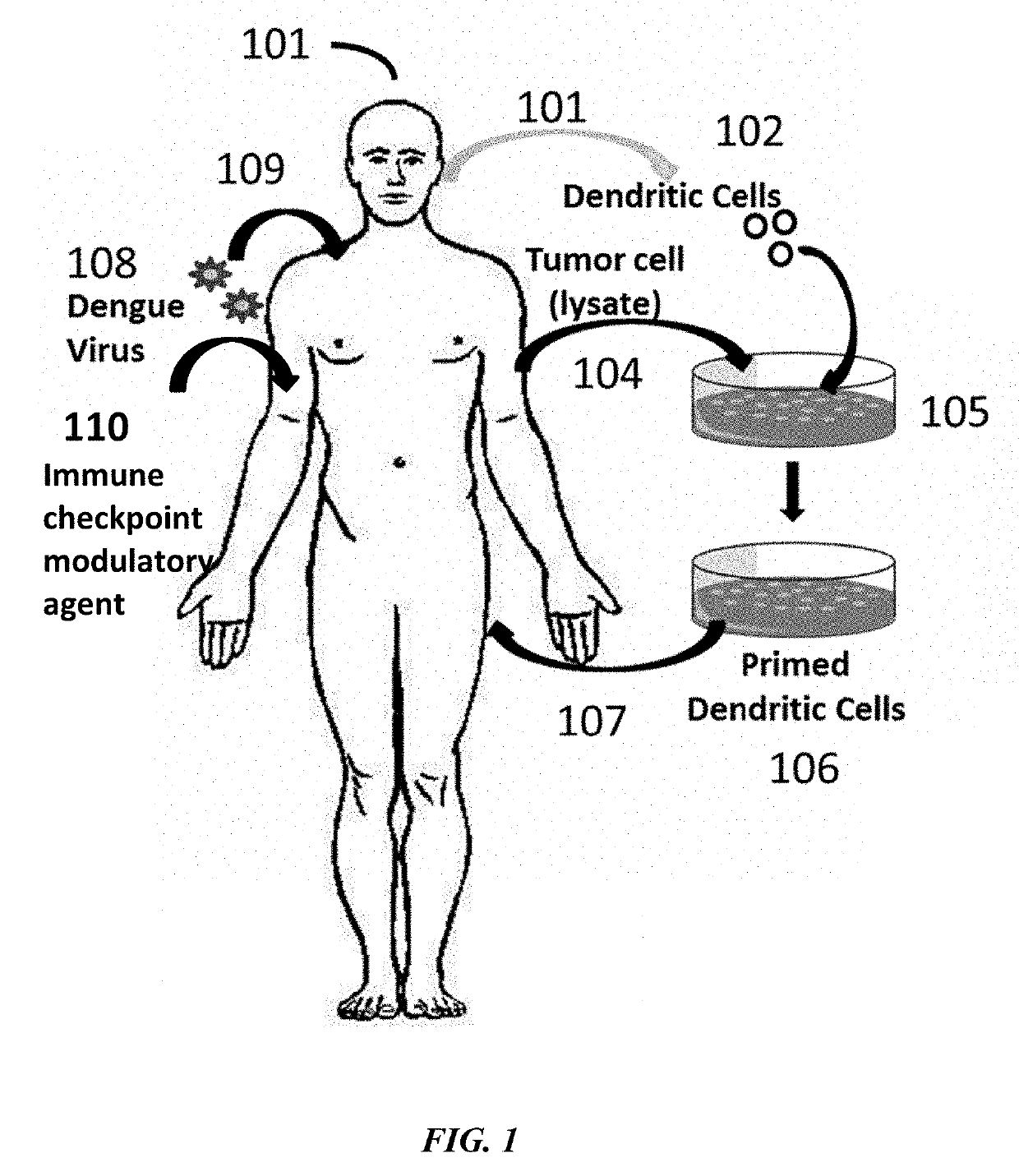
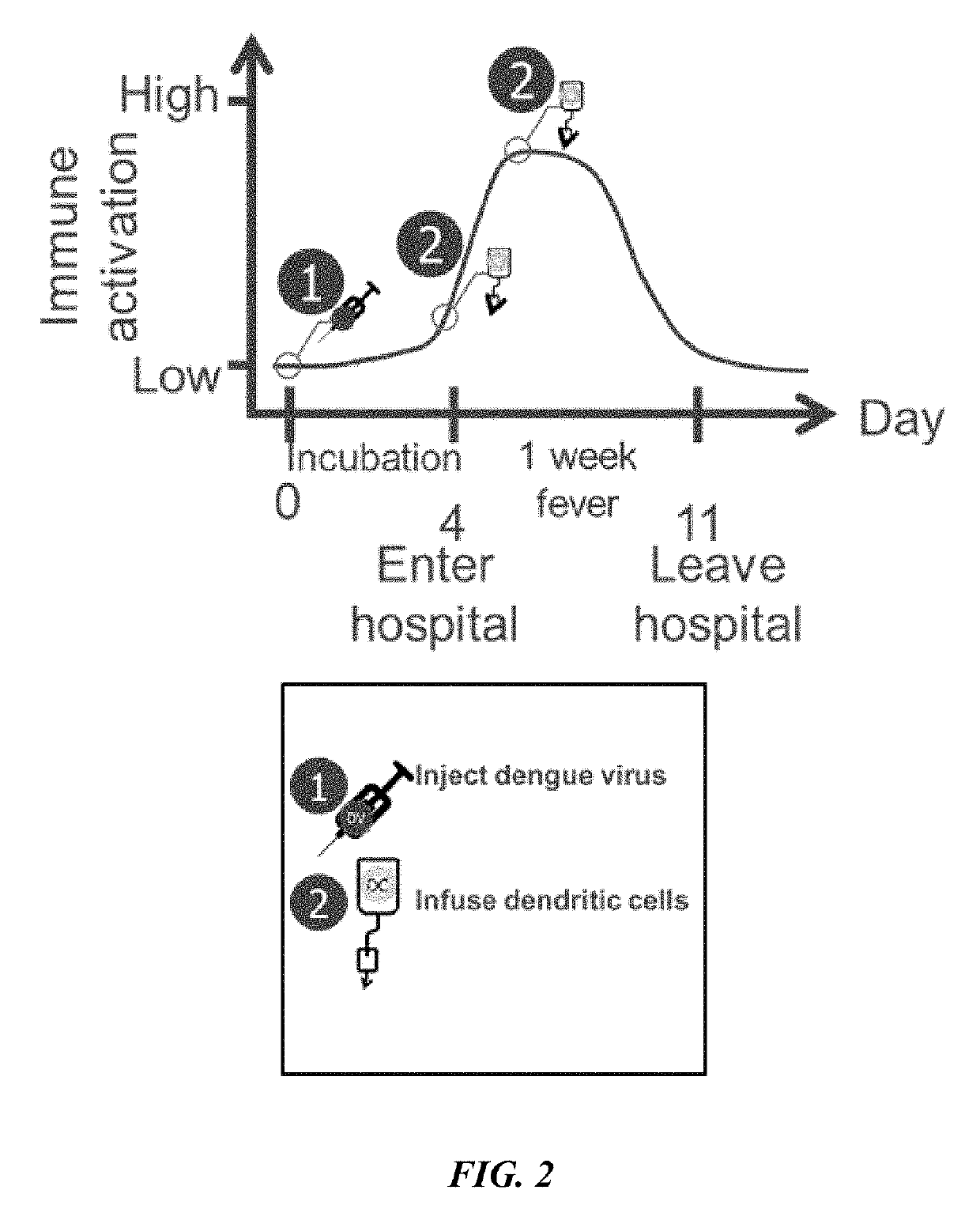
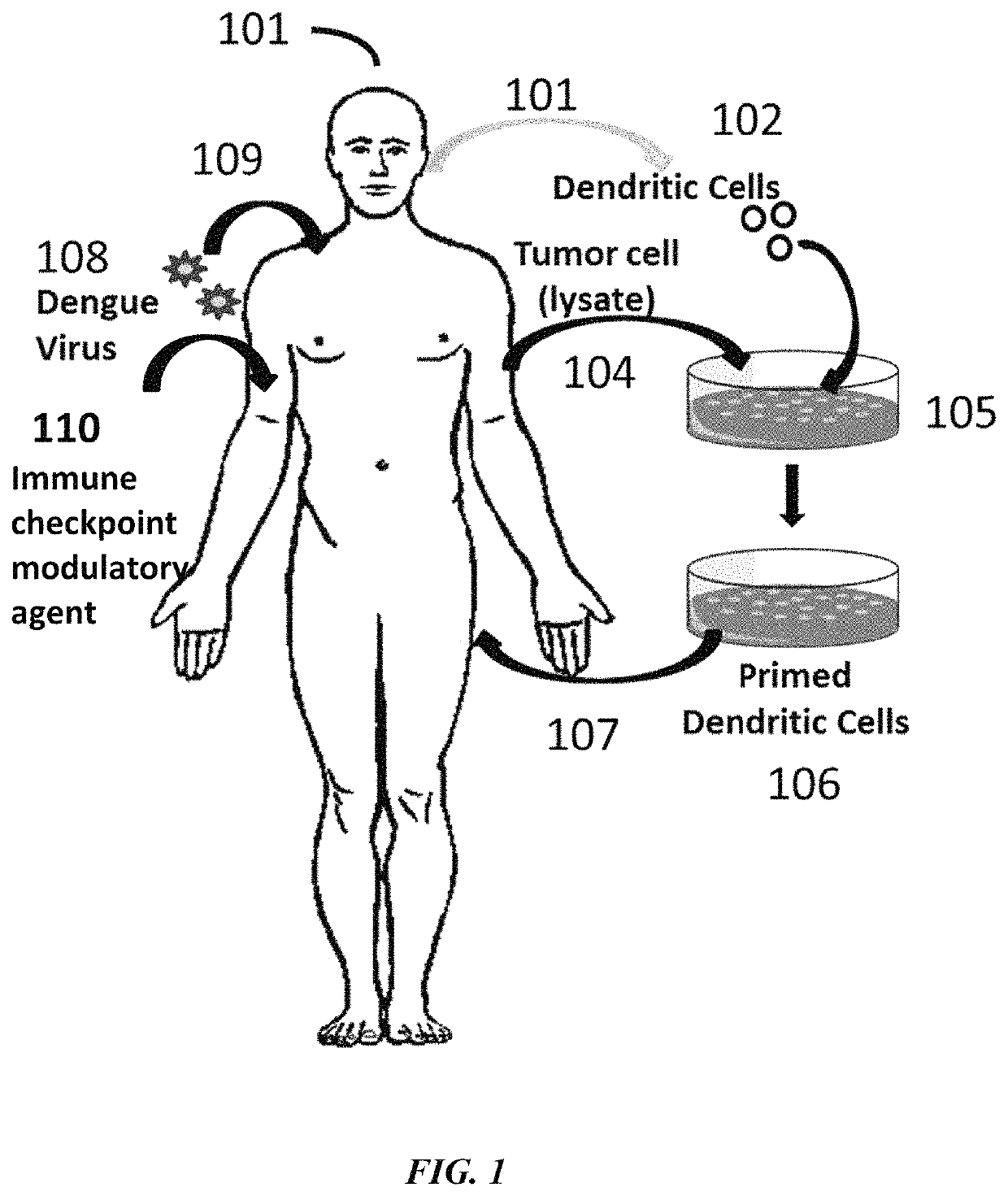
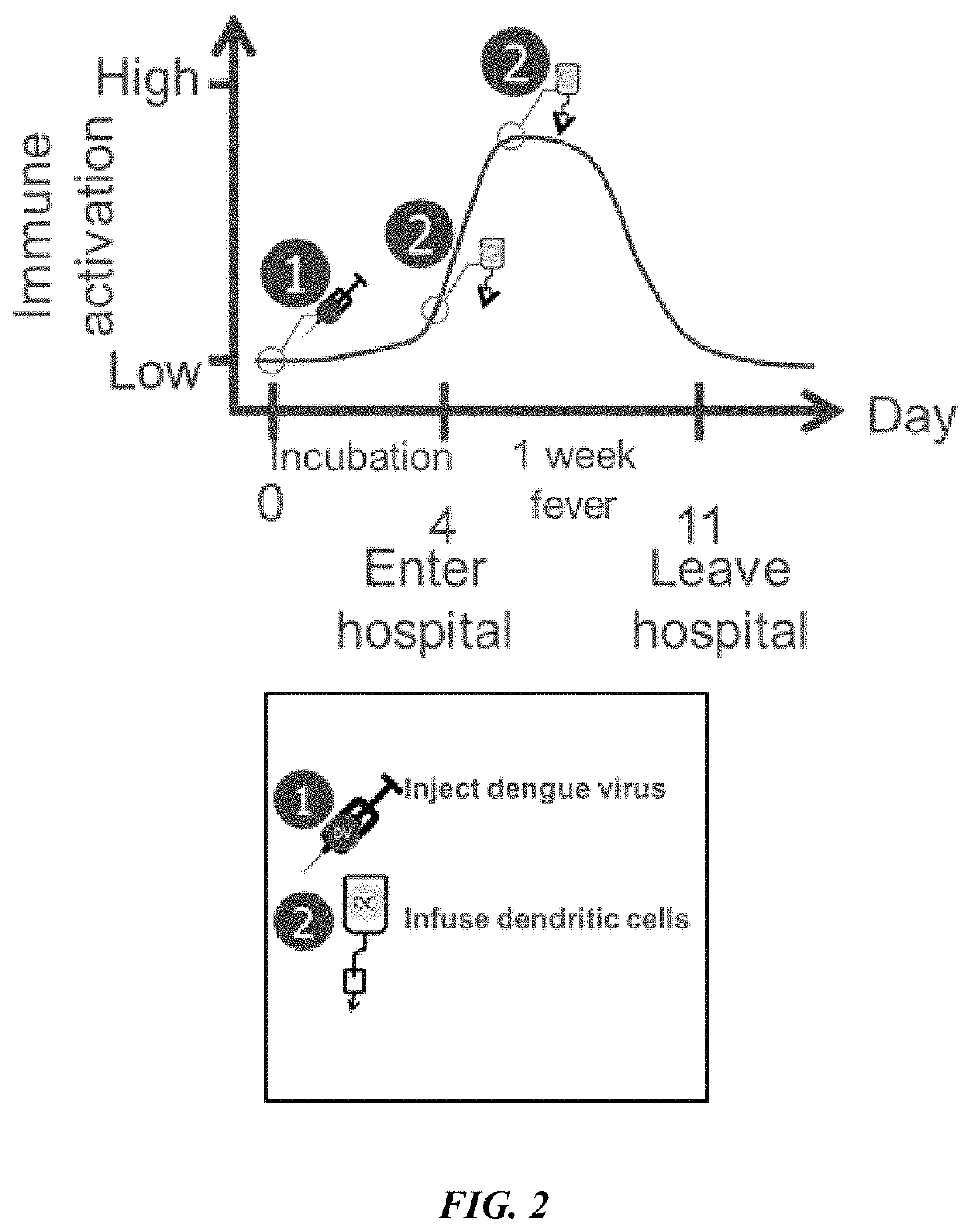
Described herein are compositions and methods for treating a disease, particularly a cancer, with an immune checkpoint modulatory agent and a strain of an Arbovirus or a strain of an Alphavirus. Also provided herein are also methods for combination therapy comprising administration of an immune checkpoint modulatory agent, tumor antigen primed dendritic cells and an Alphavirus or an Arbovirus.
Owner:PRIMEVAX IMMUNO ONCOLOGY INC
Targeted Combination Immunotherapy of Cancer and Infectious Diseases
InactiveUS20080031813A1Miniimizes patient toxicityLow toxicityBiocidePeptide/protein ingredientsAbnormal tissue growthMedicine
The present invention is directed to methods for treating cancer wherein more than one therapeutic agent is used, with each of the therapeutic agents having different tumor-killing capabilities, and wherein the therapeutic agents are delivered to the tumor sites using combined targeting and pre-targeting methods. The methods of the present invention achieve good tumor to non-tumor ratios of the therapeutic agents, and are effective for cancer therapy.
Owner:IMMUNOMEDICS INC
Combination tumor immunotherapy
PendingUS20200281953A1Promotes the egress of activated T cellsImprove anti-tumor effectImmunoglobulins against virusesImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesWhite blood cell
Owner:CHECKMATE PHARM INC
Combination immunotherapies for treatment of cancer
Described herein are compositions and methods for treating a disease, particularly a cancer, with an immune checkpoint modulatory agent and a strain of an Arbovirus or a strain of an Alphavirus. Also provided herein are also methods for combination therapy comprising administration of an immune checkpoint modulatory agent, tumor antigen primed dendritic cells and an Alphavirus or an Arbovirus.
Owner:PRIMEVAX IMMUNO ONCOLOGY INC
Inhibition of CTLA-4 and/or PD-1 For Regulation of T Cells
PendingUS20210179714A1Negative impact on T-cell responsesHeightened T-cell primingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsOncologyT cell
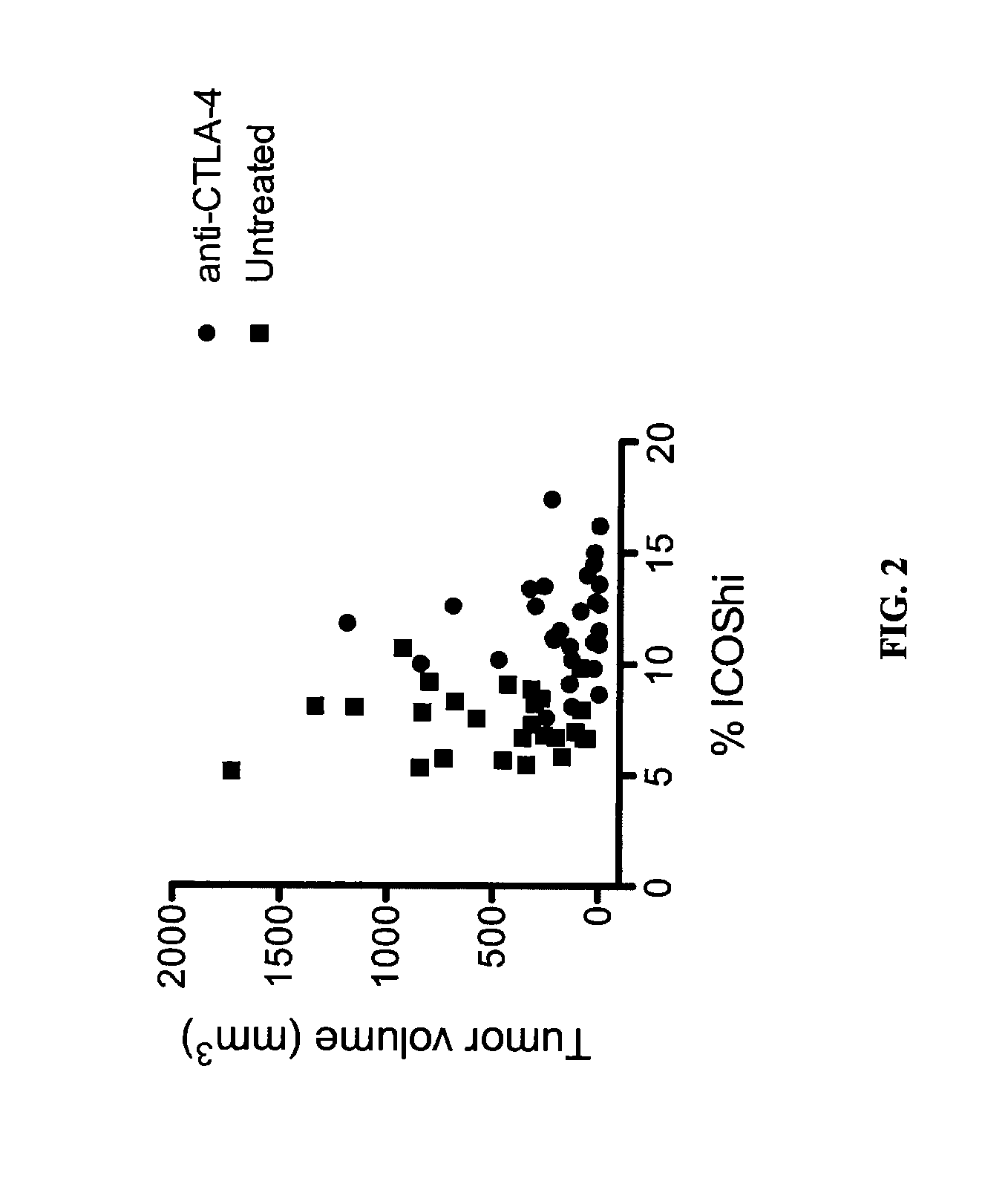
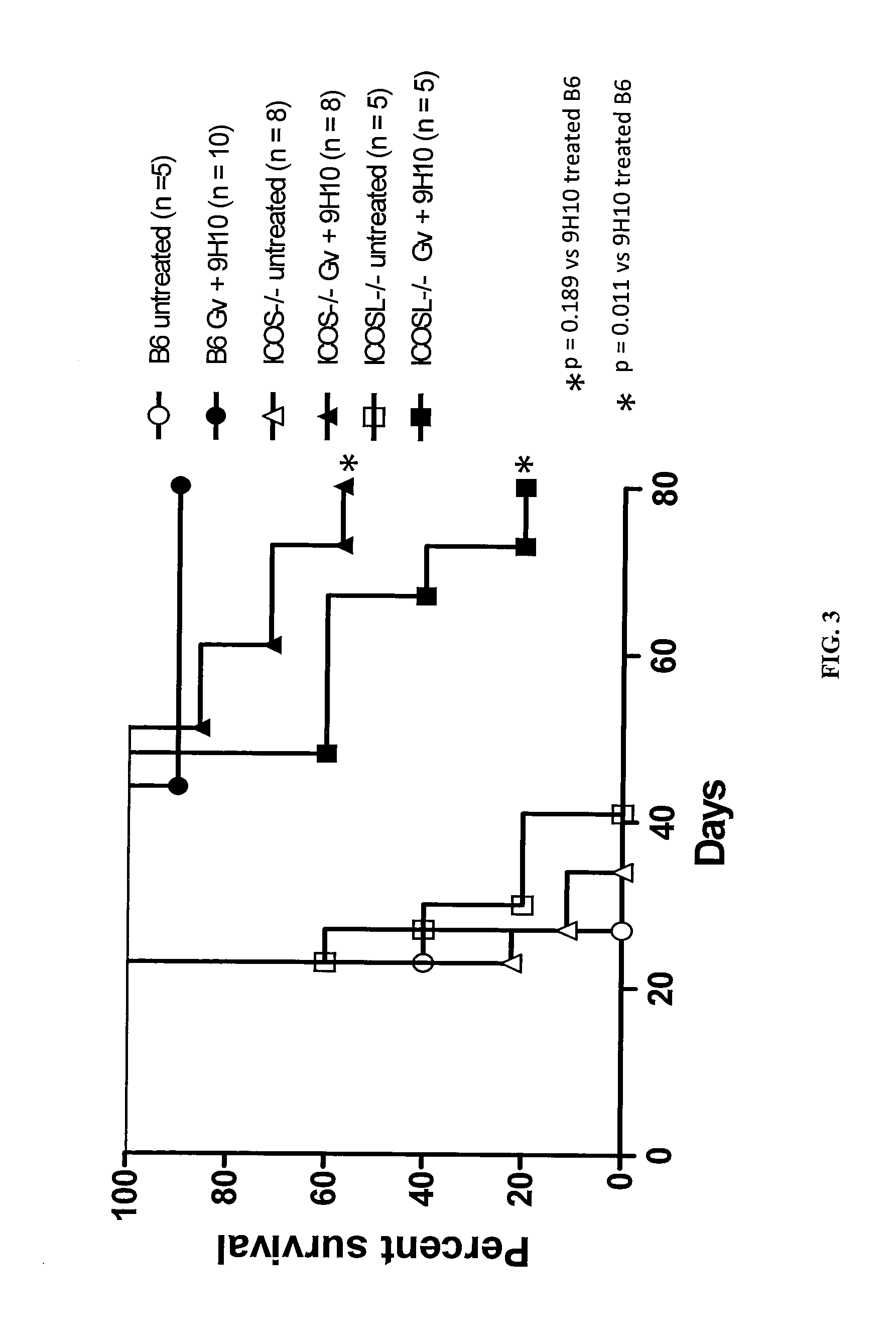
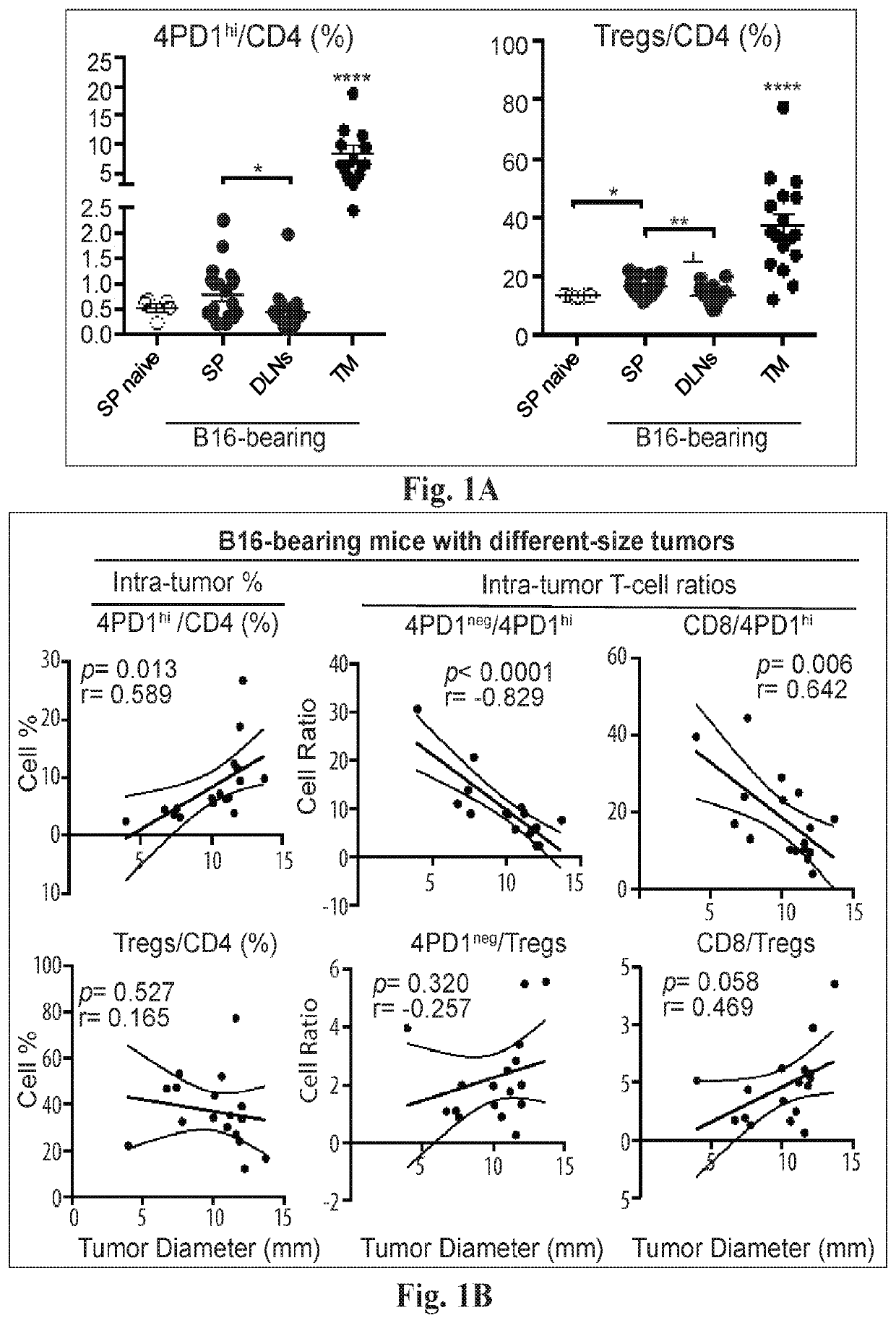
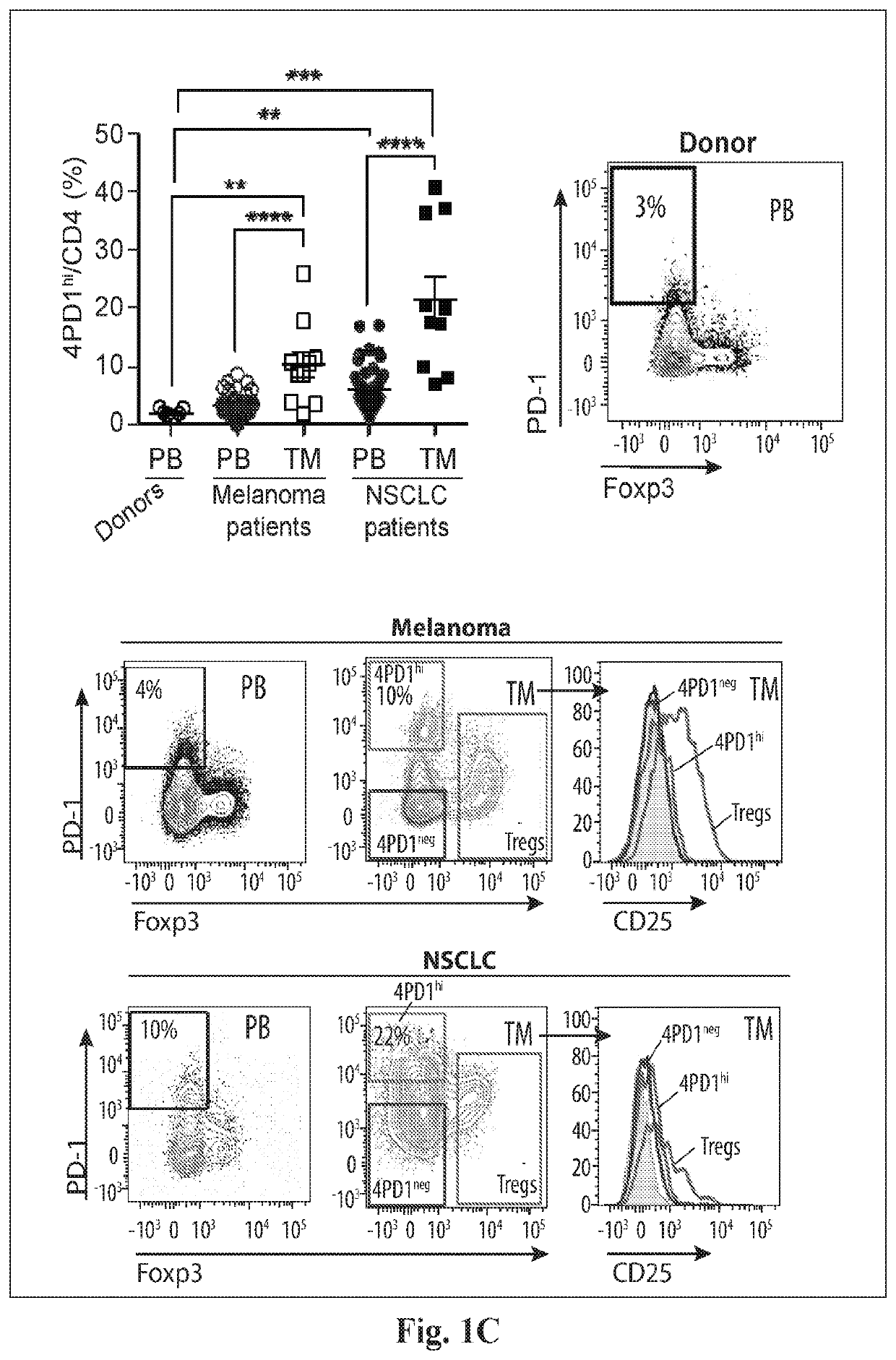
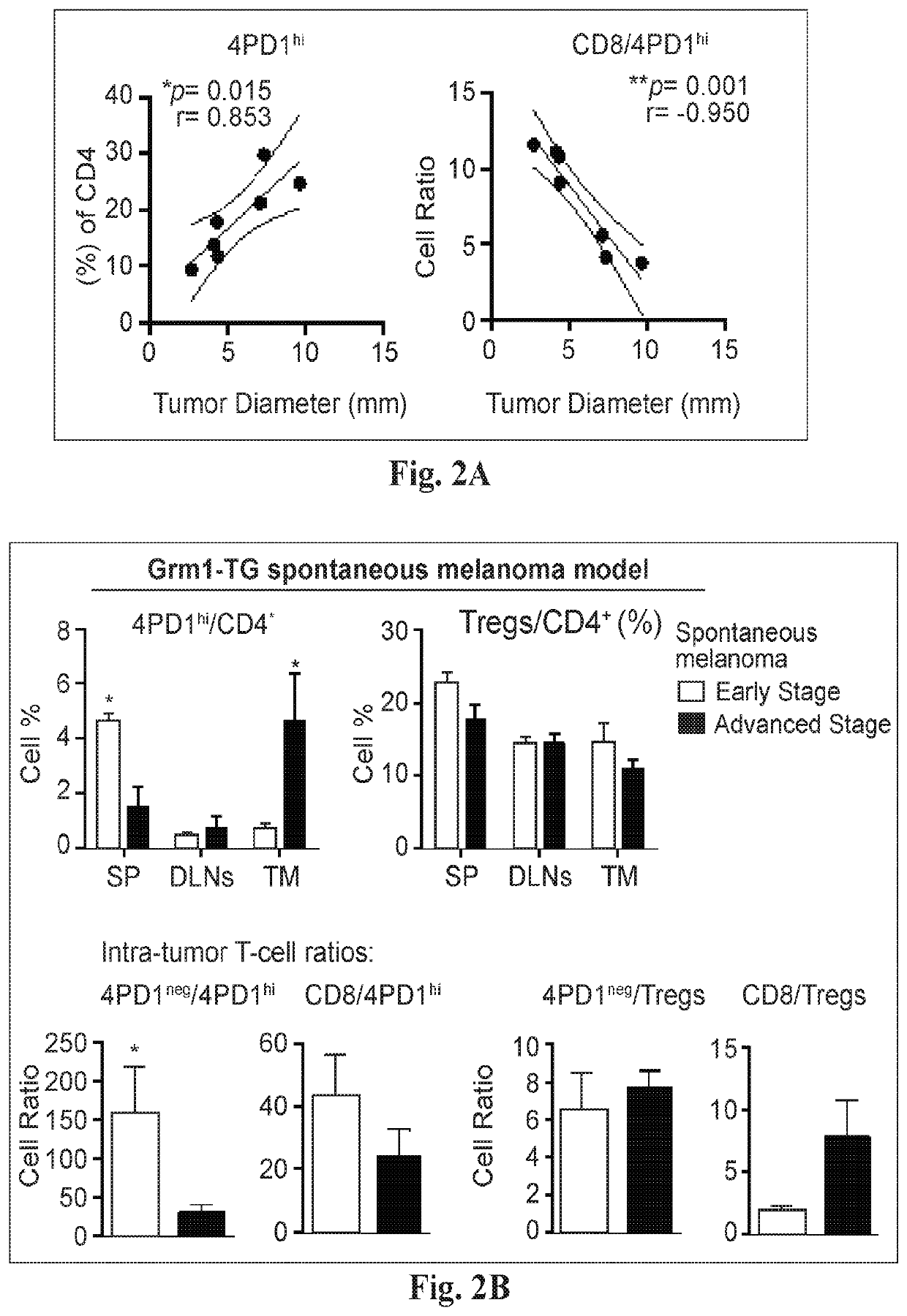
Increases in CD4+Foxp3−PD-Ihi T cells (4PD1hi) in tumor-bearing hosts after CTLA-4 blockade show that these cells constitute an unconventional T-cell inhibitory subset with TFH-like features, which can affect the outcome of cancer immunotherapy. Evidence is provided that anti-PD-1 / PD-L1 antibodies arc a viable option to control these cells. Furthermore, treating cancer by administering immune checkpoint blockade therapy and monitoring circulating 4PD1hi provides a more precise or personalized design of combination immunotherapies.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Methods and compositions for combination immunotherapy
ActiveUS11352642B2Enhance immune responsePre-existing immunityOrganic active ingredientsTumor rejection antigen precursorsImmunopotencyVaccination
Methods for generating immune responses using adenovirus vectors that allow multiple vaccinations with the same adenovirus vector and vaccinations in individuals with preexisting immunity to adenovirus are provided.
Owner:ETUBICS CORP
Combination immunotherapy for treatment of triple-negative breast cancer
ActiveUS20200121789A1Peptide/protein ingredientsPharmaceutical delivery mechanismMajor histocompatibilityPharmaceutical drug
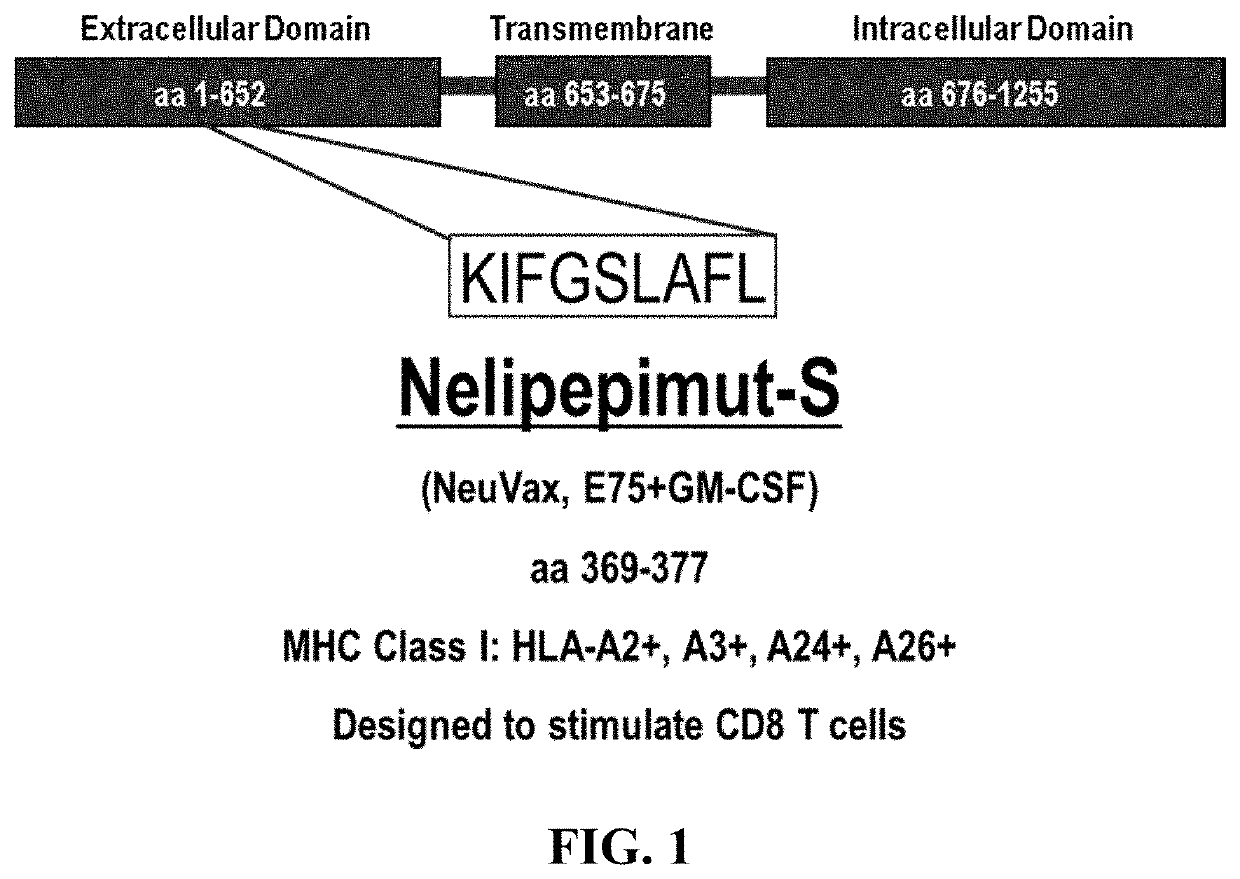
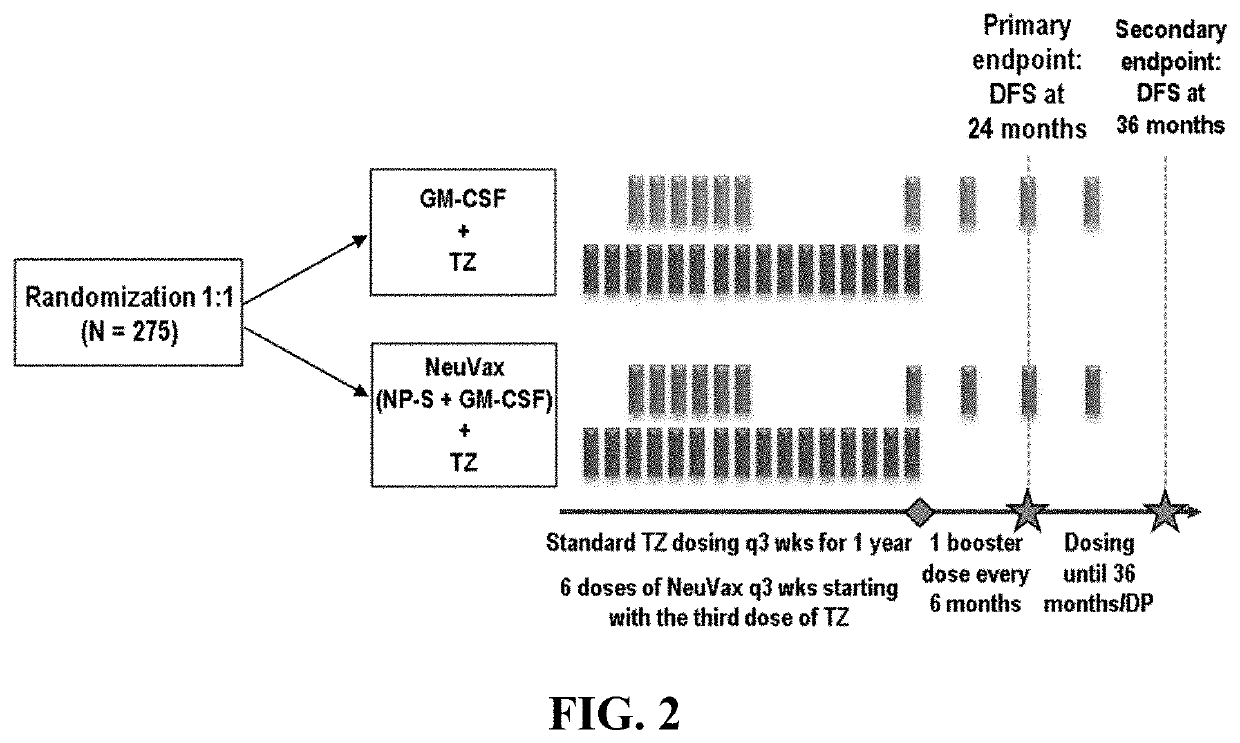
The present invention concerns a method for treating triple-negative breast cancer (TNBC) in an individual, and / or for inducing an immune response to HER2 / neu in an individual with a triple-negative breast cancer expressing low levels of HER2 / neu, the method comprising administering to the individual: (a) an effective amount of trastuzumab, or derivative thereof; and (b) an effective amount of nelipepimut-S, or variant thereof, optionally with an immunological adjuvant. Preferably, the method includes a preparatory or priming phase comprising a frequency and duration of trastuzumab or trastuzumab derivative administration sufficient to substantially increase the major histocompatibility complex (MHC)-mediated presentation of HER2 peptide fragments to the patient immune system. The invention also includes medicaments and kits for treating TNBC in an individual, and / or for inducing an immune response to HER2 / neu in an individual with a TNBC expressing HER2 / neu.
Owner:SLSG LTD LLC
Combination immunotherapy for treatment of triple-negative breast cancer
PendingCN113543805APeptide/protein ingredientsImmunoglobulins against animals/humansPharmaceutical drugOncology
The present invention concerns a method for treating triple-negative breast cancer (TNBC) in an individual, and / or for inducing an immune response to HER2 / neu in an individual with a triple-negative breast cancer expressing low levels of HER2 / neu, the method comprising administering to the individual: (a) an effective amount of trastuzumab, or derivative thereof; and (b) an effective amount of nelipepimut-S, or variant thereof, optionally with an immunological adjuvant. Preferably, the method includes a preparatory or priming phase comprising a frequency and duration of trastuzumab or trastuzumab derivative administration sufficient to substantially increase the major histocompatibility complex (MHC)-mediated presentation of HER2 peptide fragments to the patient immune system. The invention also includes medicaments and kits for treating TNBC in an individual, and / or for inducing an immune response to HER2 / neu in an individual with a TNBC expressing HER2 / neu.
Owner:エスエルエスジーリミテッドエルエルシー
Combination immunotherapy
ActiveUS11236139B2Lower Level RequirementsReduce the burden onChemokinesPeptide/protein ingredientsAntiendomysial antibodiesSecondary lymphoid organs
The invention is based on the disclosure provided herein that secondary lymphoid organ chemokine (SLC) inhibits the growth of syngeneic tumors in vivo. Thus, the invention provides a method of treating cancer in a mammal subject by administering a therapeutically effective amount of an SLC to the mammal in combination with a checkpoint inhibitor, including monoclonal antibodies and small molecule inhibitors. Exemplary checkpoint molecules include CTLA-4, a CTLA-4 receptor, PD-1, PD1-L1, PD1-L2, 4-1BB, OX40, LAG-3, TIM-3 or a combination thereof. SLCs useful in the methods of the invention include SLC polypeptides, variants and fragments and related nucleic acids.
Owner:RGT UNIV OF CALIFORNIA +1
Method to Treat Cancer with Engineered T-Cells
PendingUS20200297769A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyDiseaseT cell
Novel adoptive immunotherapy compositions comprising co-cultured lentiviral vector-transduced autologous antigen presentation cells and T cells are provided herein as well as are methods of use of same in a patient-specific combination immunotherapy that can be used to treat cancers and other diseases and conditions.
Owner:LENTIGEN TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com