Methods and compositions for combination immunotherapy
a combination immunotherapy and composition technology, applied in the direction of immunoglobulins, drug compositions, peptides, etc., can solve the problems of immunologically potent vaccines, limited strategies, and high pre-existing frequency, so as to improve the immune response, prevent or delay the onset, and increase the risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Injections of Ad5Null Adenovirus Vector Produces Anti-Adenovirus Antibodies
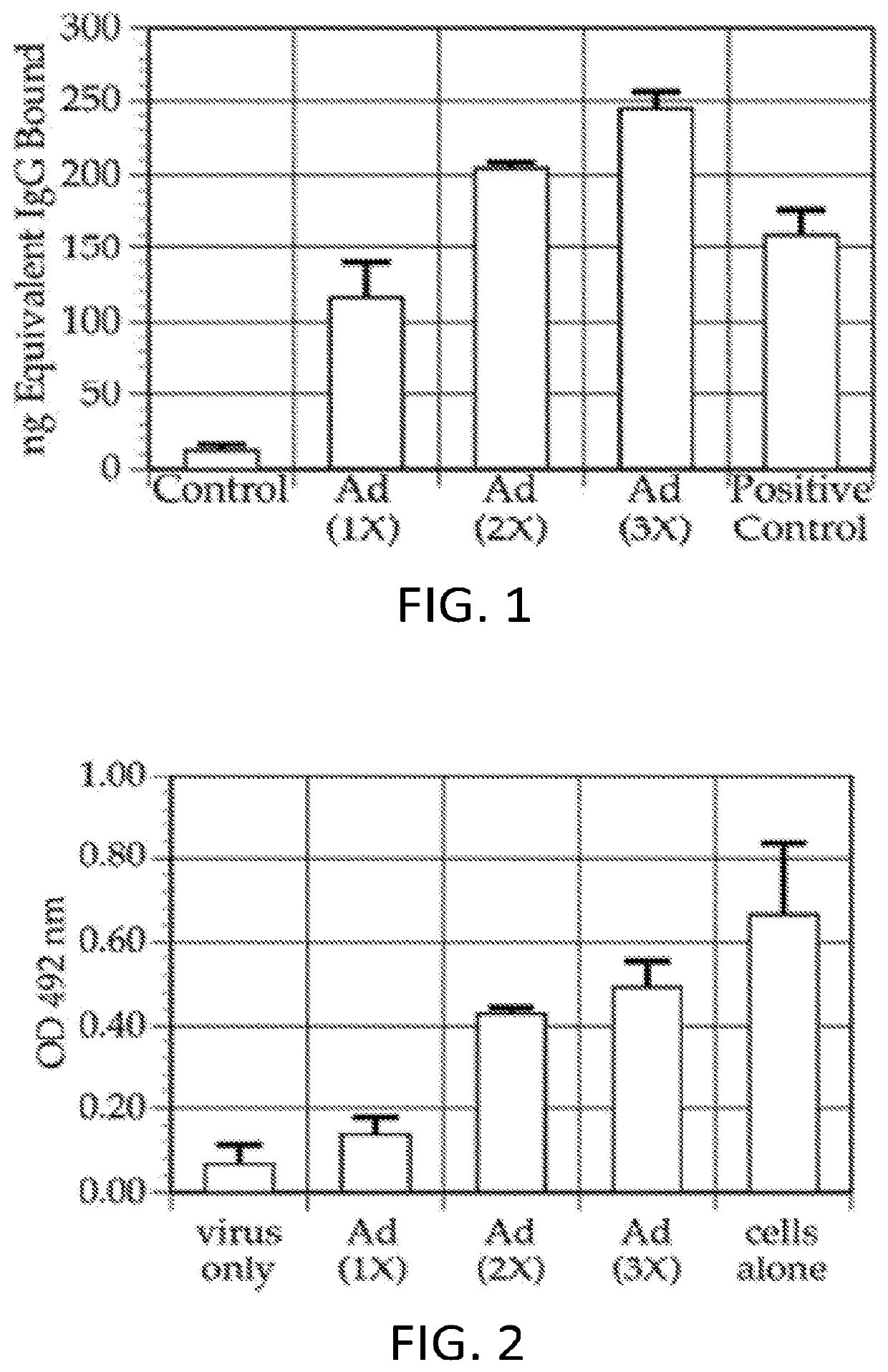
[0349]This example shows that multiple injections of Ad5-null results in the production of anti-adenovirus antibodies in the injected subjects.
[0350]It was demonstrated that the Ad5-null adenovirus vector that does not contain any heterologous nucleic acid sequences, generated a neutralizing immune response in mice. In one experiment, female Balb / c mice aged 5-7 weeks were immunized with Ad5Null viral particles at 14 day intervals. To determine the presence of anti-adenovirus antibodies, an enzyme linked immunosorbent assay (ELISA) was used. For this ELISA, 109 viral particles were coated onto microtiter wells in 100 μL of 0.05M carbonate / bicarbonate buffer, pH 9.6, and incubated overnight at room temperature. For a standard immunoglobulin G (IgG) reference curve, 200 ng, 100 ng, 50 ng, 25 ng, and 0 ng of purified mouse IgG were coated onto microtiter wells as described above. After incubation, all wells were...
example 2
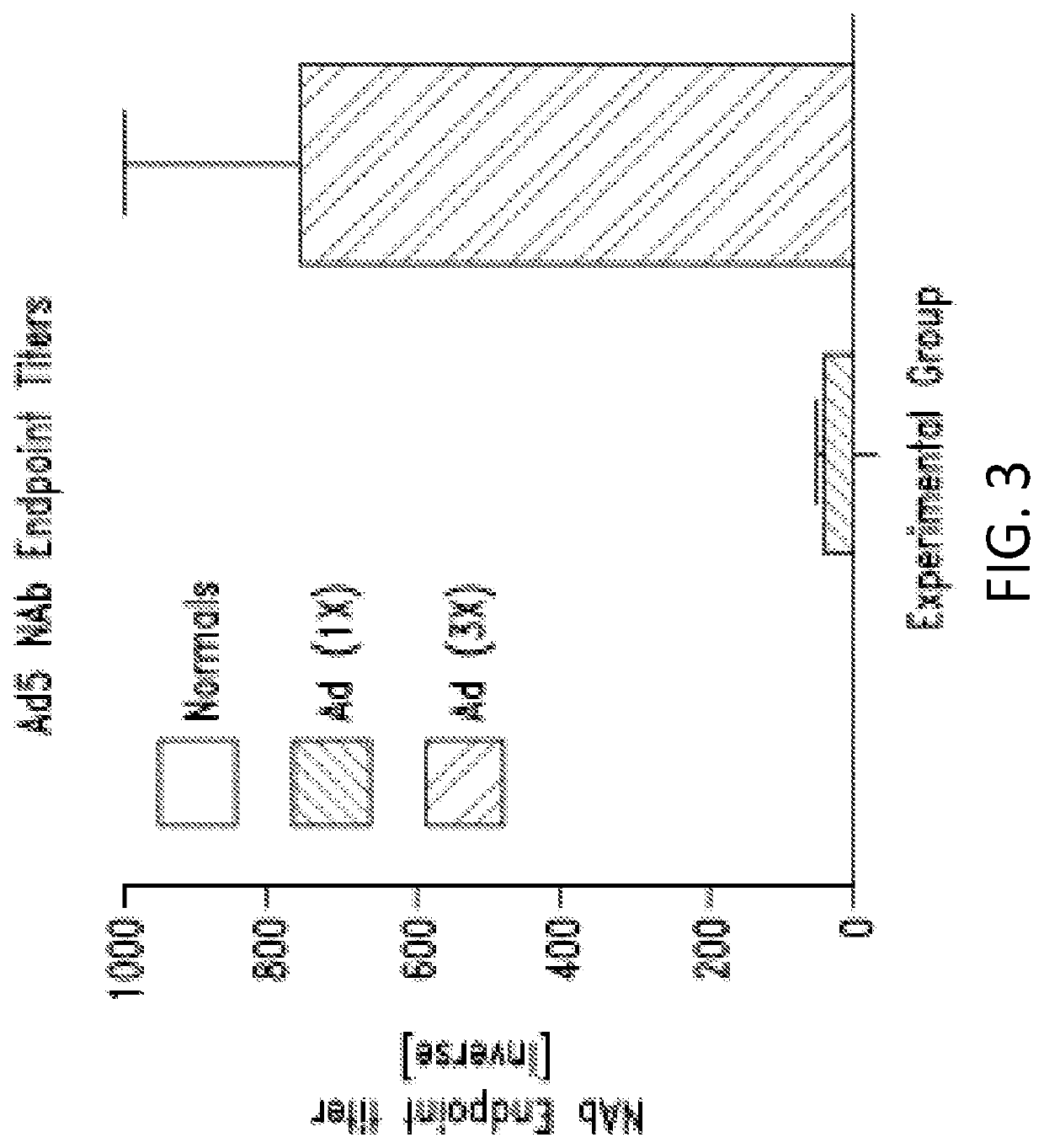
E1-]-CEA Vector Vaccine Induces CEA Specific Immune Response Upon Re-Immunization in Ad5 Immune Mice
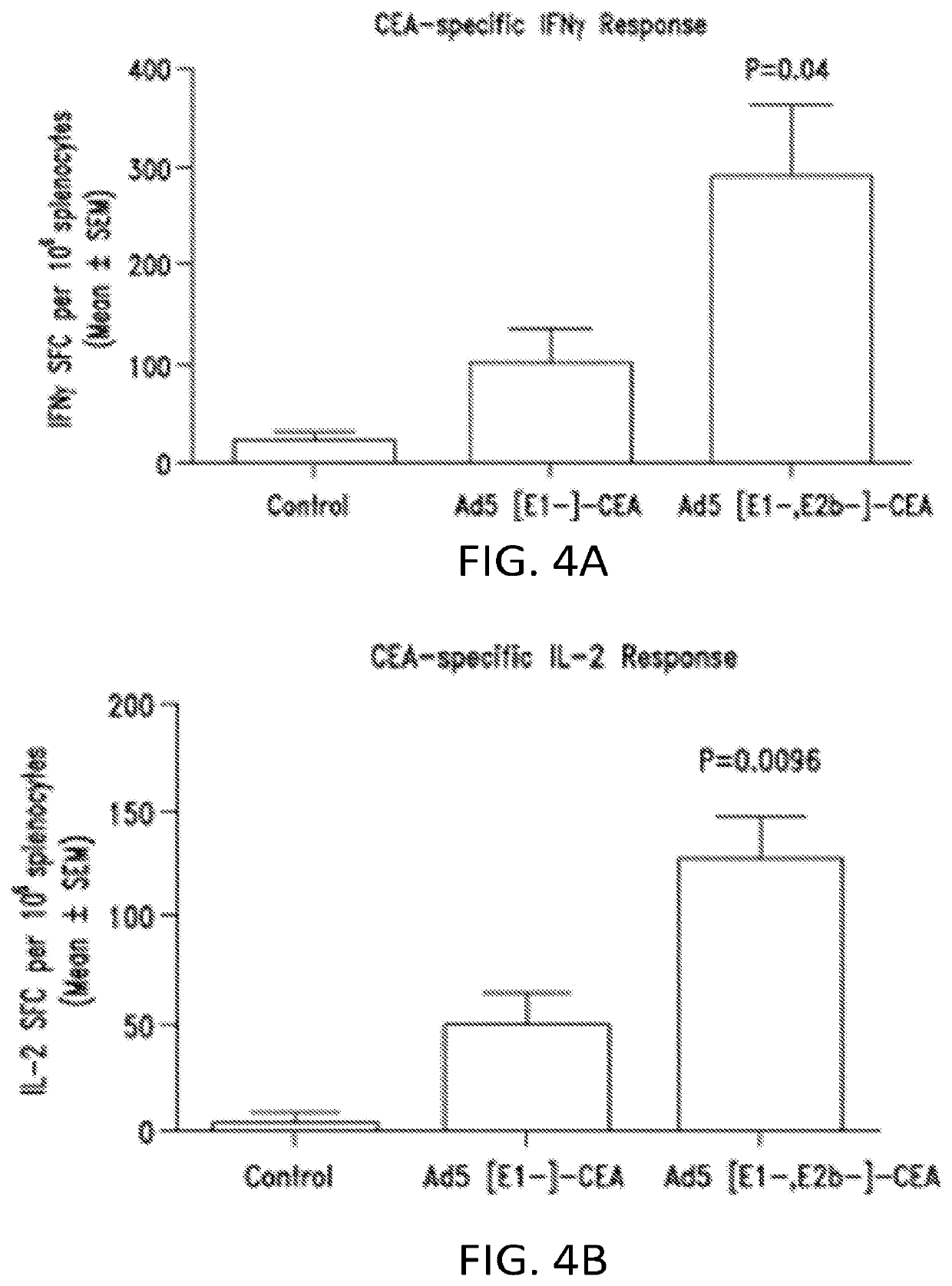
[0355]This example shows that the Ad5 [E1-, E2b-] vector platform induces CMI responses against the tumor associated antigen (TAA) carcinoembryonic antigen (CEA) in the presence of pre-existing Ad5 immunity in mice.
Characterization of Ad5 CEA Vectors
[0356]Initial studies were performed to confirm CEA gene expression of two Ad5-CEA vector platforms. It was first determined that the CEA antigen could be expressed on cells transfected with the vaccine vector platforms. A549 cells were obtained from ATCC and transfected with Ad5 [E1-]-CEA or Ad5 [E1-, E2b-]-CEA. Western blot analysis revealed that cells transfected with the vector platforms expressed CEA antigen. (FIG. 35)
Methods
[0357]A549 cells were inoculated at a MOI of 555 VPs / cell with Ad5 [E1-, E2b-]-CEA. Cells were incubated for 48 hours at 37° C. in 5% CO2. After 48 hours cells were harvested and washed with PBS and freeze / thawed ...
example 3
ive ELISA for CEA Expression on A549 Cells after Infection
[0365]This example shows a dose response evaluation using the Ad5 [E1-, E2b-] CEA vector to transduce the human cancerous lung cell line, A-549. The results show that the CEA antigen can be expressed in a dose dependent manner.
Experimental Design
[0366]On day one, of the assay a BD Falcon Tissue Culture 96-well plate was seeded with A549 cells passaged three days prior (lot #30Jul02, passage p+23), (7.7×103 cells / well) and placed into a 37±2° C. incubator with a 5±2% CO2 atmosphere overnight.
[0367]The next day, a dilution series of the test article were prepared and replicate wells were inoculated at levels ranging from 1.56×103 to 2.5×104 viral particles / well. Untreated A549 cells were used to serve as the mock sample. On day four of the assay wells were treated with a 10% Triton X-100 solution for analysis by ELISA to measure CEA concentration. For the ELISA, a microtiter plate was coated overnight with an anti-CEA capture a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com