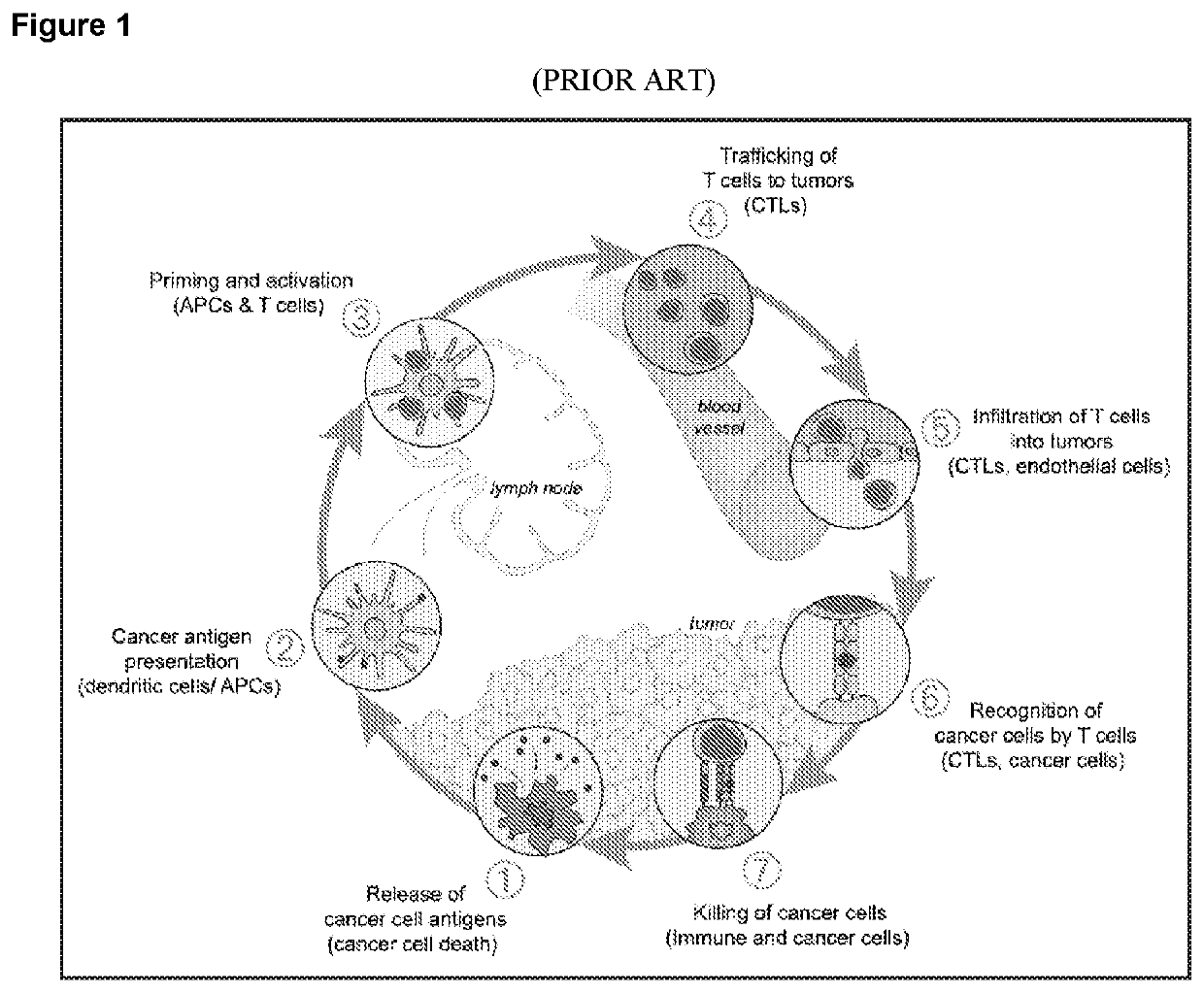
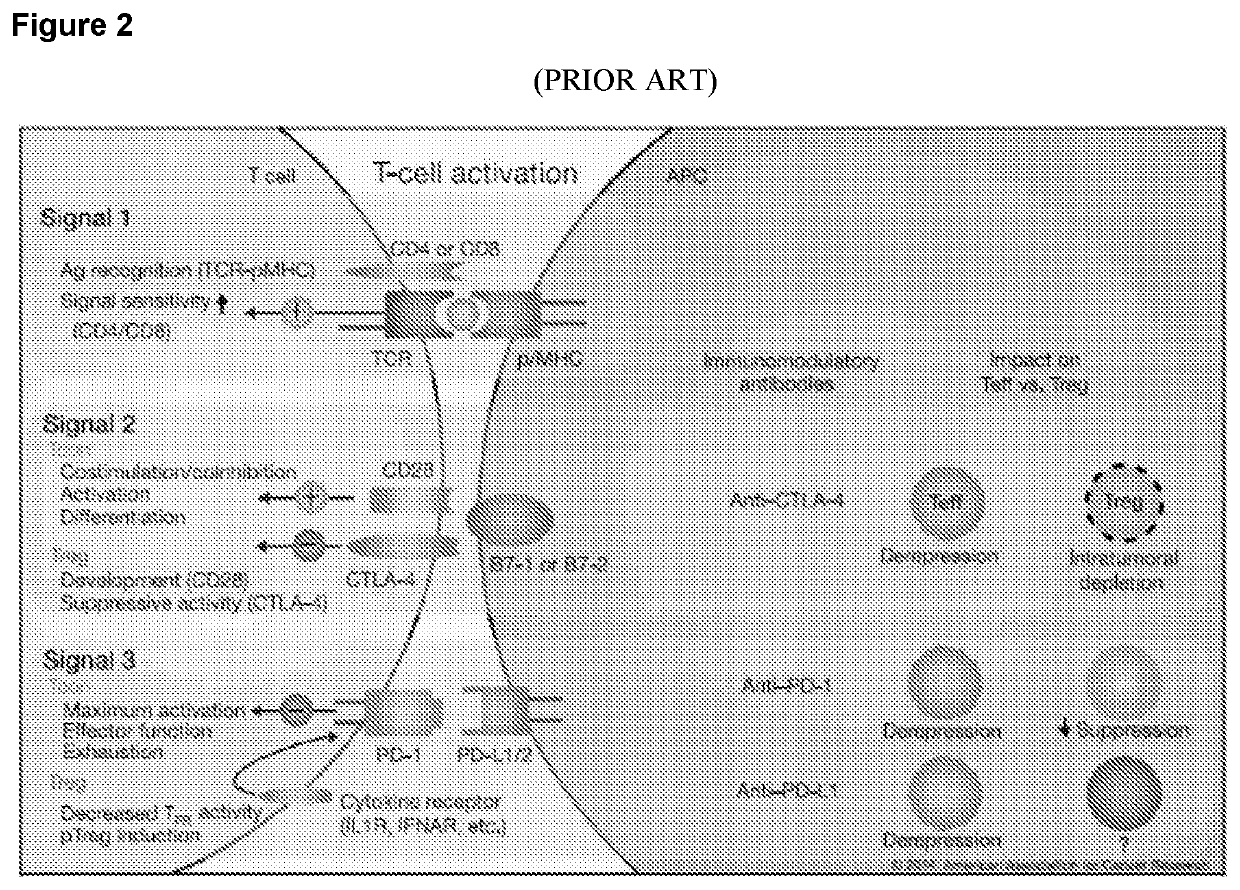
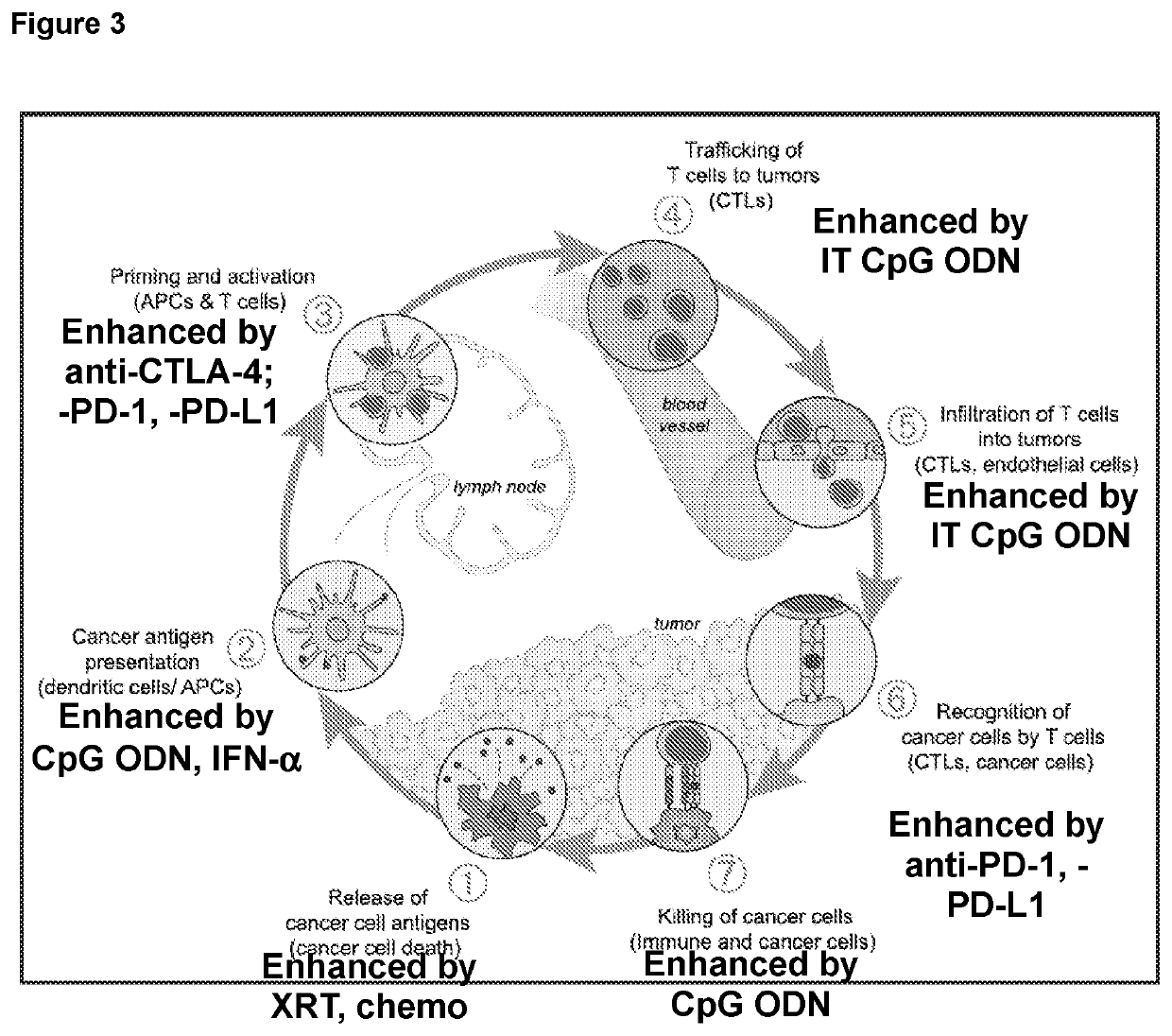
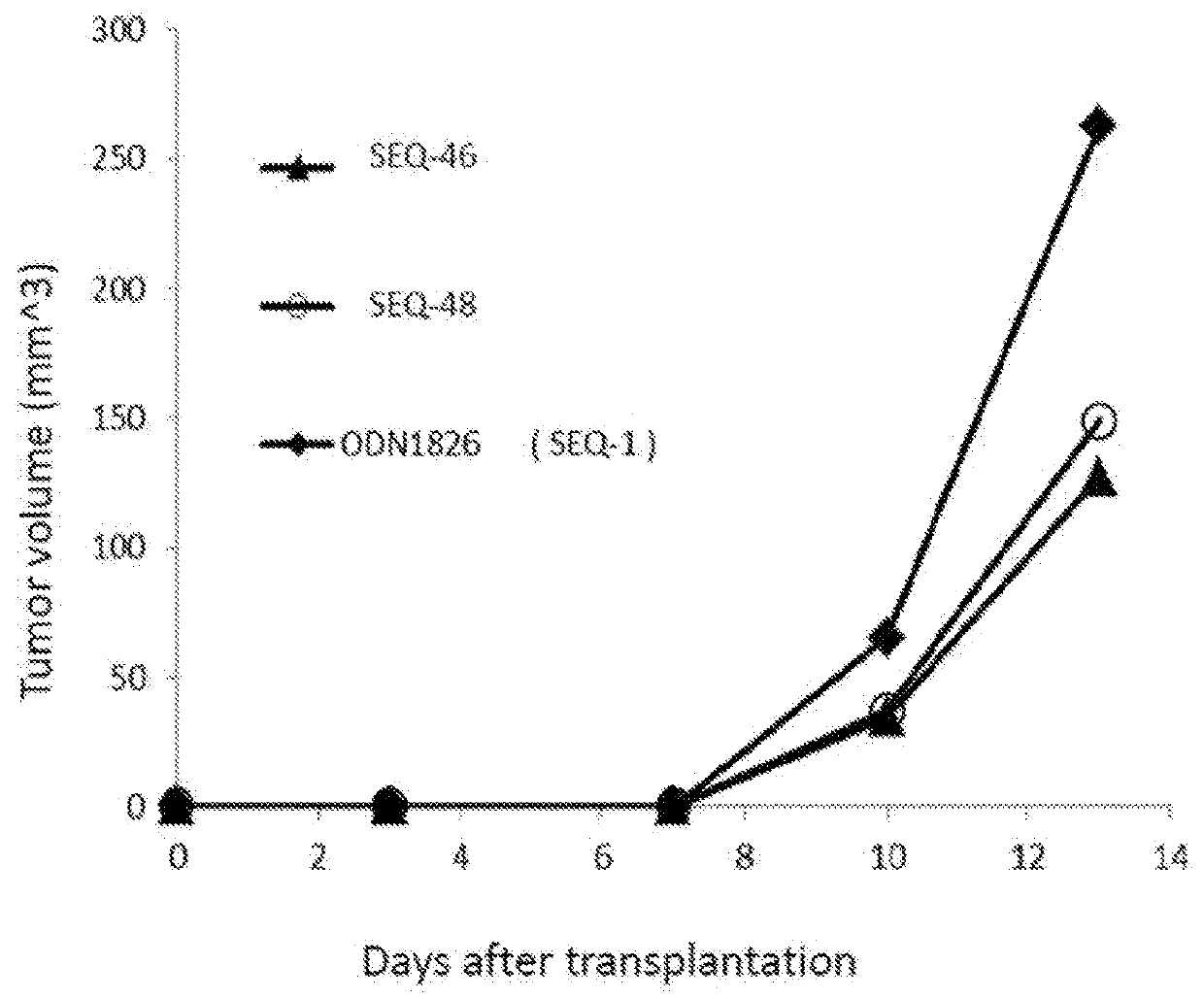
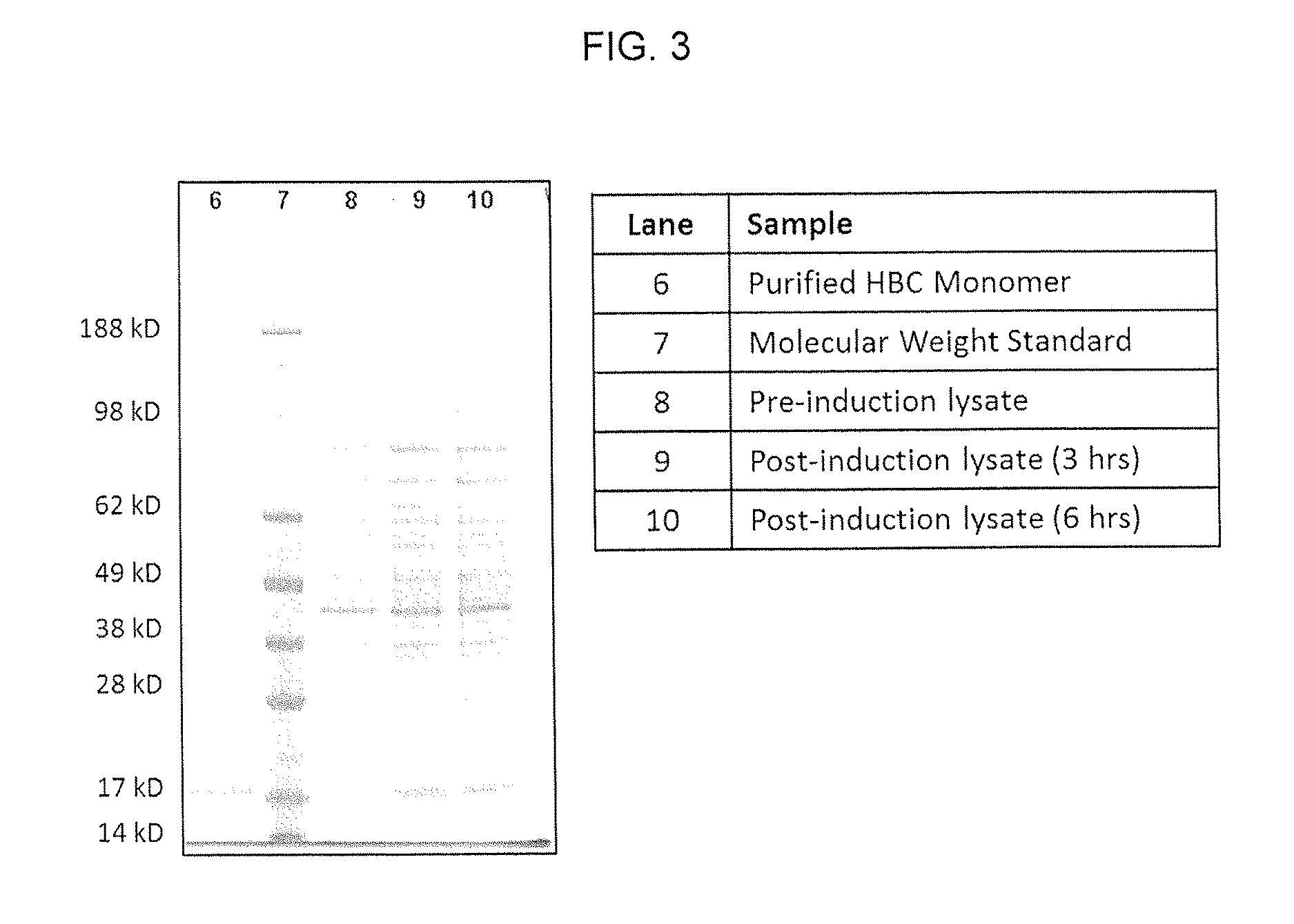
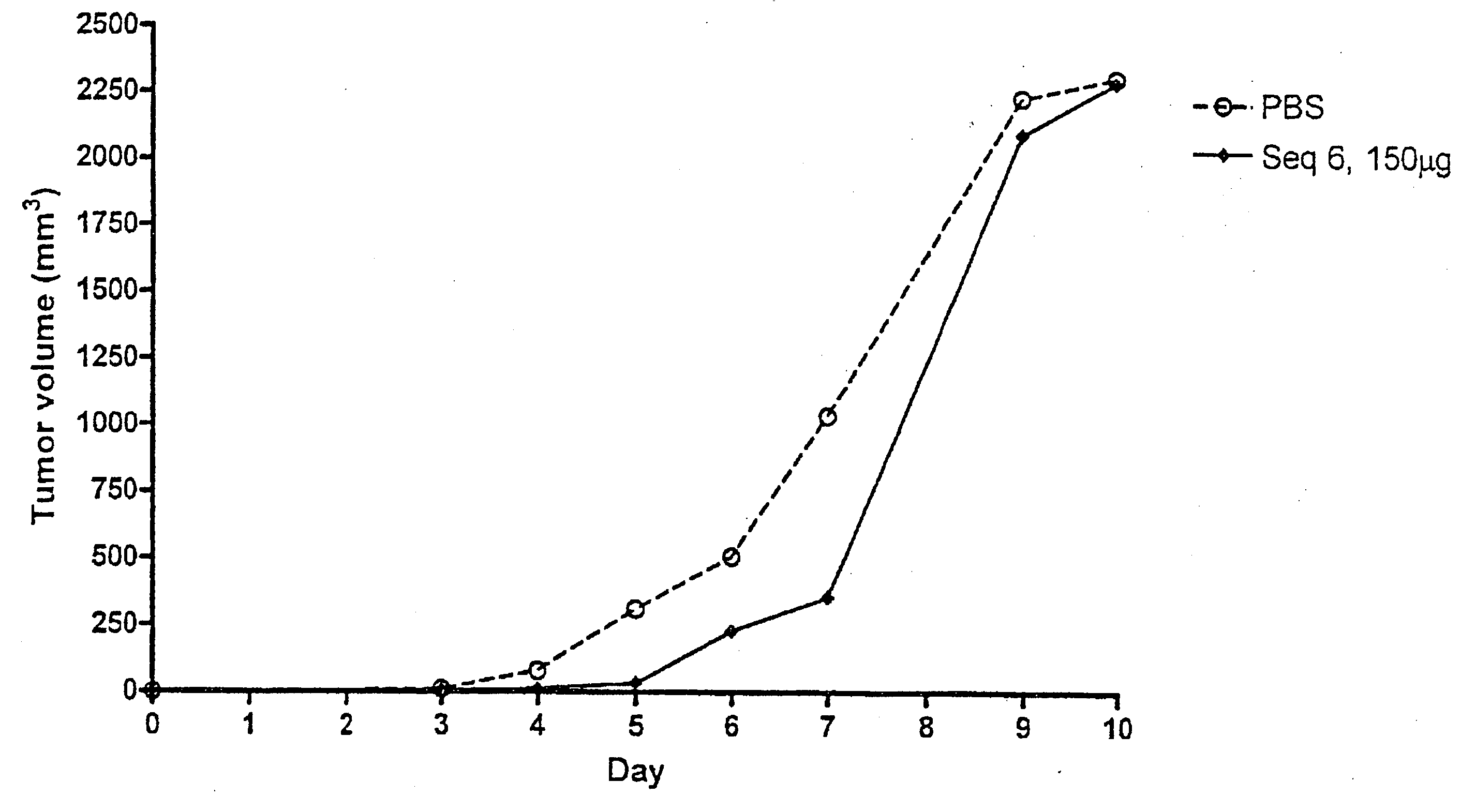
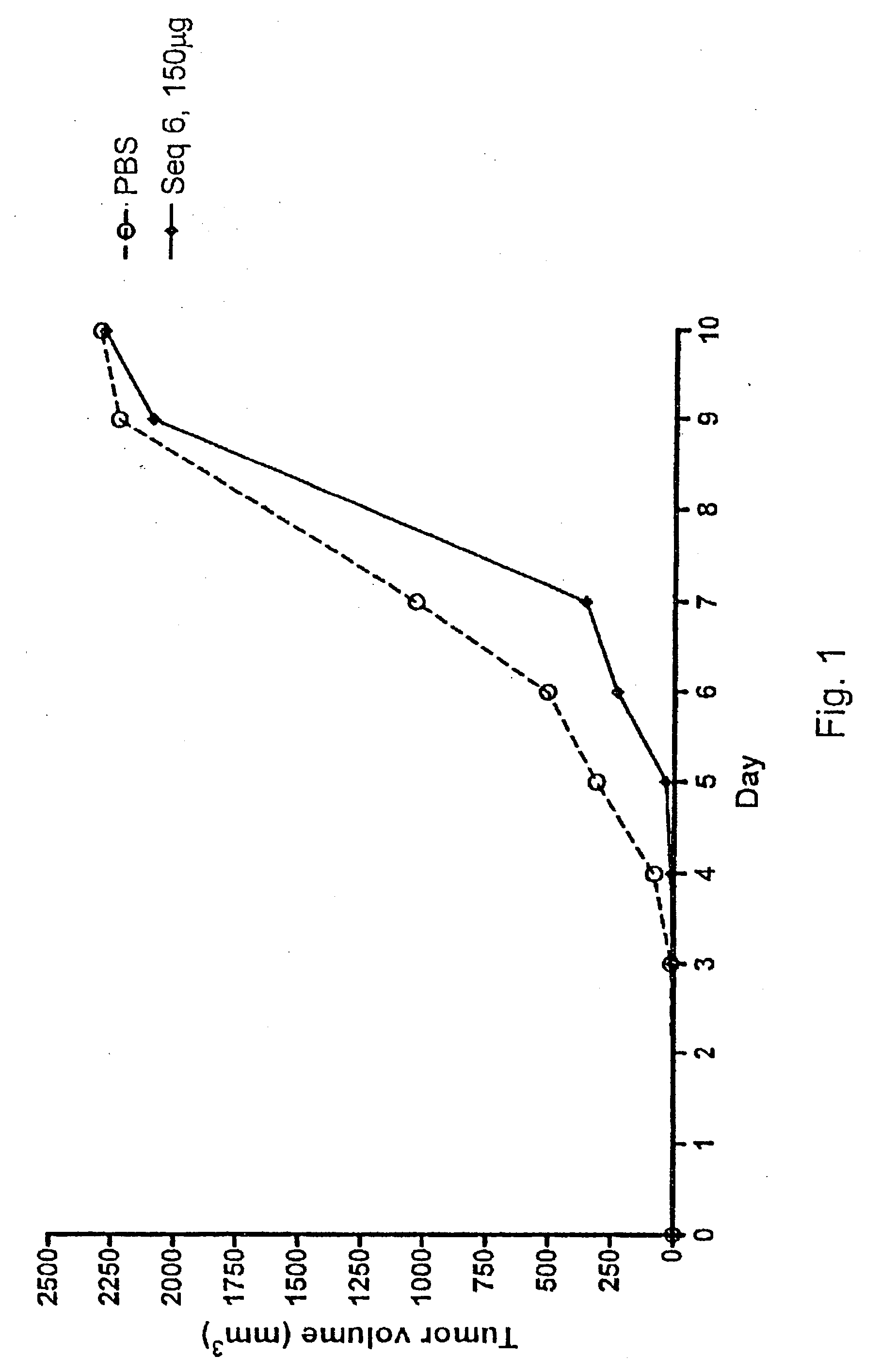
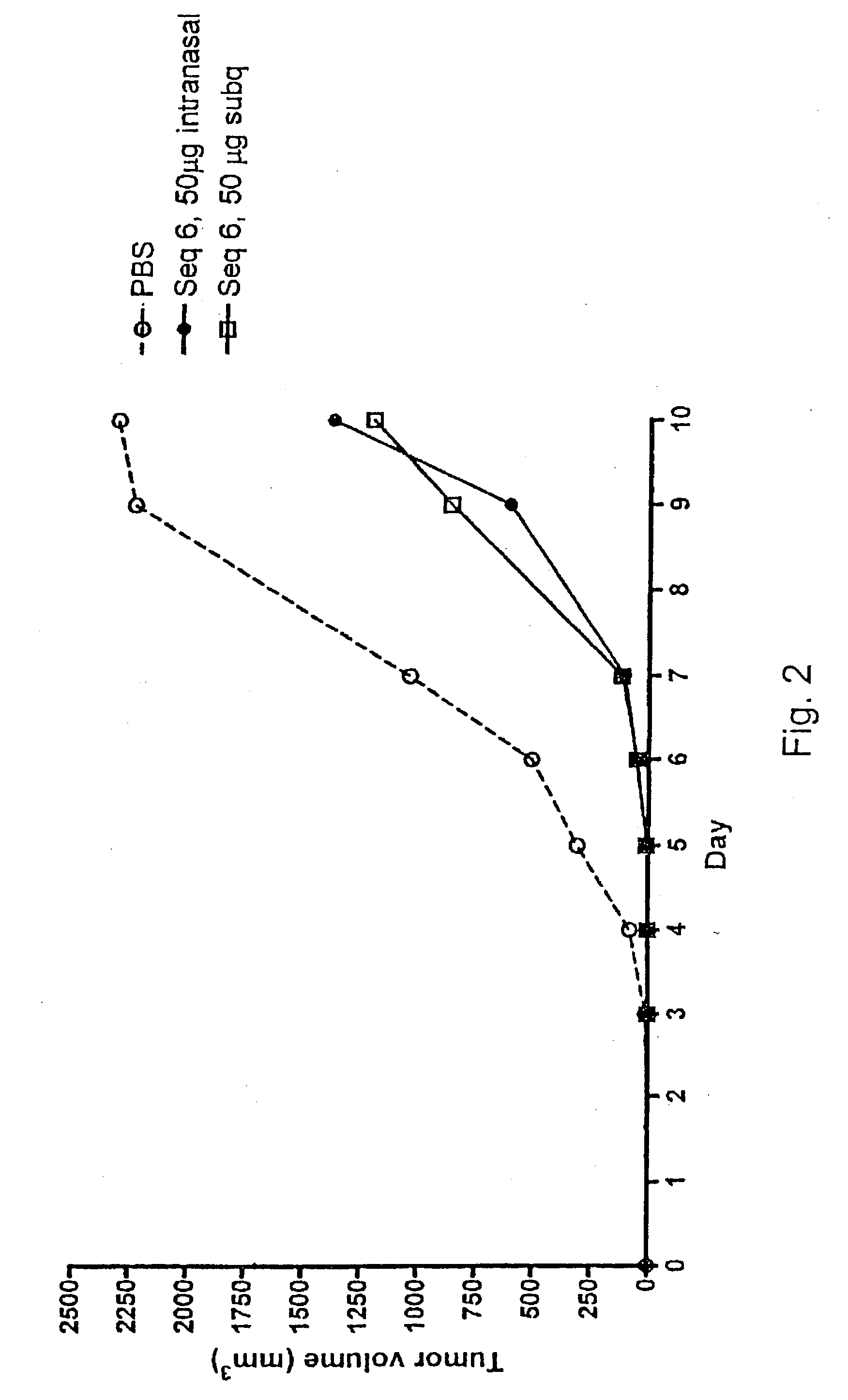
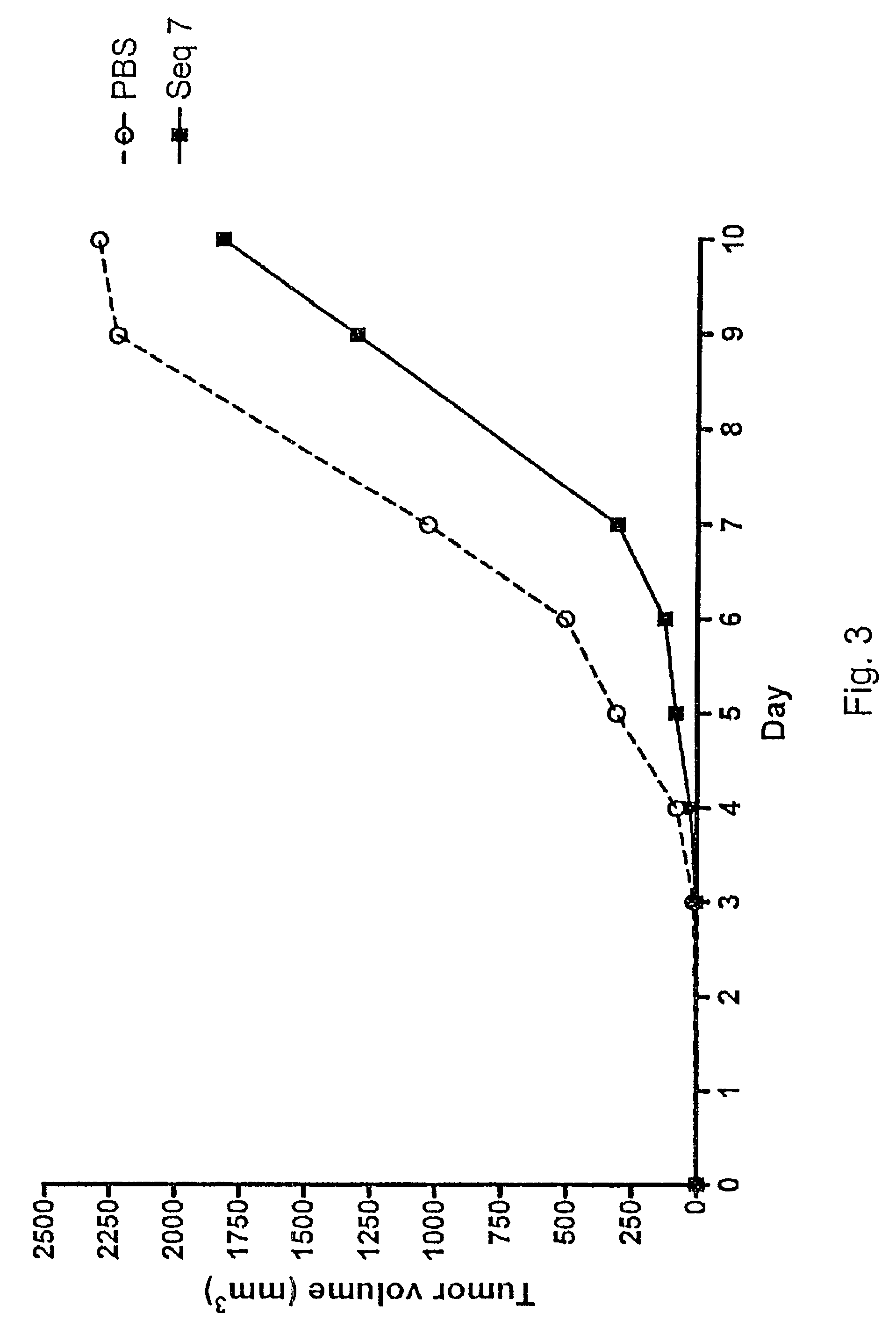
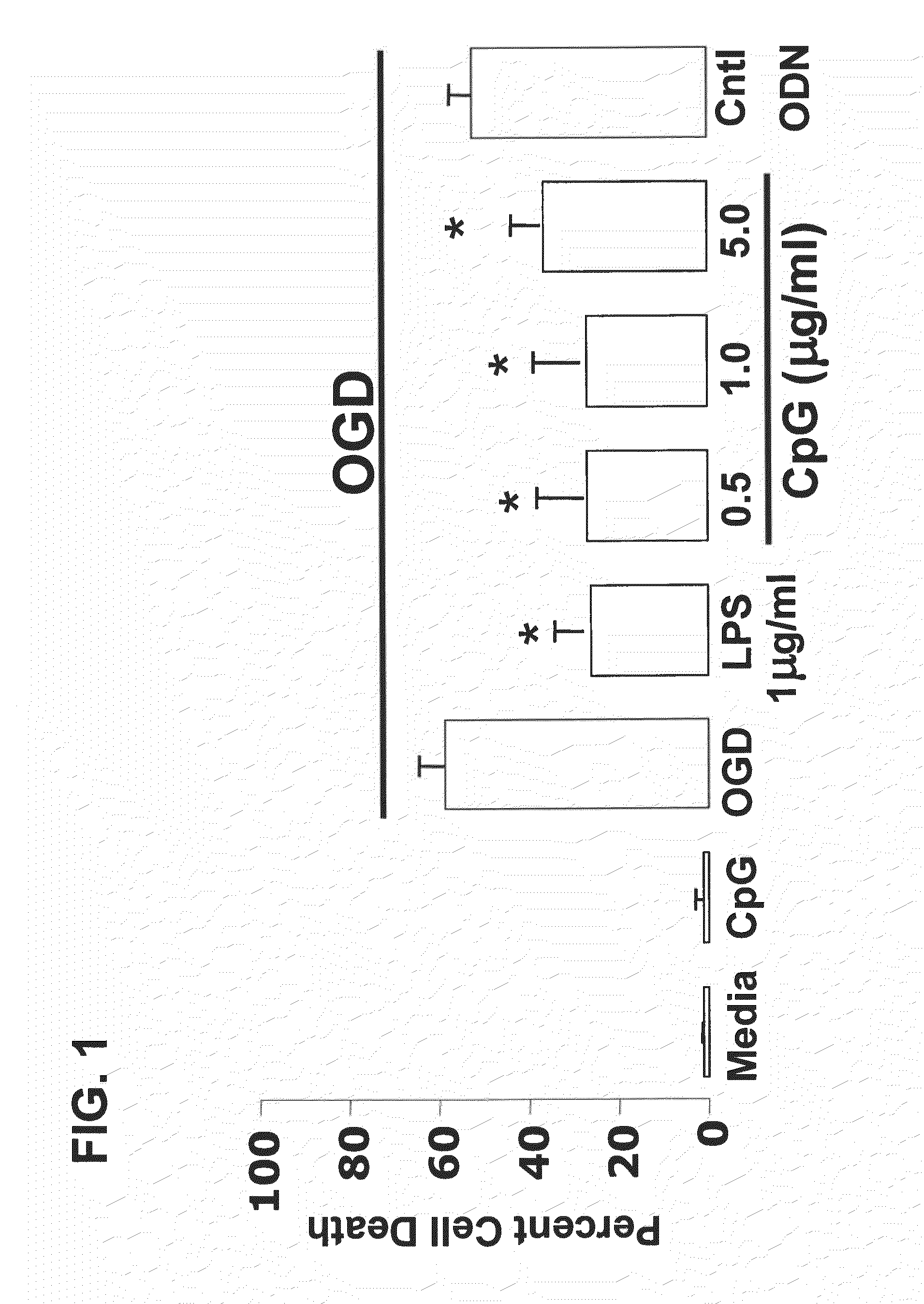
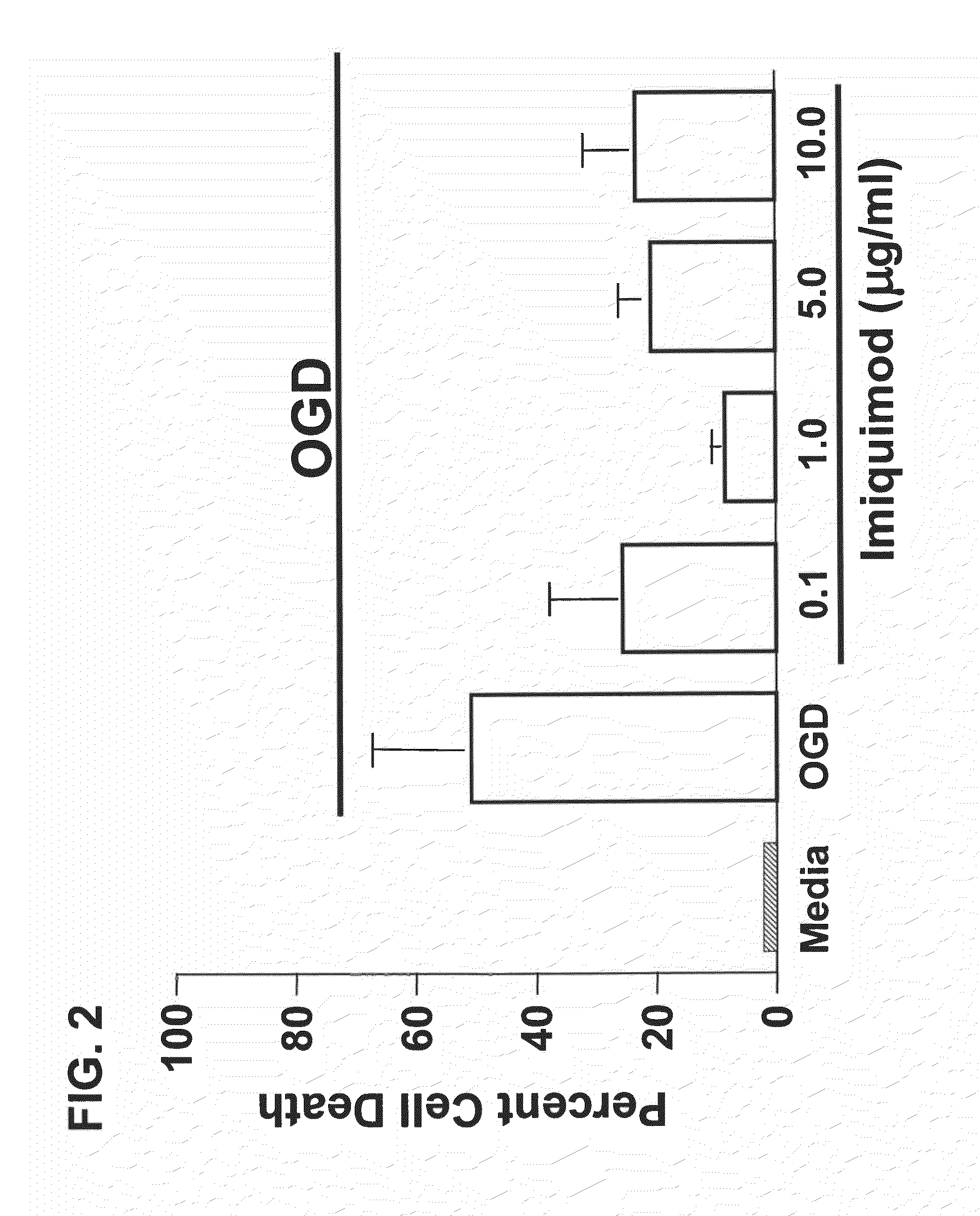
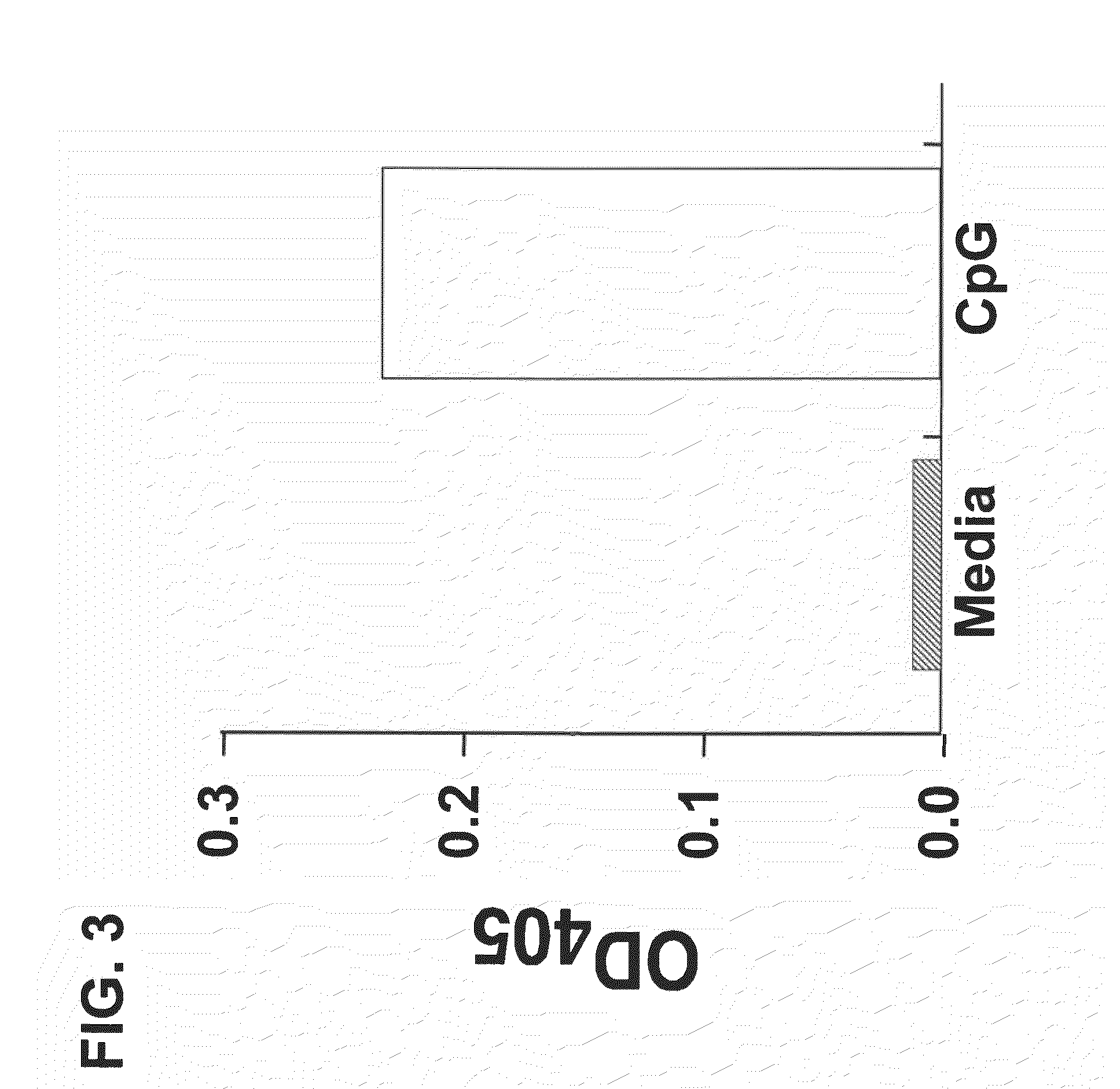
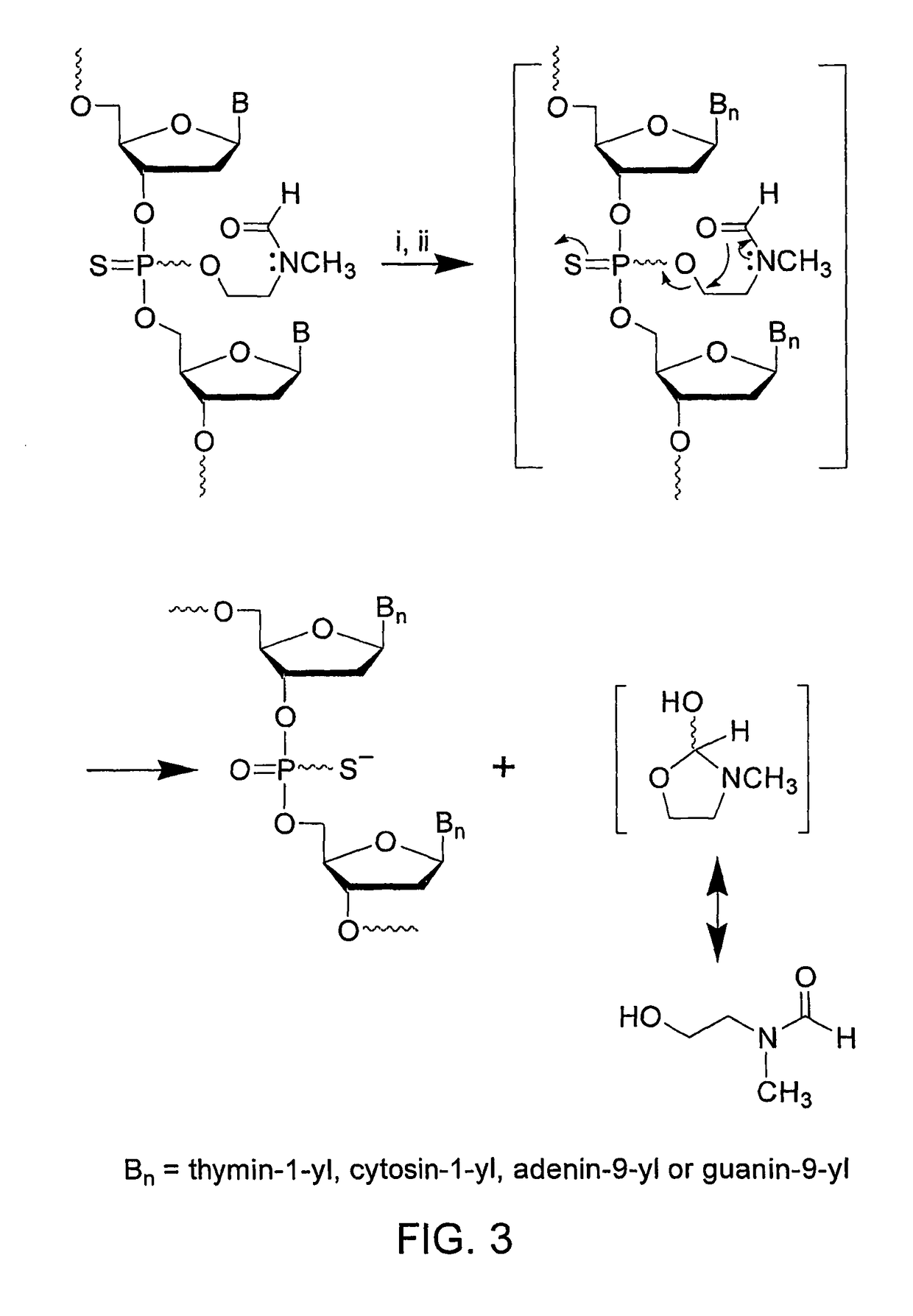
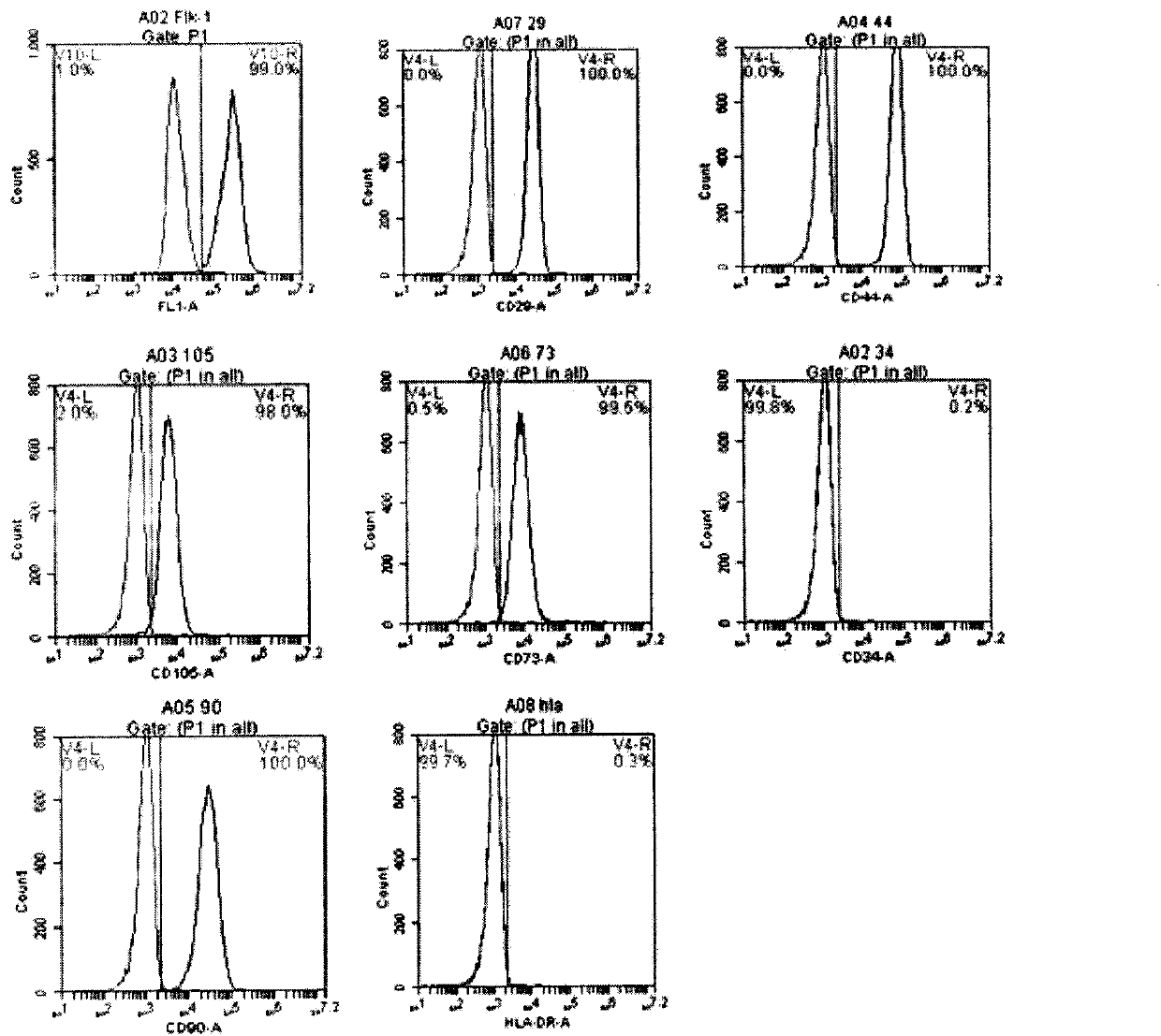
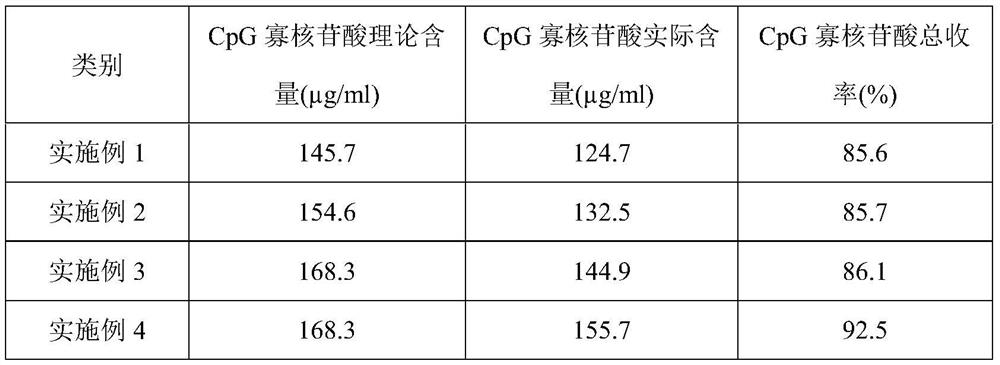
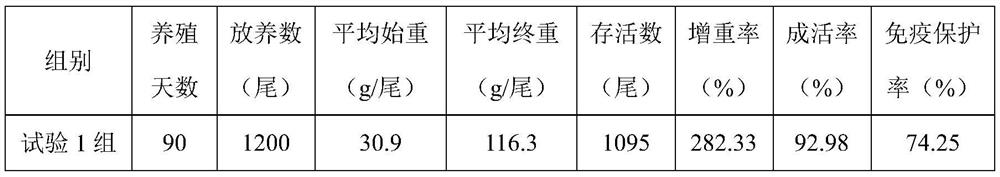
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Cpg oligonucleotides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CpG oligonucleotides (ODNs) are synthetic ODNs that contain unmethylated CpG dinucleotides in specific sequence contexts (CpG motifs). These CpG motifs are present at a 20-fold greater frequency in bacterial DNA than in mammalian DNA. CpG ODNs activate Toll-like receptor 9 (TLR9), leading to strong immunostimulatory effects.
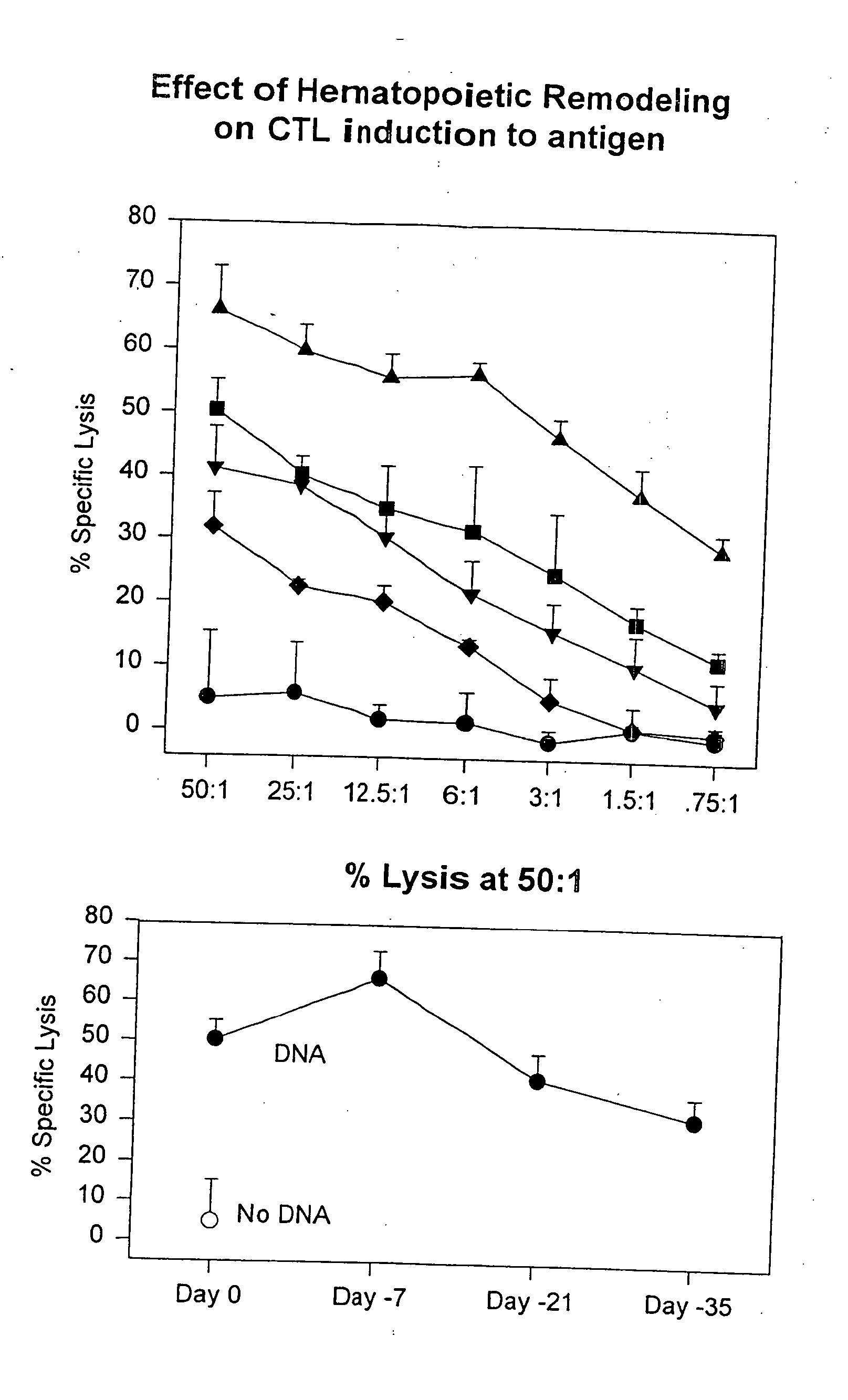
Methods for regulating hematopoiesis using CpG-oligonucleotides
InactiveUS20070184465A1Induce hematopoiesisInduces thrombocytopeniaSugar derivativesMicrobiological testing/measurementCpg oligonucleotidesPlatelet
The invention relates to methods for regulating hematopoiesis using CpG containing oligonucleotides. In particular, the invention relates to methods of treating thrombopoiesis and anemia by regulating hematopoiesis. The invention also relates to methods of regulating immune system remodeling by administering CpG oligonucleotides to control hematopoiesis.
Owner:COLEY PHARMA GMBH
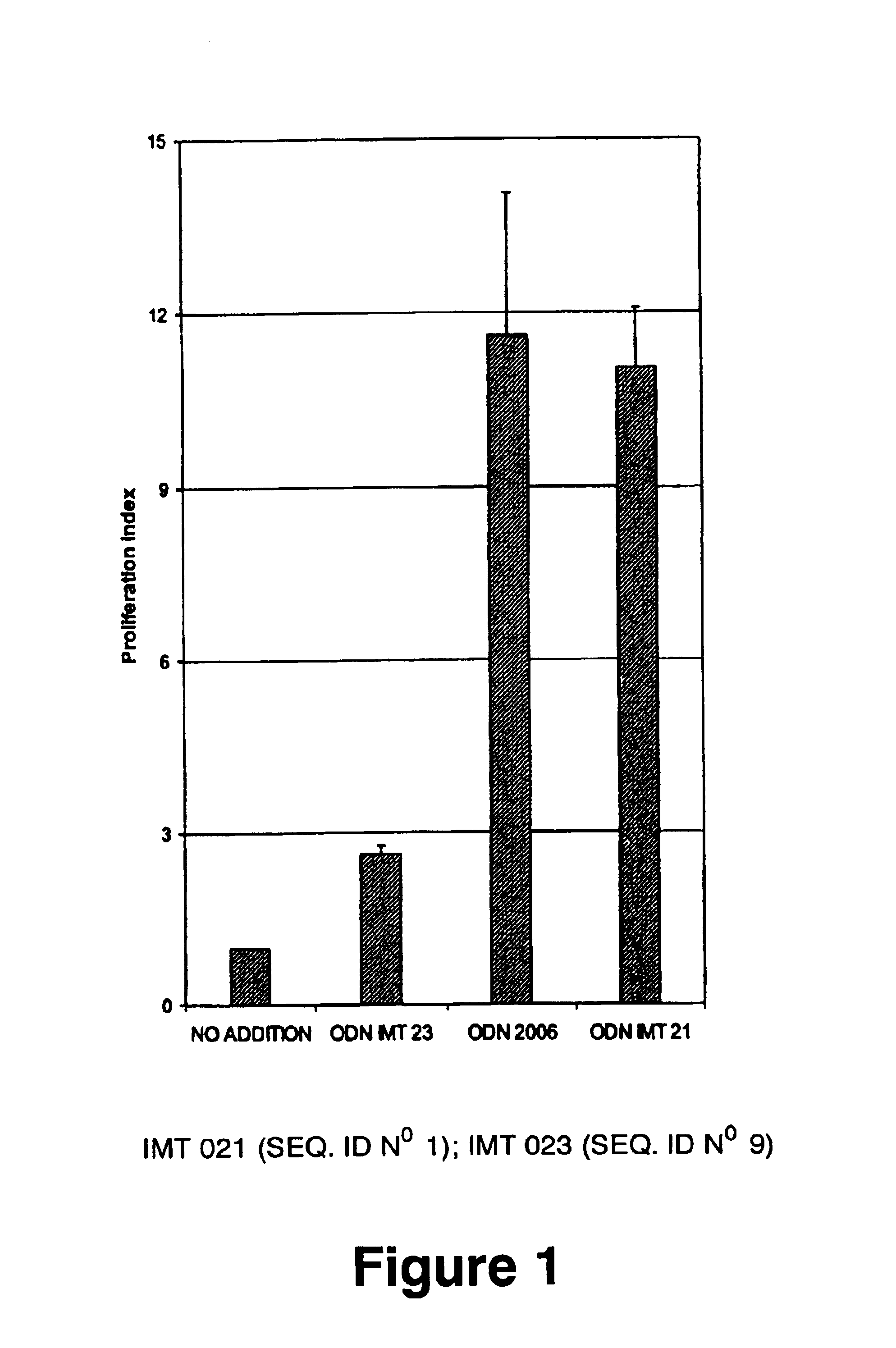
Immunostimulatory oligonucleotides and uses thereof
Oligonucleotides containing the non-palindromic sequence motif:X1X2X3X4X5X6X7X8,wherein X1 is C,T,G or A (preferably T or C); wherein X2 is C,T,G or A; wherein X7 is C,T,G or A (preferably G); at least three, and preferably all, of X3, X4, X5, X6 and X8 are T; and with the proviso that, in the motif, a C does not precede a G (in other terms, the nucleic acid motif does not consist of a CpG oligonucleotide), that modulate the immune response of animals of the order Primate, including humans, are disclosed. This immune modulation is characterized by stimulation of proliferation, differentiation, cytokine production and antibody production on B-cells and cell differentiation on plasmacytoid dendritic cells.
Owner:DAVID HORN LLC
Therapeutic use of cpg oligodeoxynucleotide for skin disease
InactiveUS20090062224A1Good conditionReduce expressionOrganic active ingredientsSugar derivativesDiseaseCpg oligonucleotides
Disclosed is the therapeutic use of CpG oligodeoxynucleotides for skin diseases. The CpG oligodeoxynucleotides (CpG ODNs) of the present invention show excellent immunoactive effects against skin diseases in both cases of CpG ODNs with a phosphorothioate backbone and CpG ODNs with a phosphodiester backbone.
Owner:BIO CLUE & SOLUTION +2
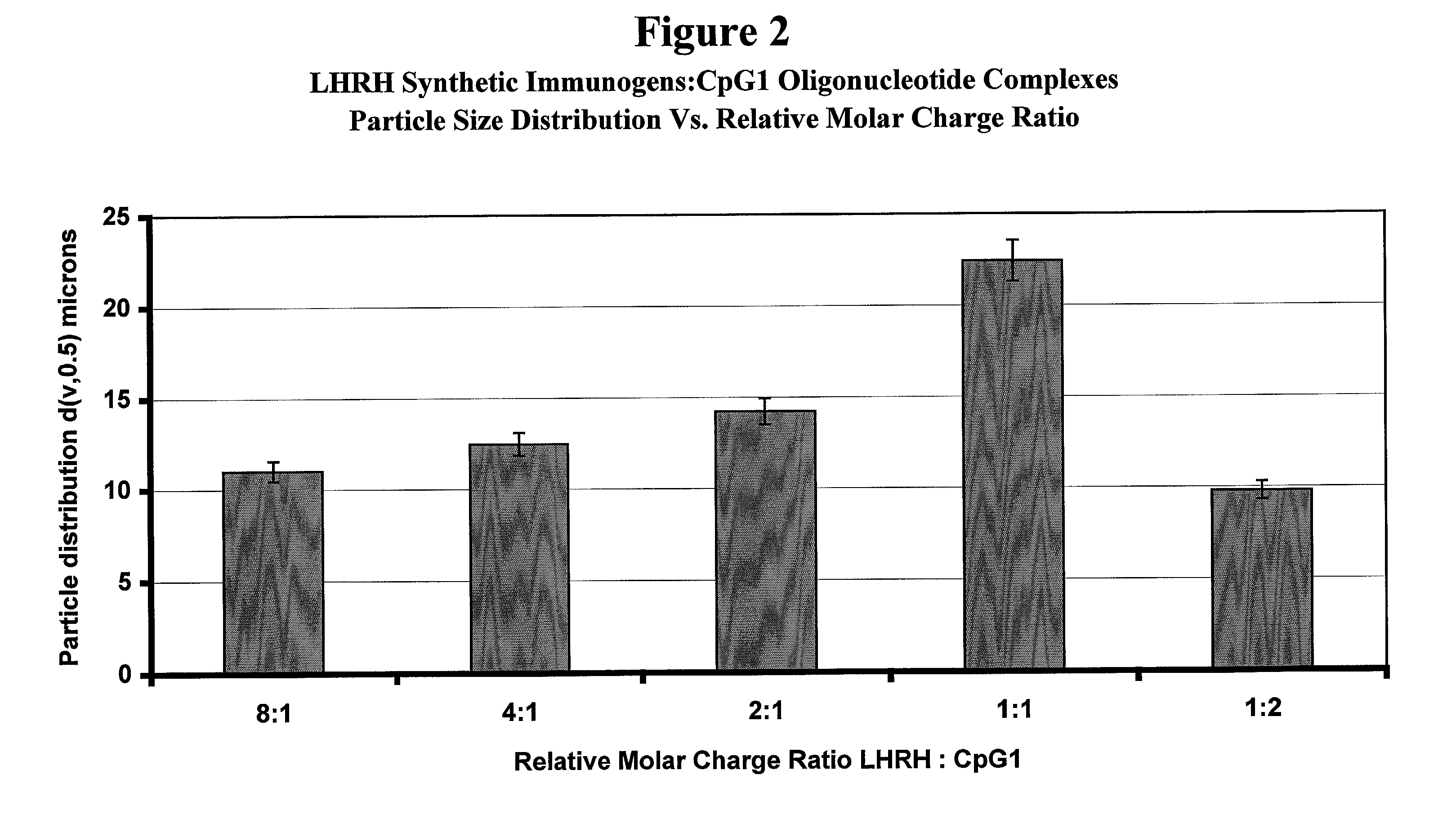
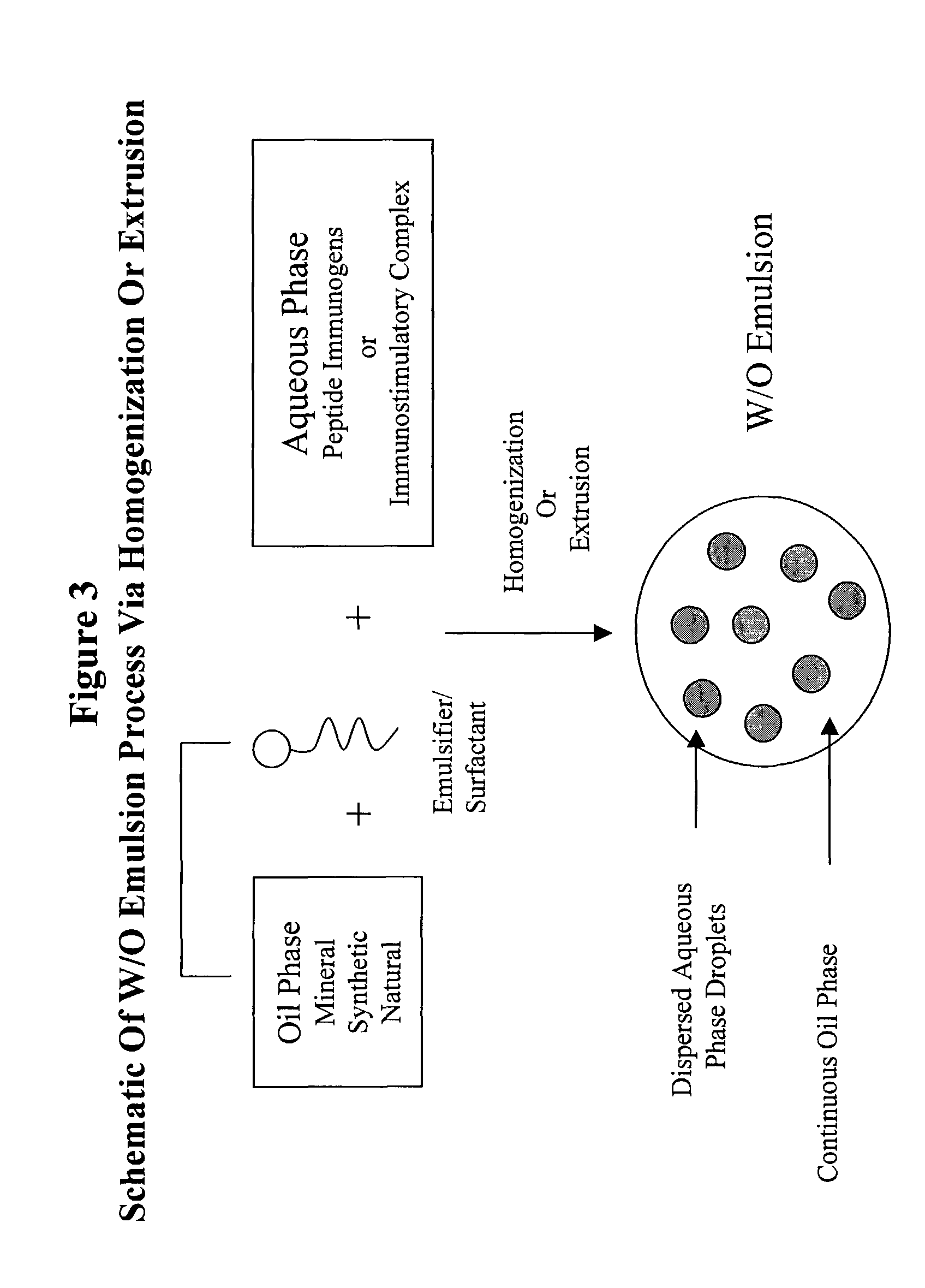
Stabilized synthetic immunogen delivery system
ActiveUS8088388B2Improve propertiesEffectively upregulating immune responseImpression capsPeptide/protein ingredientsSynthetic ImmunogensParticulates
Owner:UNITED BIOMEDICAL INC
CpG OLIGONUCLEOTIDE PRODRUGS, COMPOSITIONS THEREOF AND ASSOCIATED THERAPEUTIC METHODS
ActiveUS20090263405A1Improve the immunityOrganic active ingredientsSugar derivativesNucleotideCpg oligonucleotides
The present invention provides a CpG oligonucleotide prodrug that includes a thermolabile substituent on at least one nucleotide thereof. The present invention also provides compositions that include a carrier and a therapeutically effective amount of at least one CpG oligonucleotide prodrug. The present invention further provides therapeutic methods of using such thermolabile CpG oligonucleotide prodrugs and compositions thereof. The present invention further provides a method of inhibiting tetrad formation in a CpG oligonucleotide by functionalizing the CpG oligonucleotide with one or more thermolabile substituents.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Combination tumor immunotherapy
ActiveUS10682365B2Easy to operateInhibited anti-tumor immune responsePeptide/protein ingredientsImmunoglobulins against virusesAntiendomysial antibodiesWhite blood cell
Provided are methods for treating cancer using local administration of certain CpG oligonucleotides (CpG ODN) and systemic administration of a checkpoint inhibitor such as an anti-PD-1 antibody, an anti-PD-L1 antibody, and / or an anti-CTLA-4 antibody. In preferred embodiments, the CpG ODN are selected based on their propensity to induce high amounts of interferon alpha (IFN-α) and T-cell activation relative to interleukin-10 (IL-10) and B-cell activation. In certain embodiments, the methods further include pretreatment with radiotherapy, to potentiate the combination immunotherapy.
Owner:CHECKMATE PHARM INC
Nucleic acid derivative having immunostimulatory activity
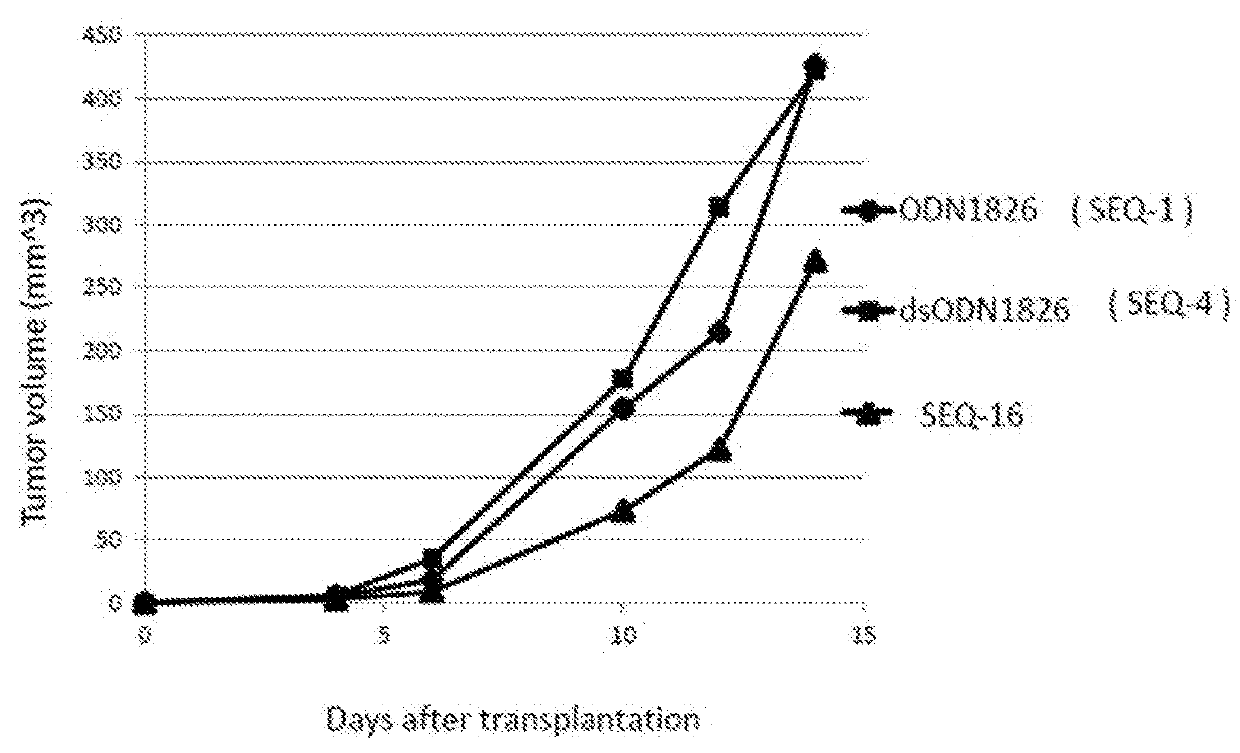
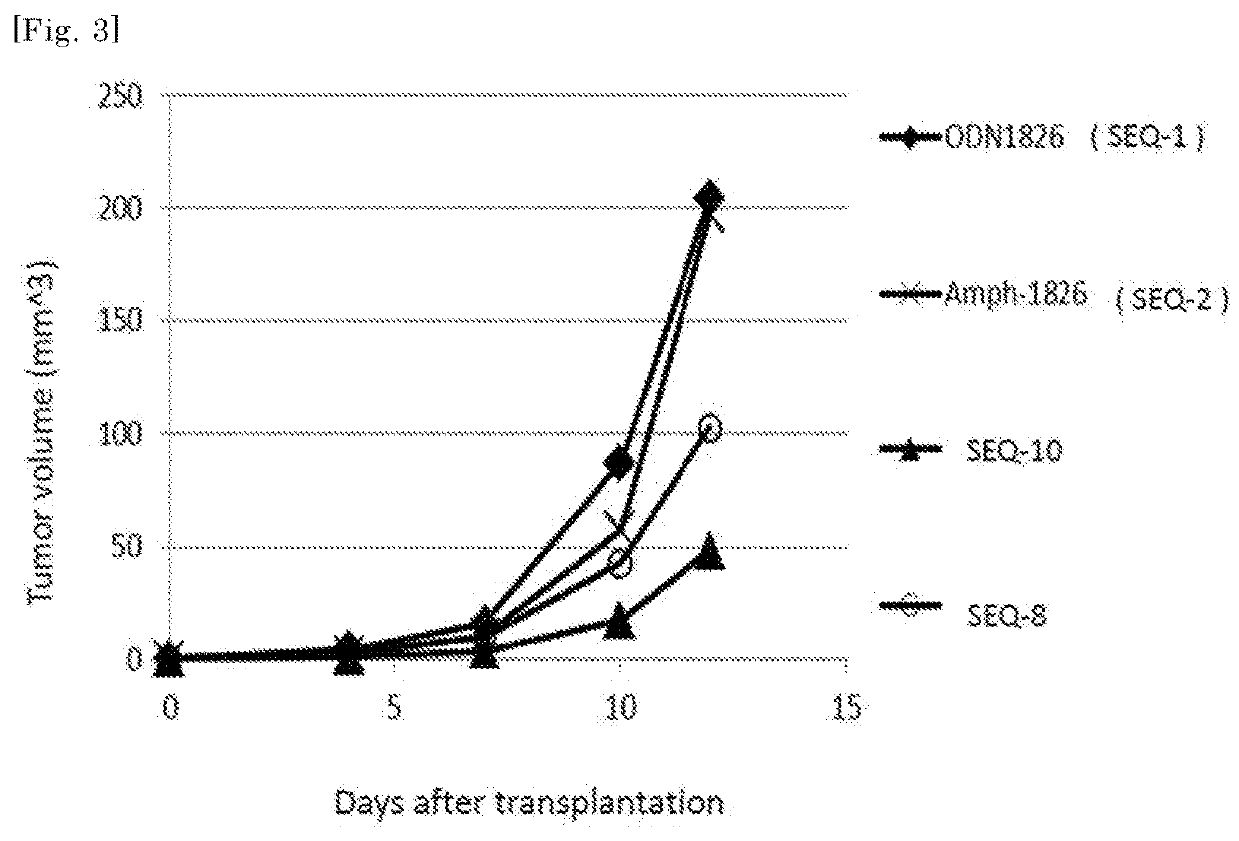
ActiveUS20180264105A1High activityAntibacterial agentsCancer antigen ingredientsAdjuvantCpg oligonucleotides
The purpose of the present invention is to provide double-stranded oligonucleotides comprising the CpG oligonucleotide mentioned below, as a nucleic acid derivative having an immunostimulatory activity.An adjuvant comprising a double-stranded oligonucleotide, whereina first strand is a CpG oligonucleotide consisting of 8 to 50 nucleotides,a second strand is an oligonucleotide consisting of 8 to 60 nucleotides and comprisinga sequence capable of hybridizing with the first strand, and a lipid binds to the second strand through a linker.
Owner:SHIONOGI & CO LTD
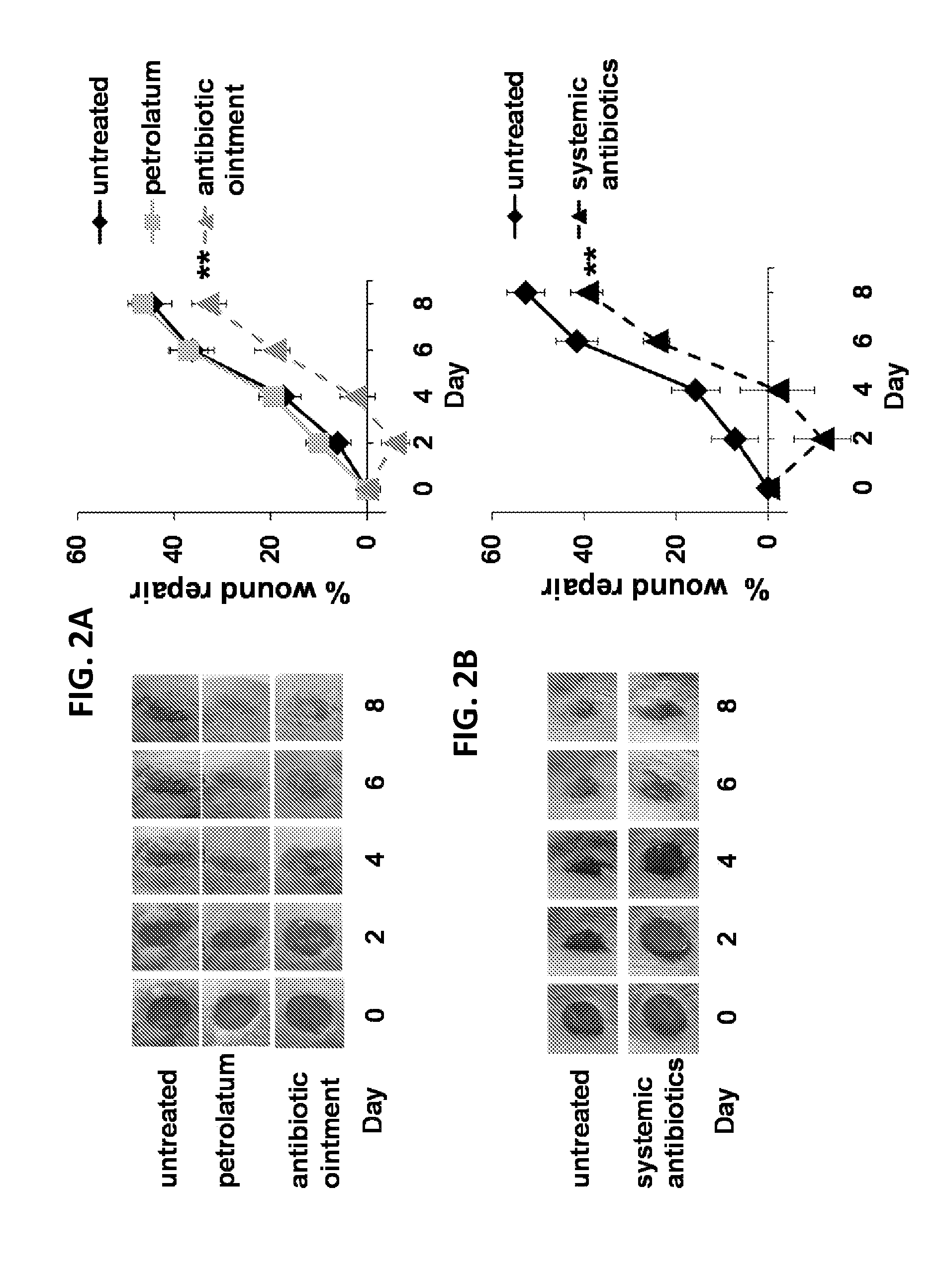
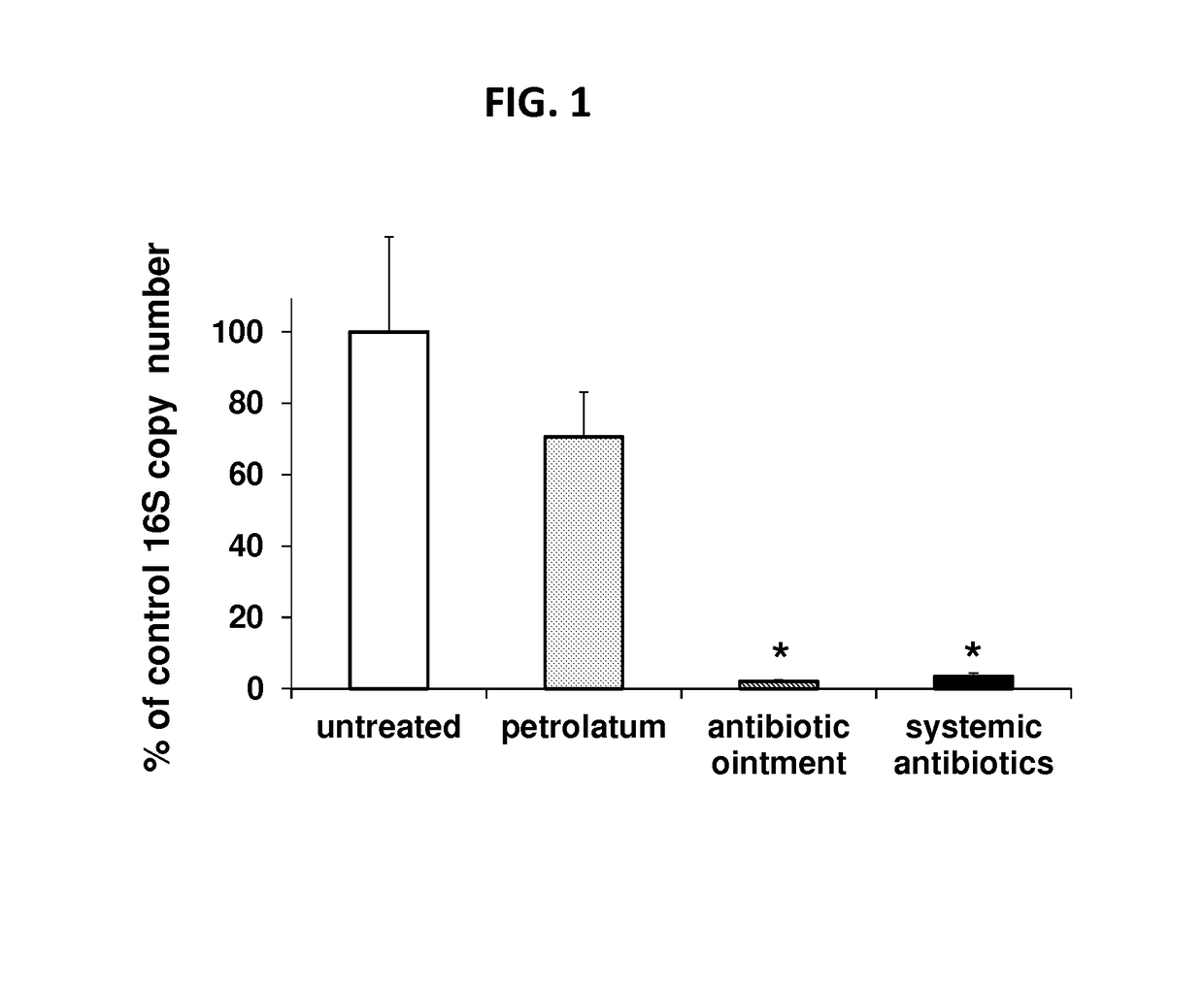
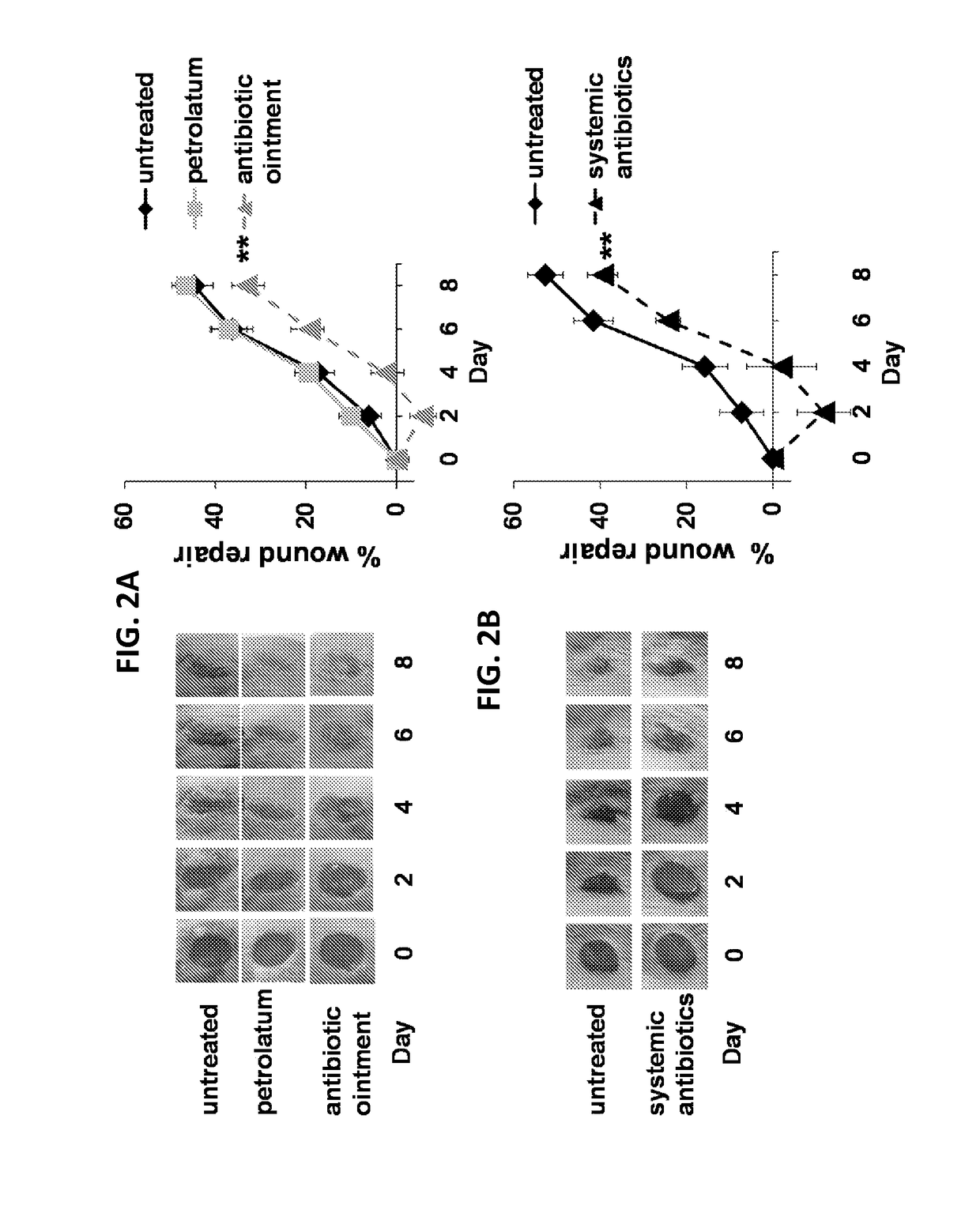
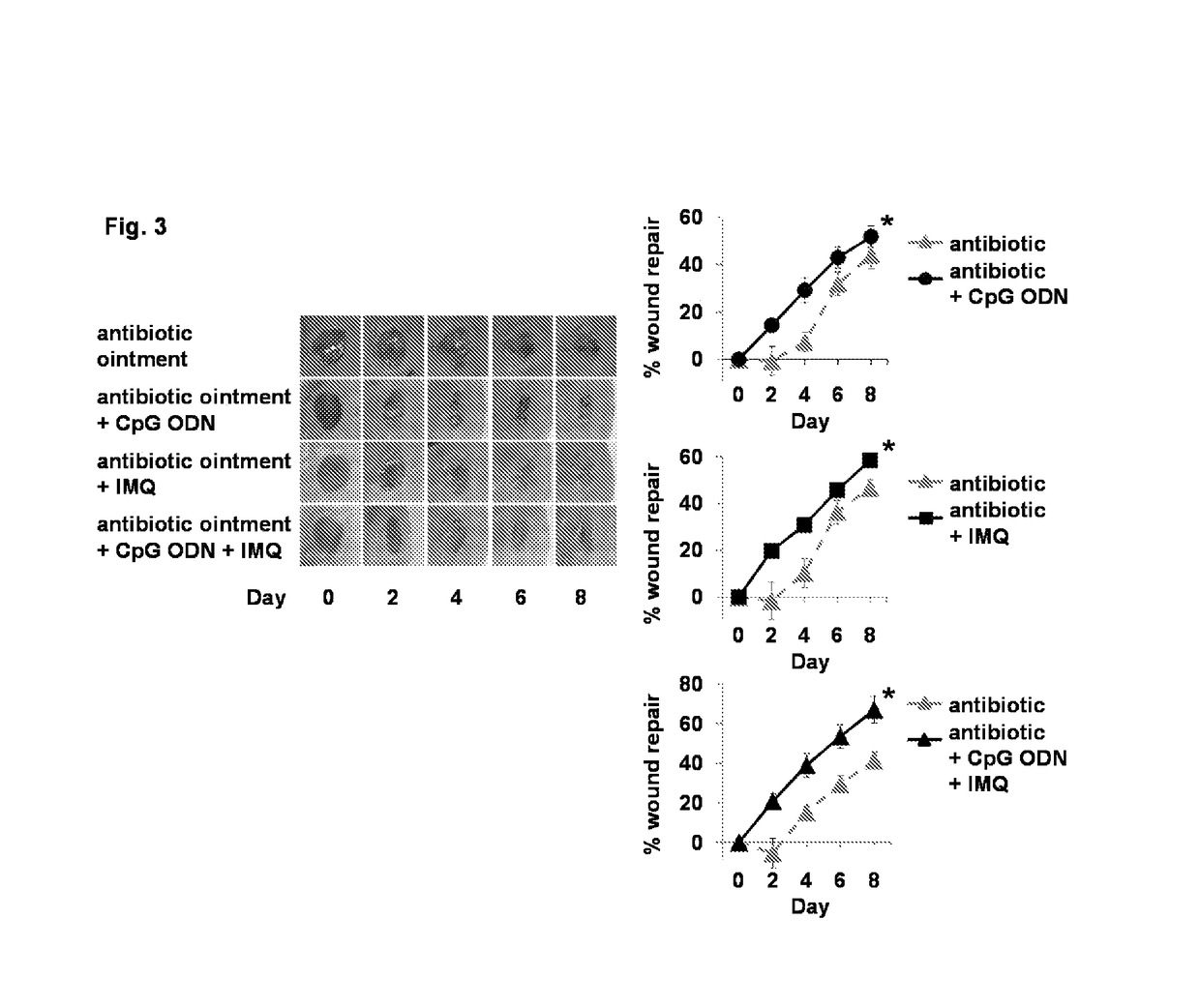
Use of cpg oligonucleotides co-formulated with an antibiotic to accelerate wound healing
ActiveUS20150104482A1Delay wound healingDelayed resistanceOrganic active ingredientsOintment deliveryReceptorNucleotide
Pharmaceutical compositions are provided that include an antibiotics, but that include ingredients that counteract the effect of that antibiotic on wound healing, without altering the bactericidal properties of the antibiotic. These pharmaceutical compositions include an effective amount of 1) an imidazoquinoline having toll-like receptor 7 (TLR7) ligand activity, 2) an immunostimulatory K-type CpG oligodeoxynucleotide (ODN) comprising an unmethylated CpG motif, 3) an antibiotic, and 4) a surfactant, wherein the composition is formulated for topical administration. Methods for accelerating wound healing are also provided. These methods include topically administering the disclosed compositions. The wound can be in the skin or in the eye.
Owner:UNITED STATES OF AMERICA
SPECIFIC VIRUS-LIKE PARTICLE-CpG OLIGONUCLEOTIDE VACCINES AND USES THEREOF
InactiveUS20170035864A1Viral antigen ingredientsCancer antigen ingredientsMedicineCpg oligonucleotides
The invention provides vaccines containing, as its only active ingredient, a VLP having a CpG oligonucleotide attached thereto and a non-toxic pharmaceutically acceptable carrier or diluent and uses thereof. The invention further provides a pharmaceutical composition comprising a vaccine consisting of a VLP having a CpG oligonucleotide, one or more non-toxic pharmaceutically acceptable carrier or diluent, and a therapeutic agent admixture therewith and uses thereof.
Owner:BULLET BIOTECH
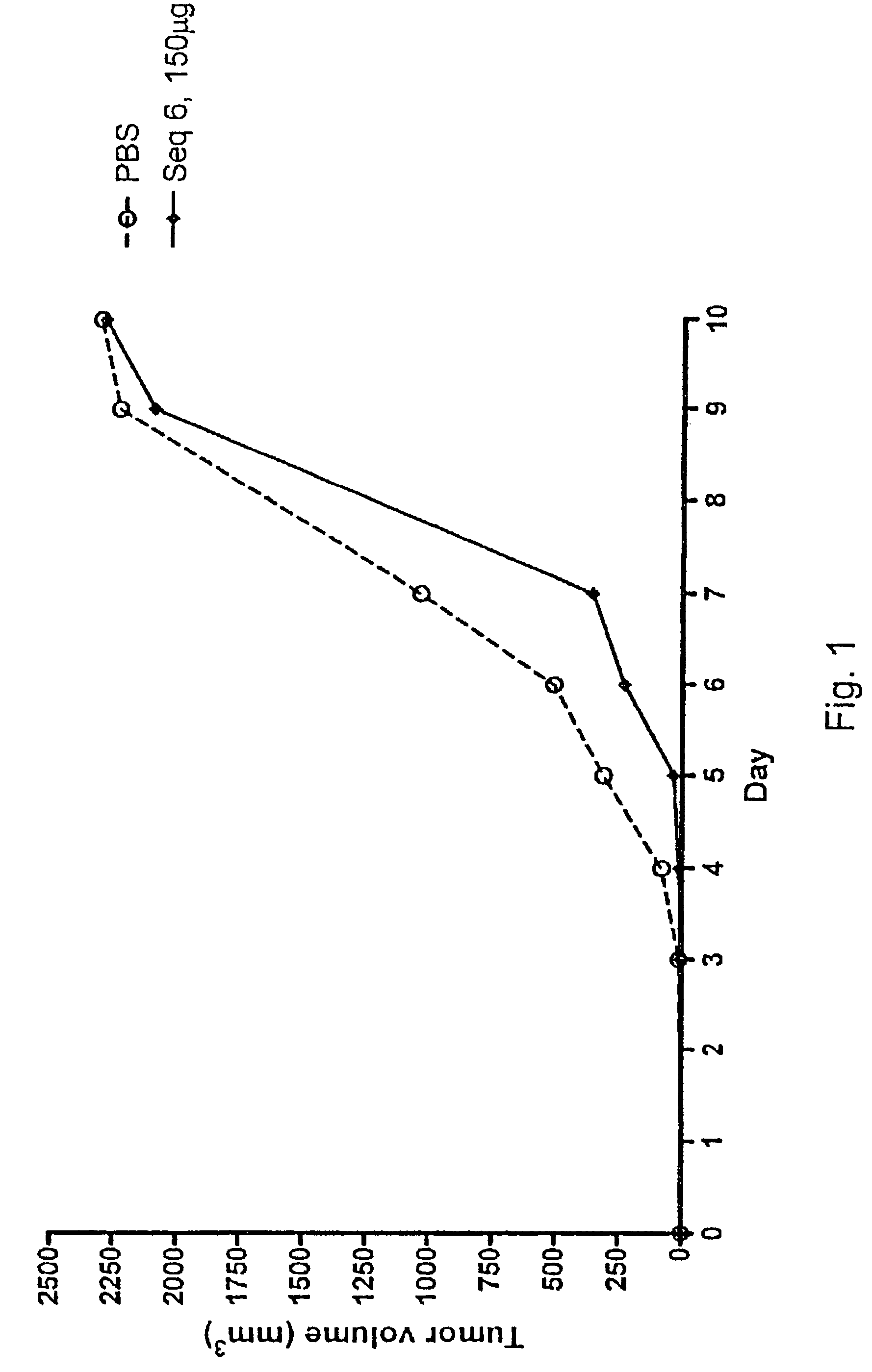
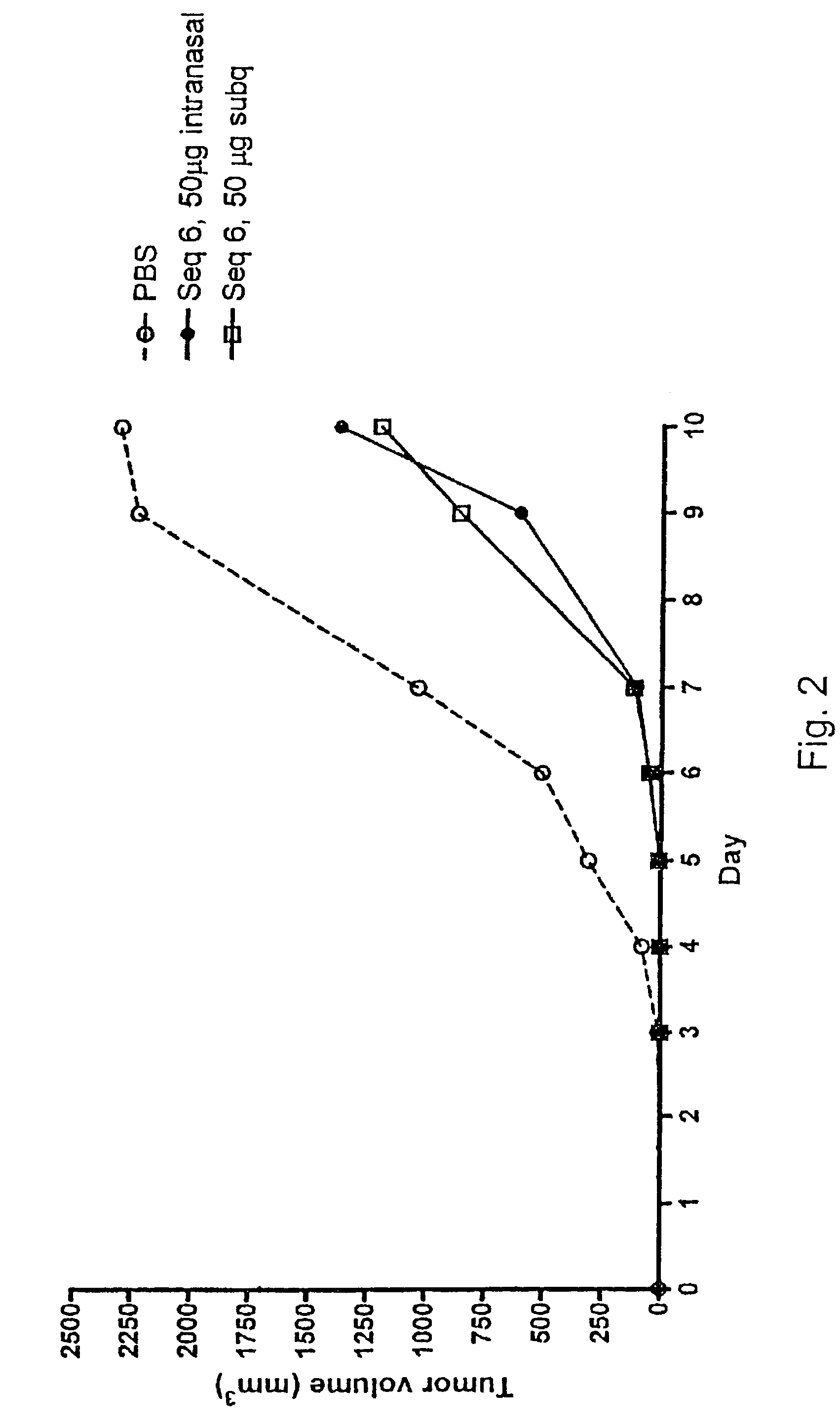
Tumour Growth Inhibitory Compounds and Methods of their Use
ActiveUS20100196356A1Various formsOrganic active ingredientsSugar derivativesHealthy subjectsHormone
Specific CpG oligonucleotide sequences, when given subcutaneously and in particular when administered on a mucous membrane, e.g. intranasally, intravaginally, or rectally, have a profound effect on various human cancer forms as confirmed in vivo, in animal studies, and in vitro, in human PBMCs collected from blood from healthy subjects and from patients suffering from CLL or FL. The compounds are also preferably used in combination with a cancer therapy chosen among radiation treatment, hormone treatment, surgical intervention, chemotherapy, immunological therapies, photodynamic therapy, laser therapy, hyperthermia, cryotherapy, angiogenesis inhibition, or a combination of any of these, and is most preferably an immunological treatment and comprises the administration of an antibody to the patient.
Owner:INDEX PHARMA
Stabilized synthetic immunogen delivery system
ActiveUS8084015B2Effectively upregulating immune responseEasy to demonstrateAntimycoticsImpression capsSynthetic ImmunogensParticulates
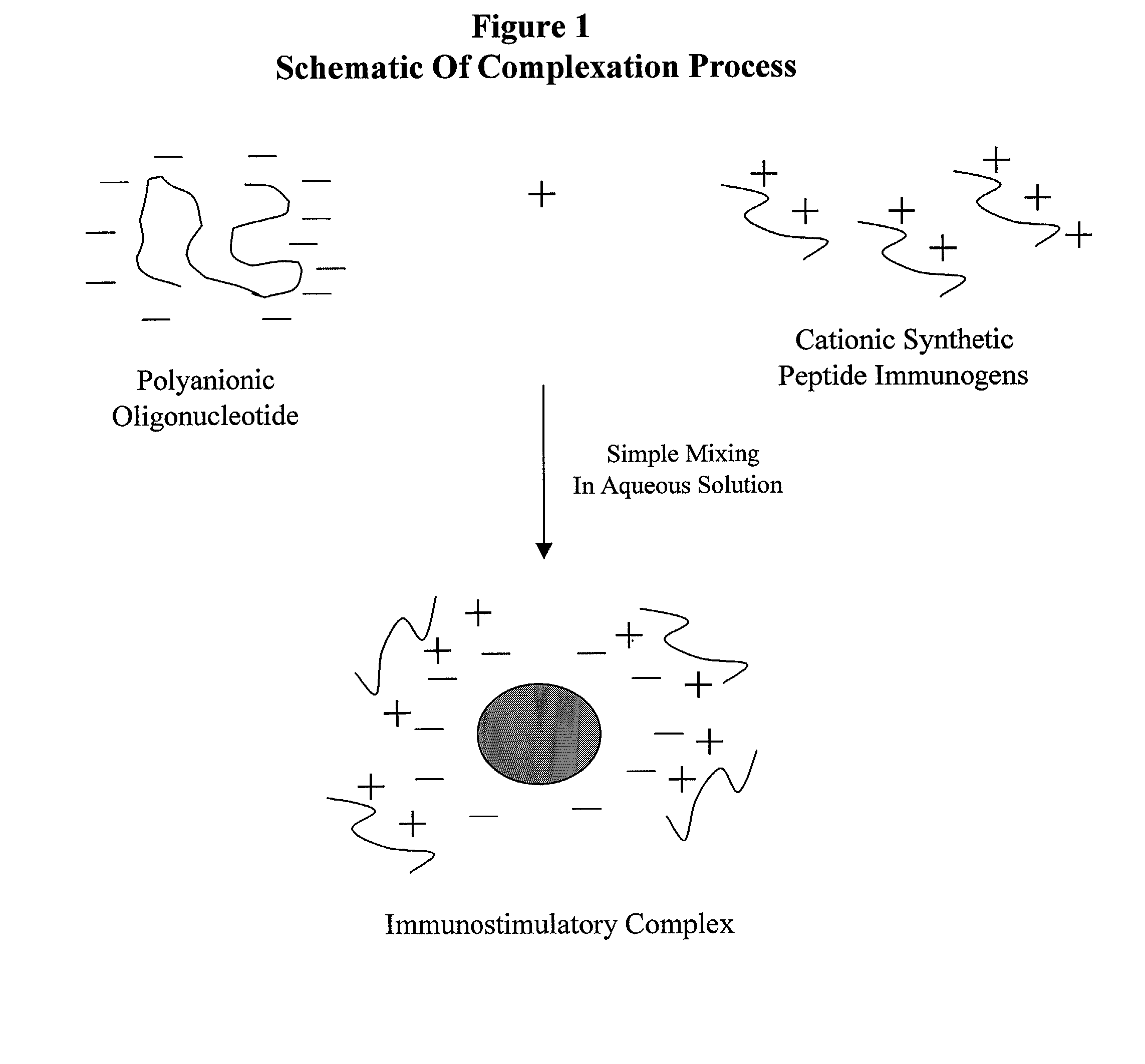
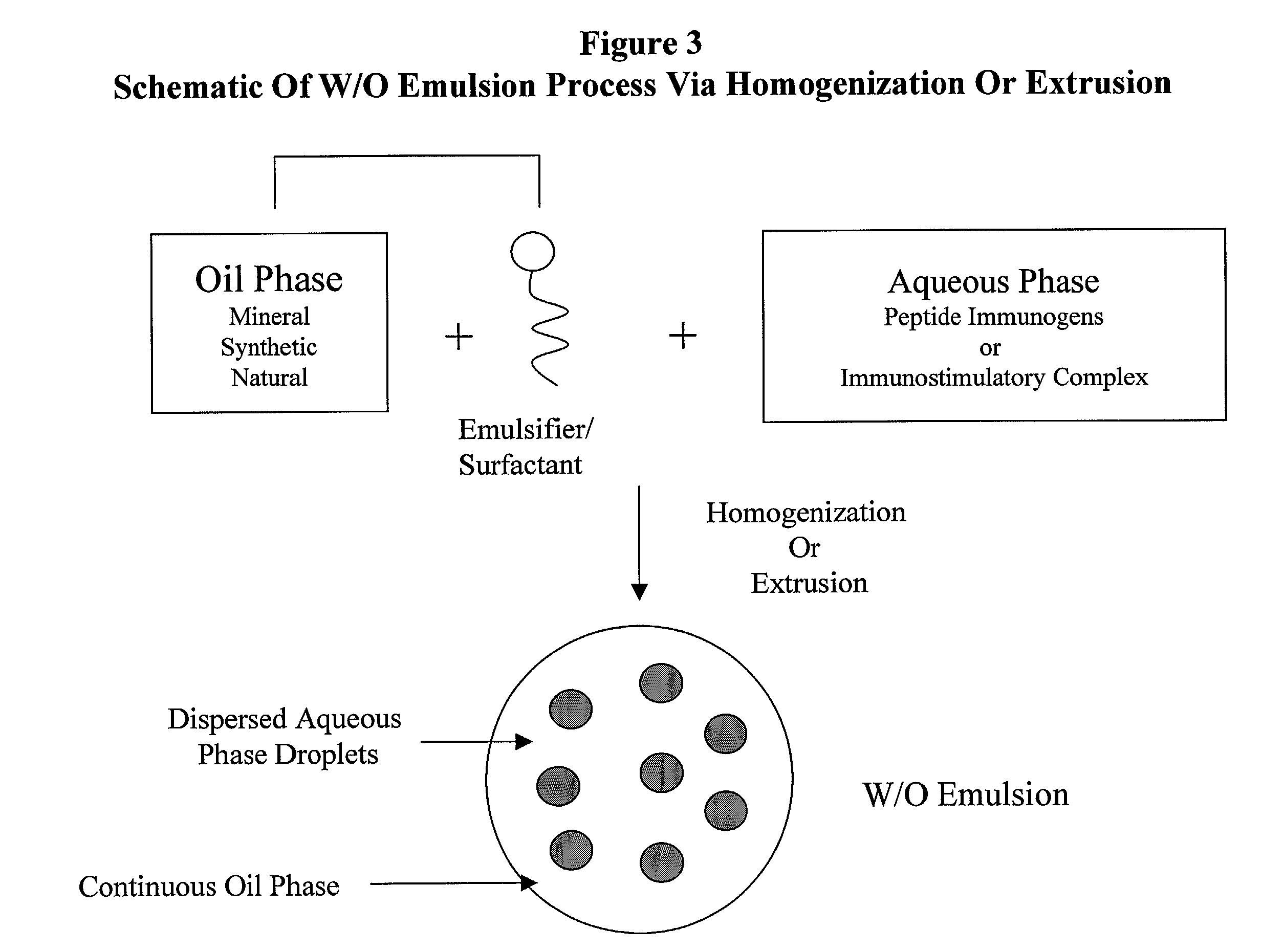
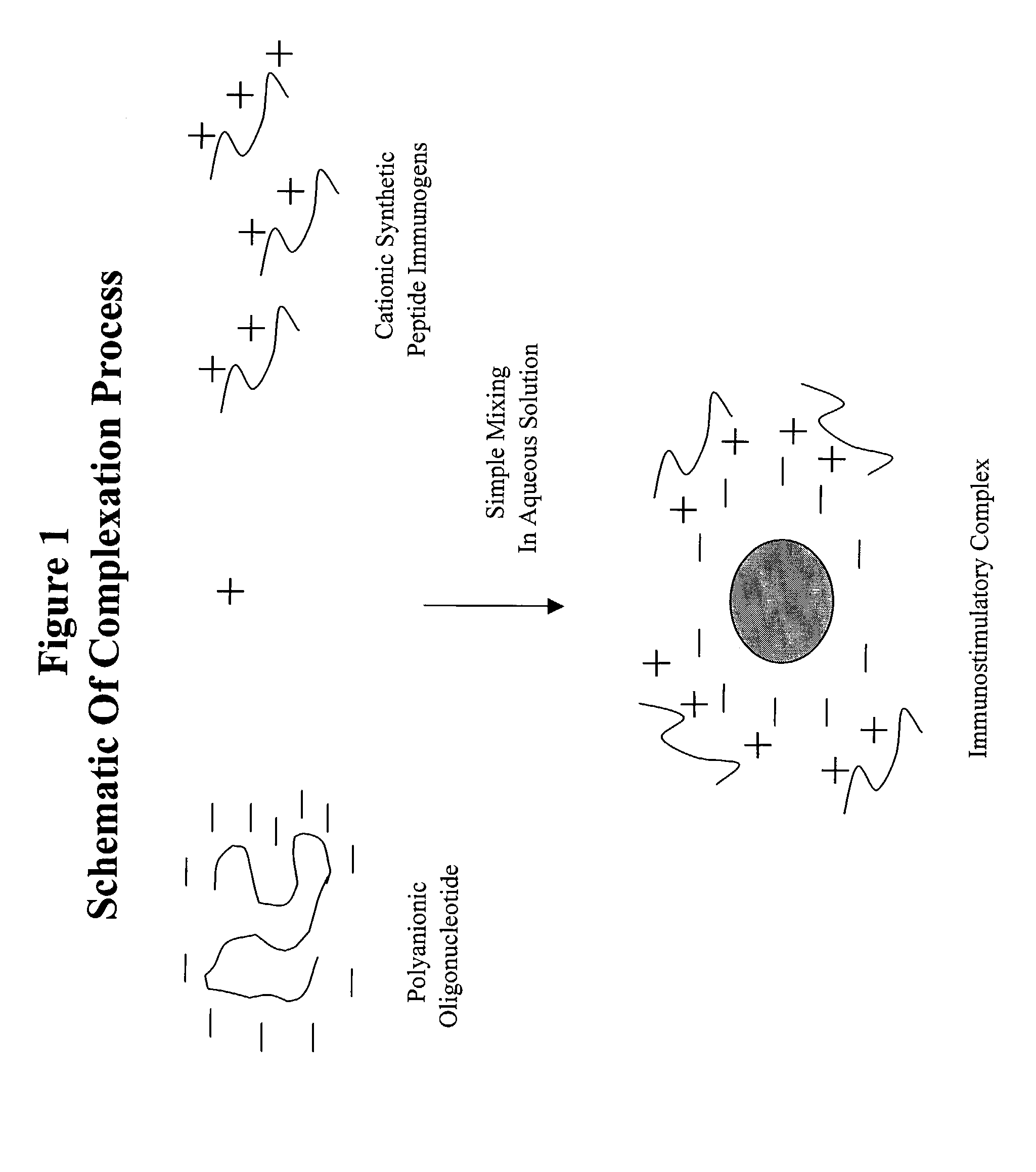
The present invention provides an immunostimulatory complex specifically adapted to act as adjuvant and as a peptide immunogen stabilizer. The immunostimulatory complex comprises a CpG oligonucleotide and a biologically active peptide immunogen. The immunostimulatory complex is particulate and can efficiently present peptide immunogens to the cells of the immune system to produce an immune response. The immunostimulatory complex may be formulated as a suspension for parenteral administration. The immunostimulatory complex may also be formulated in the form of w / o-emulsions, as a suspension in combination with a mineral salt suspension or with an in-situ gelling polymer for the efficient delivery of an immunogen to the cells of the immune system of a subject following parenteral administration, to produce an immune response which may also be a protective immune response.
Owner:UNITED BIOMEDICAL INC
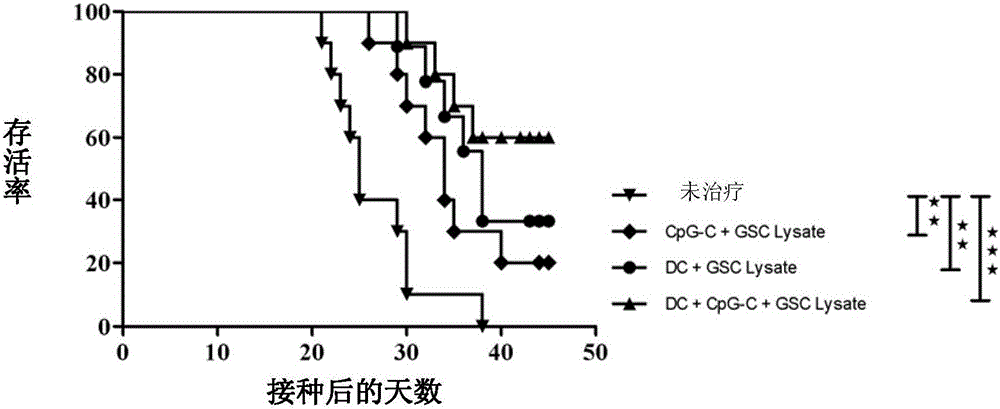
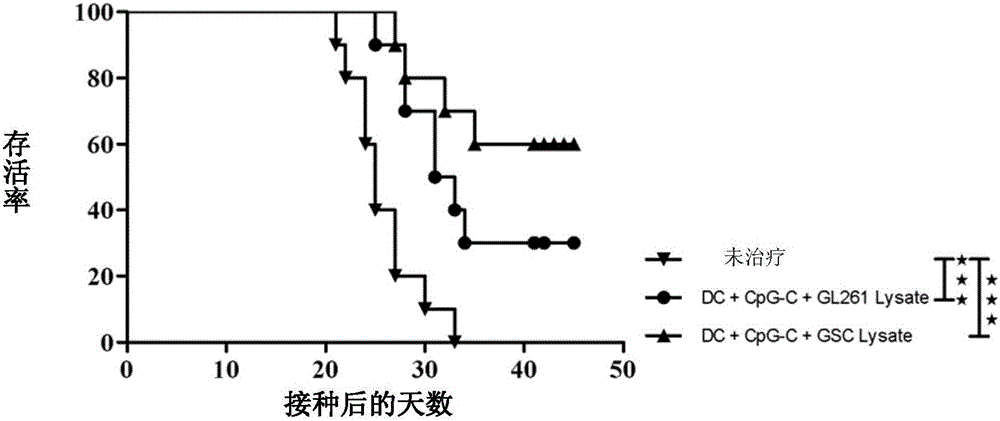
Method for preparing antigen composition capable of targeting glioma cells and glioma stem cells, and vaccine containing the antigen composition
InactiveCN107174657APrevent recurrenceEfficient killingAntibody ingredientsCancer antigen ingredientsFreeze thawingDendritic cell
The invention provides a method for preparing an antigen composition capable of targeting glioma cells and glioma stem cells. The method comprises the steps of (1) providing isolated cell populations containing the glioma stem cells and the glioma cells; (2) amplifying the glioma stem cells in the cell populations; (3) carrying out freeze-thaw cracking treatment on the amplified cell populations so as to obtain the antigen composition containing glioma stem cell lysates and glioma cell lysates. The invention also provides a vaccine containing the antigen composition, dendritic cells and CpG oligodeoxynucleotide, and application of the vaccine in preparation of medicines for treating glioma. The antigen composition and the vaccine which are provided by the invention can target the glioma cells and the glioma stem cells at the same time, thus being capable of effectively killing the residue glioma stem cells and the glioma cells and further inhibiting the recurrence of the glioma.
Owner:JILIN UNIV
Tumour growth inhibitory compounds and methods of their use
Specific CpG oligonucleotide sequences, when given subcutaneously and in particular when administered on a mucous membrane, e.g. intranasally, intravaginally, or rectally, have a profound effect on various human cancer forms as confirmed in vivo, in animal studies, and in vitro, in human PBMCs collected from blood from healthy subjects and from patients suffering from CLL or FL. The compounds are also preferably used in combination with a cancer therapy chosen among radiation treatment, hormone treatment, surgical intervention, chemotherapy, immunological therapies, photodynamic therapy, laser therapy, hyperthermia, cryotherapy, angiogenesis inhibition, or a combination of any of these, and is most preferably an immunological treatment and comprises the administration of an antibody to the patient.
Owner:INDEX PHARMA
MULTIPLE CpG OLIGODEOXYNUCLEOTIDE AND THEIR USE TO INDUCE AN IMMUNE RESPONSE
InactiveUS20110189267A1Enhance immune responseOptimization parametersAntibacterial agentsAntimycoticsCpg oligonucleotidesCpG Oligodeoxynucleotide
Compositions including multiple oligodeoxynucleotides with a CpG motif are disclosed herein. The compositions can include either D or K type oligodeoxynucleotides. These compositions are of use in inducing an immune response in a large percentage of the individuals in a population.
Owner:UNITED STATES OF AMERICA
Neuroprotectants
Owner:OREGON HEALTH & SCI UNIV
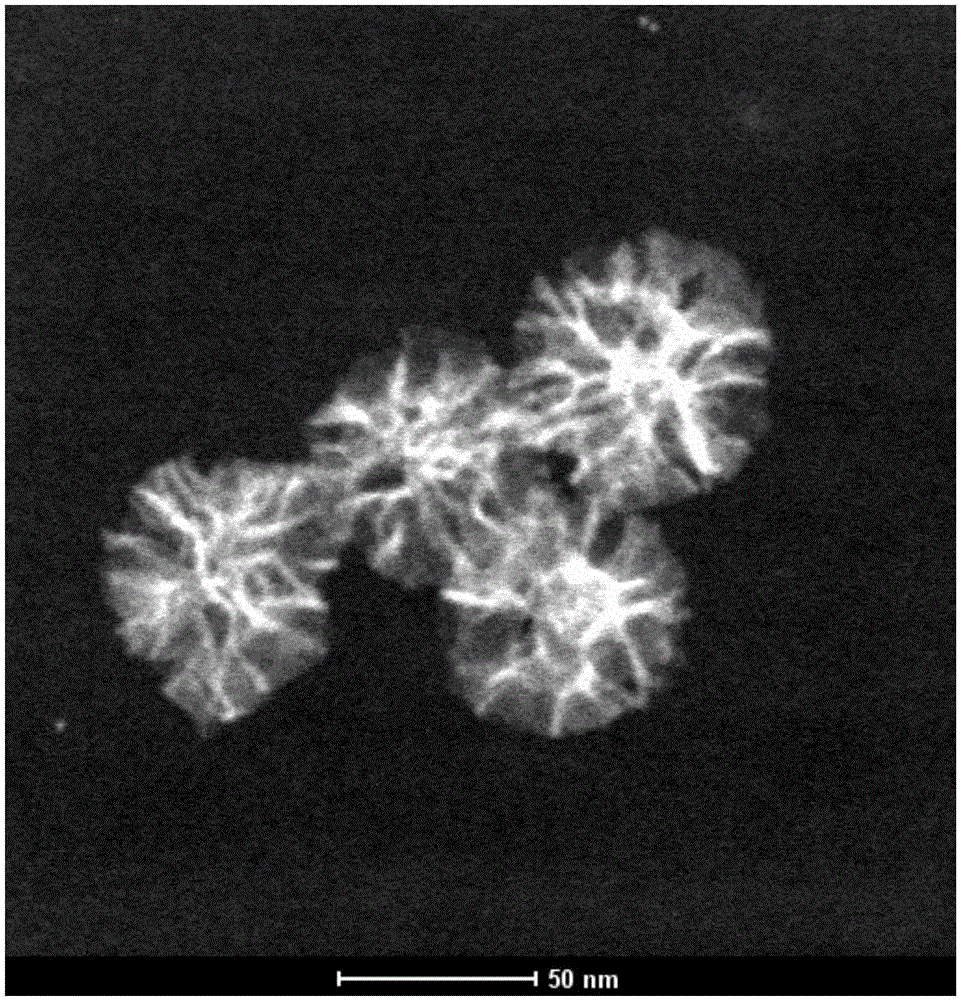
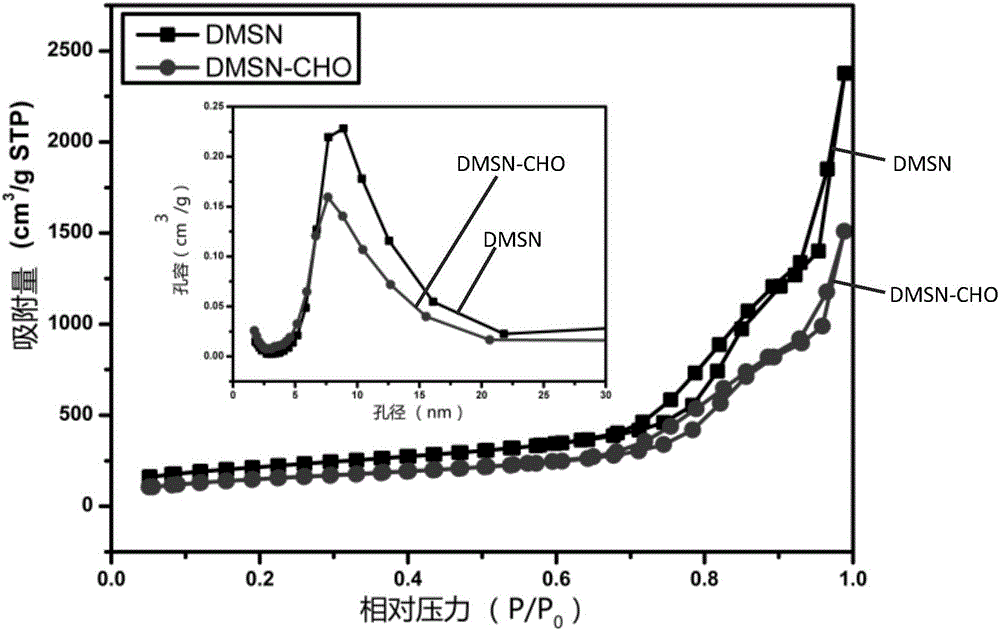
CpG nucleic acid medicine conveying system with pH response and preparation method thereof
InactiveCN105944113AAchieve controlled releasePromote secretionOrganic active ingredientsAntiinfectivesLysosomeCpg oligonucleotides
The invention provides a CpG nucleic acid medicine conveying system. The CpG nucleic acid medicine conveying system comprises a carrier and a CpG nucleic acid medicine, and is characterized in that the carrier is an aldehyde group silane coupling agent modified mesoporous silica nano grain; the CpG nucleic acid medicine is 5' terminal amino modified CpG oligonucleotide; the carrier and the CpG nucleic acid medicine are in covalent linkage through an imine linkage; and the imine linkage has pH sensitivity. The invention further provides a preparation method of the CpG nucleic acid medicine conveying system. According to the CpG nucleic acid medicine conveying system provided by the invention, the carrier and the CpG nucleic acid medicine are in covalent linkage through the pH sensitive imine linkage and can reach a lysosome after being absorbed by immune cells; and the free CpG nucleic acid medicine is released under a weak acidic condition of the lysosome, so that the immune cells are induced to secrete a series of cell factors. The CpG nucleic acid medicine conveying system can realize controllable release of the CpG nucleic acid medicine and has high secretion induction efficiency.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
CpG oligonucleotide prodrugs, compositions thereof and associated therapeutic methods
ActiveUS9809824B2Improve the immunitySugar derivativesSnake antigen ingredientsNucleotideCpg oligonucleotides
The present invention provides a CpG oligonucleotide prodrug that includes a thermolabile substituent on at least one nucleotide thereof. The present invention also provides compositions that include a carrier and a therapeutically effective amount of at least one CpG oligonucleotide prodrug. The present invention further provides therapeutic methods of using such thermolabile CpG oligonucleotide prodrugs and compositions thereof. The present invention further provides a method of inhibiting tetrad formation in a CpG oligonucleotide by functionalizing the CpG oligonucleotide with one or more thermolabile substituents.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
Combination tumor immunotherapy
PendingUS20200281953A1Promotes the egress of activated T cellsImprove anti-tumor effectImmunoglobulins against virusesImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesWhite blood cell
Owner:CHECKMATE PHARM INC
Use of CPG oligonucleotides co-formulated with an antibiotic to accelerate wound healing
Owner:UNITED STATES OF AMERICA
Regulatory b cells and uses thereof
InactiveUS20200113939A1Effective stimulationPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCpg oligonucleotidesLigation
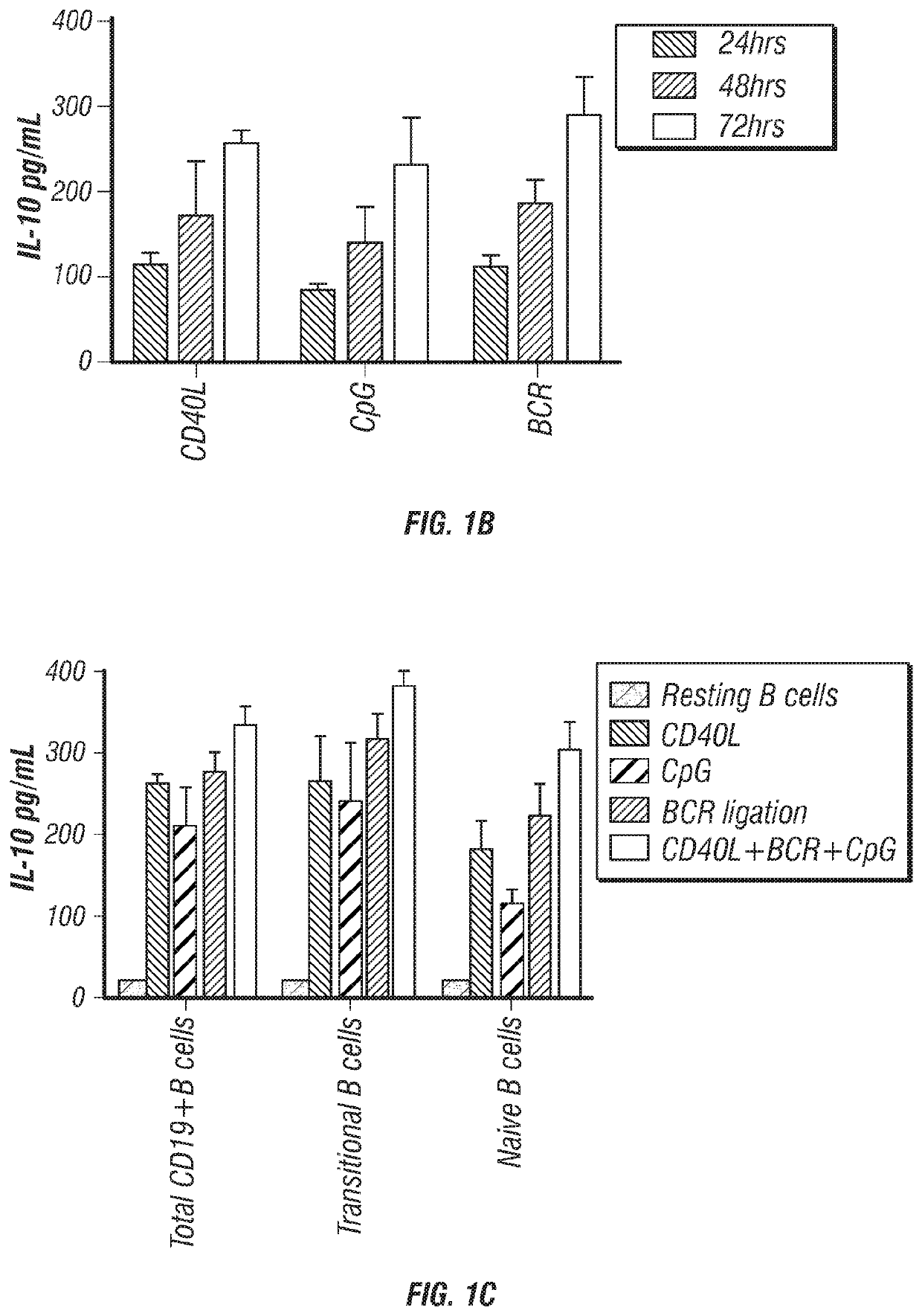
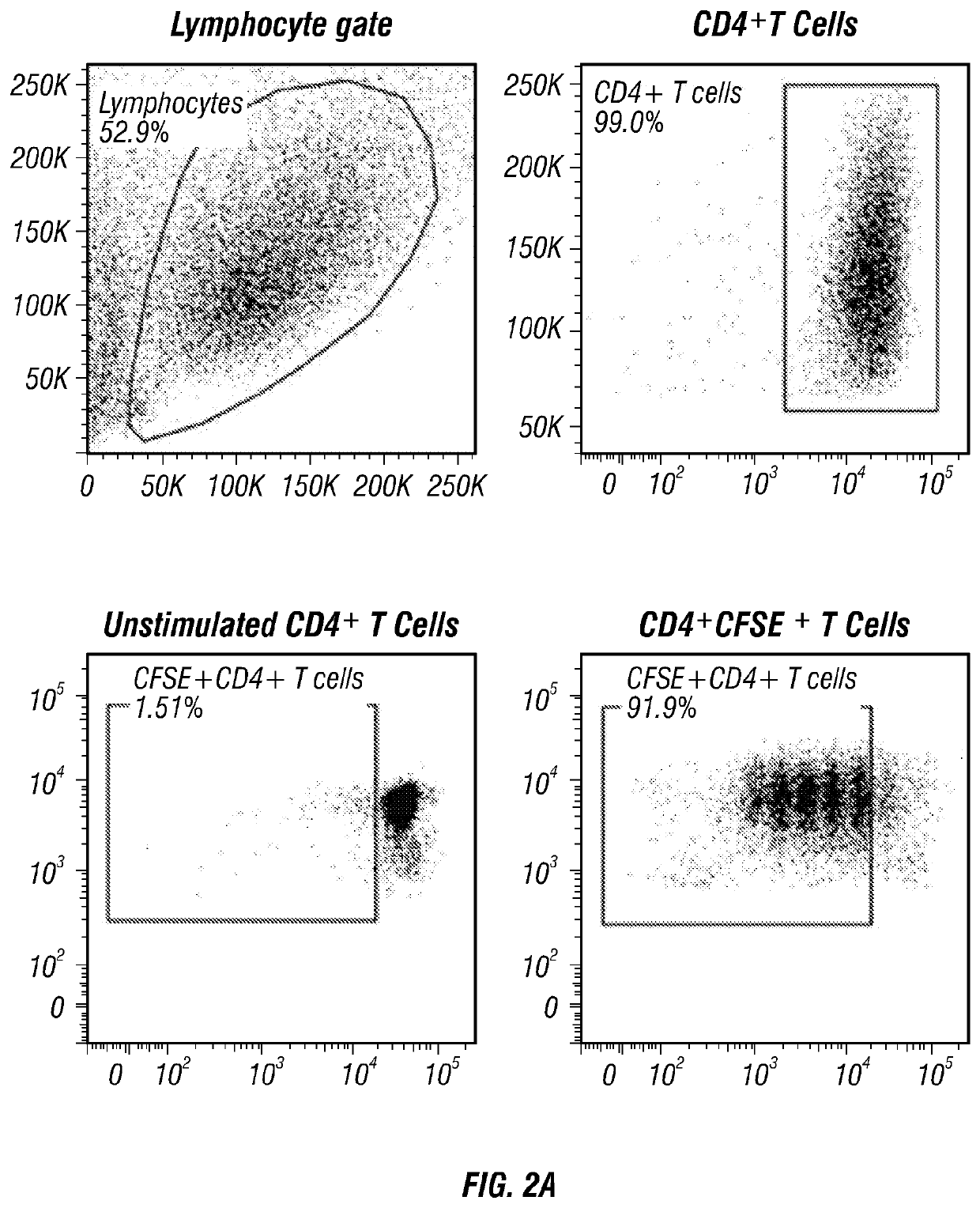
Provided herein are methods for producing stimulated populations of regulatory B cells comprising treating an isolated population of B cells with stimulatory agents, such as CpG oligonucleotides, BCR ligation, and CD40 ligand. Also provided herein are methods of treating immune disorders, such as chronic graft versus host disease, with the stimulated population of regulatory B cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
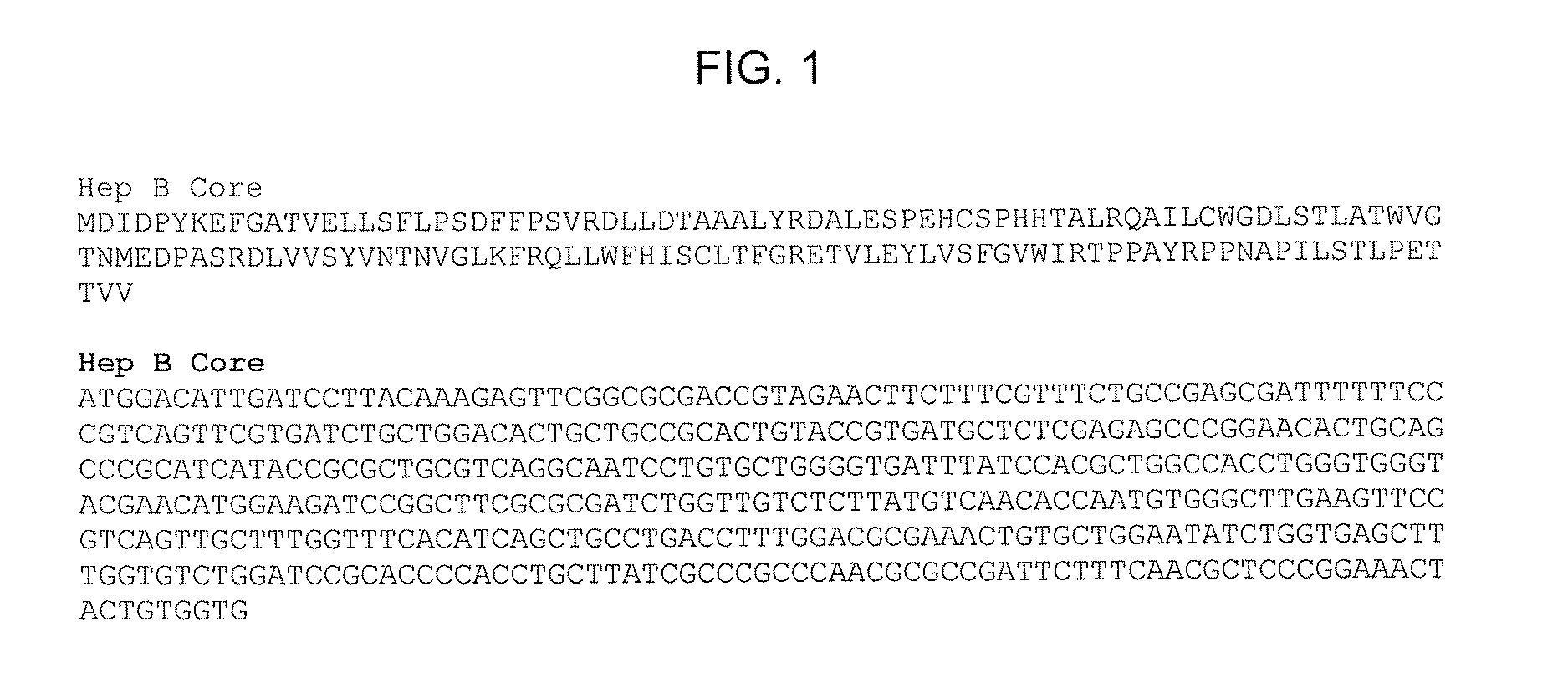
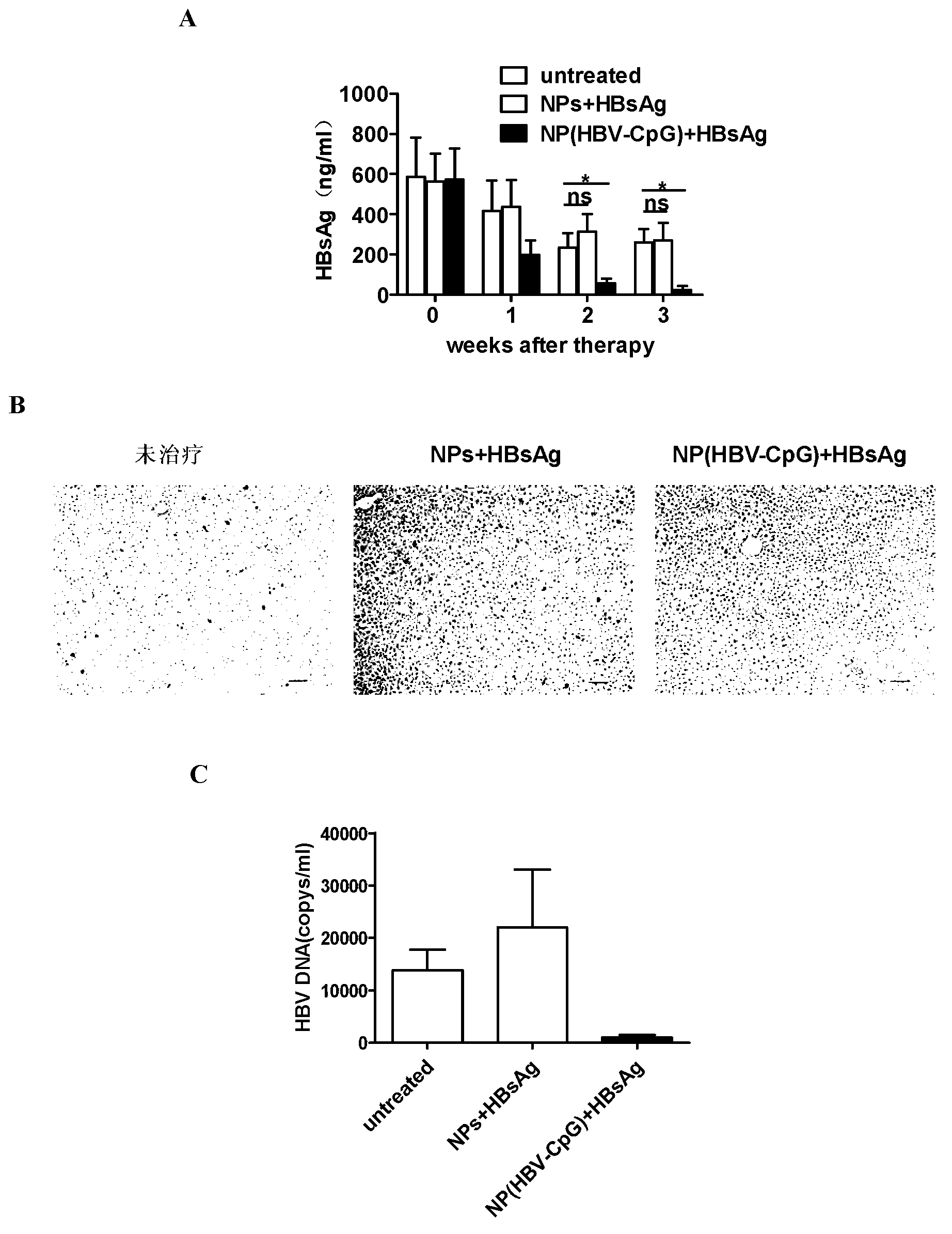
Application of PEG (polyethylene glycol)-PLA (Poly Lactic Acid) nano-material-coated HBV (Hepatitis B Virus)-CpG (Cytosine Phosphate Guanosine) in prevention and/or treatment of hepatitis B
ActiveCN103233011AImprove immunityEfficient removalDigestive systemAntiviralsPhosphatePolyethylene glycol
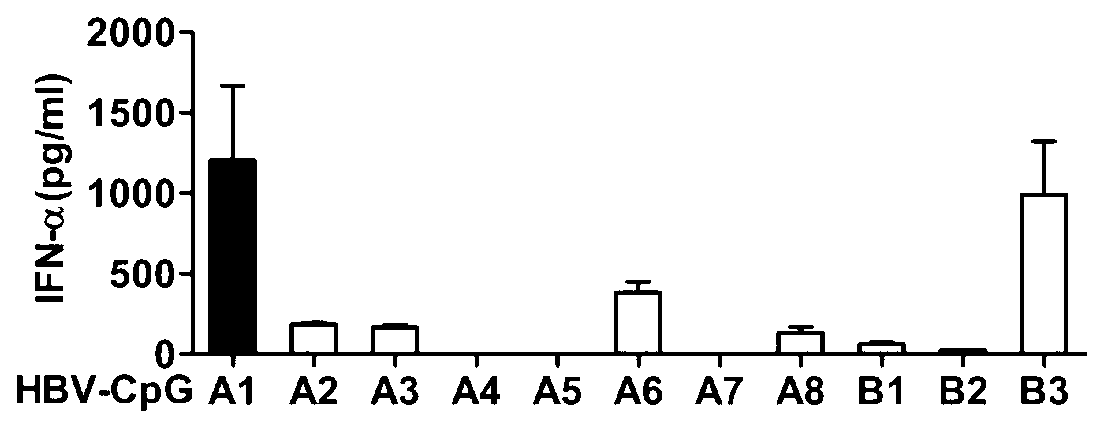
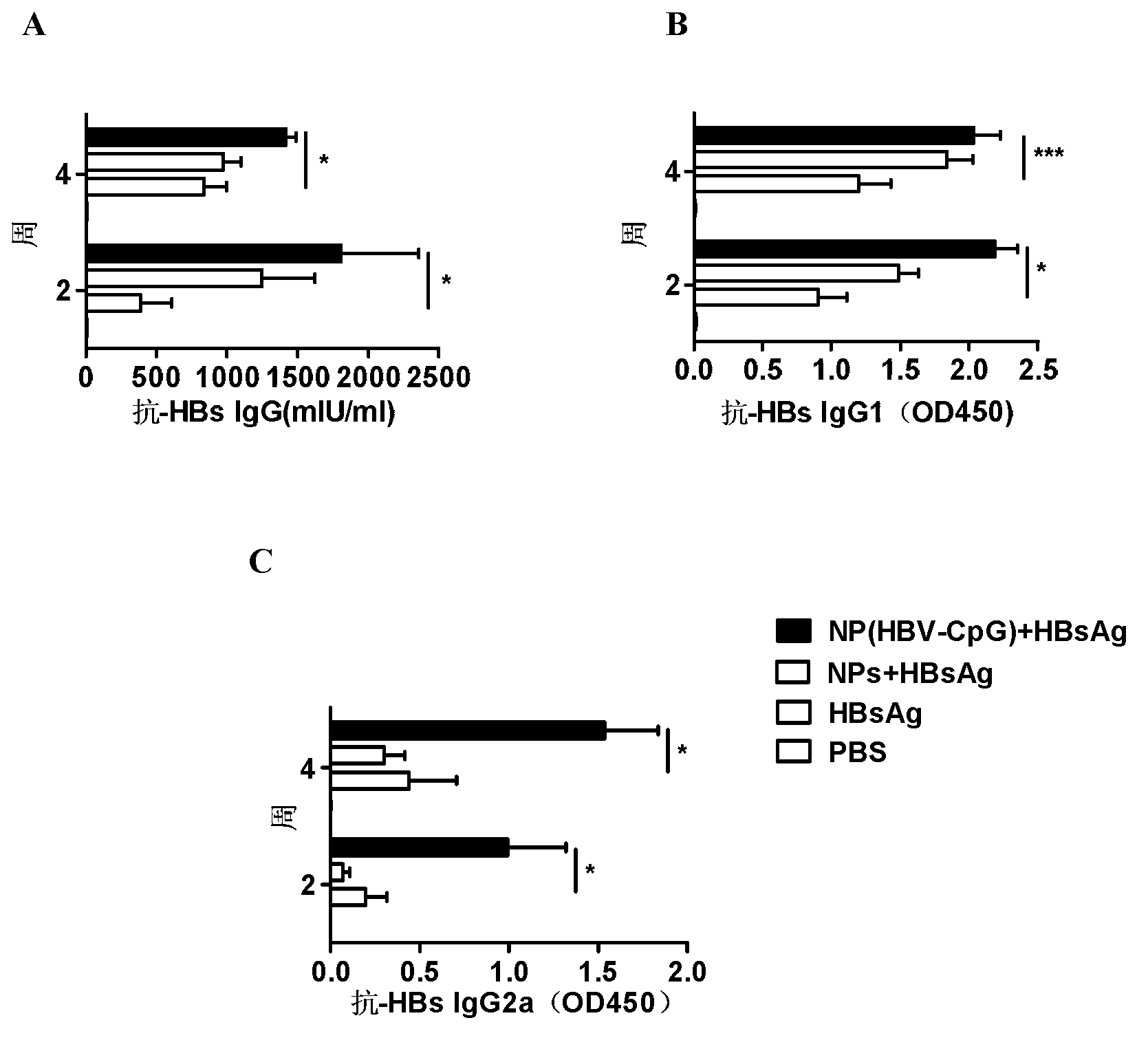
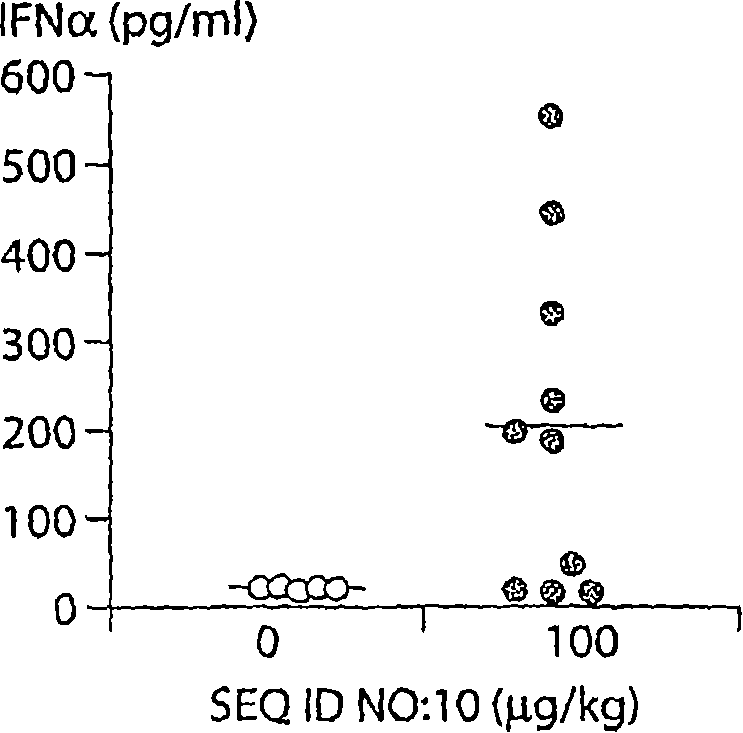
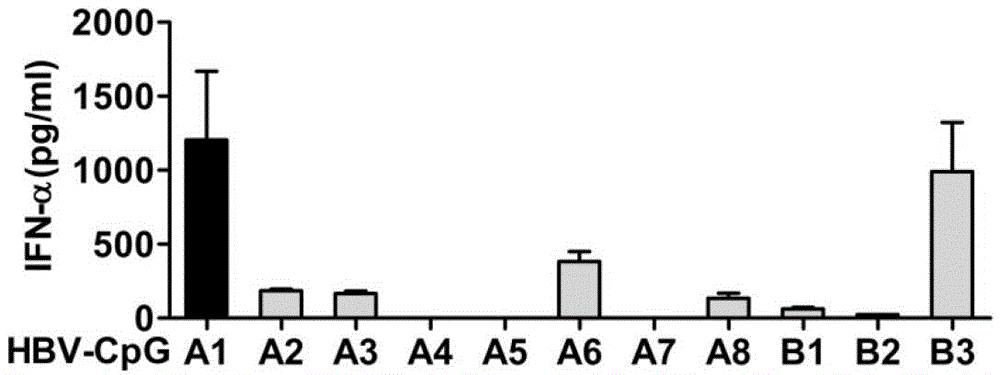
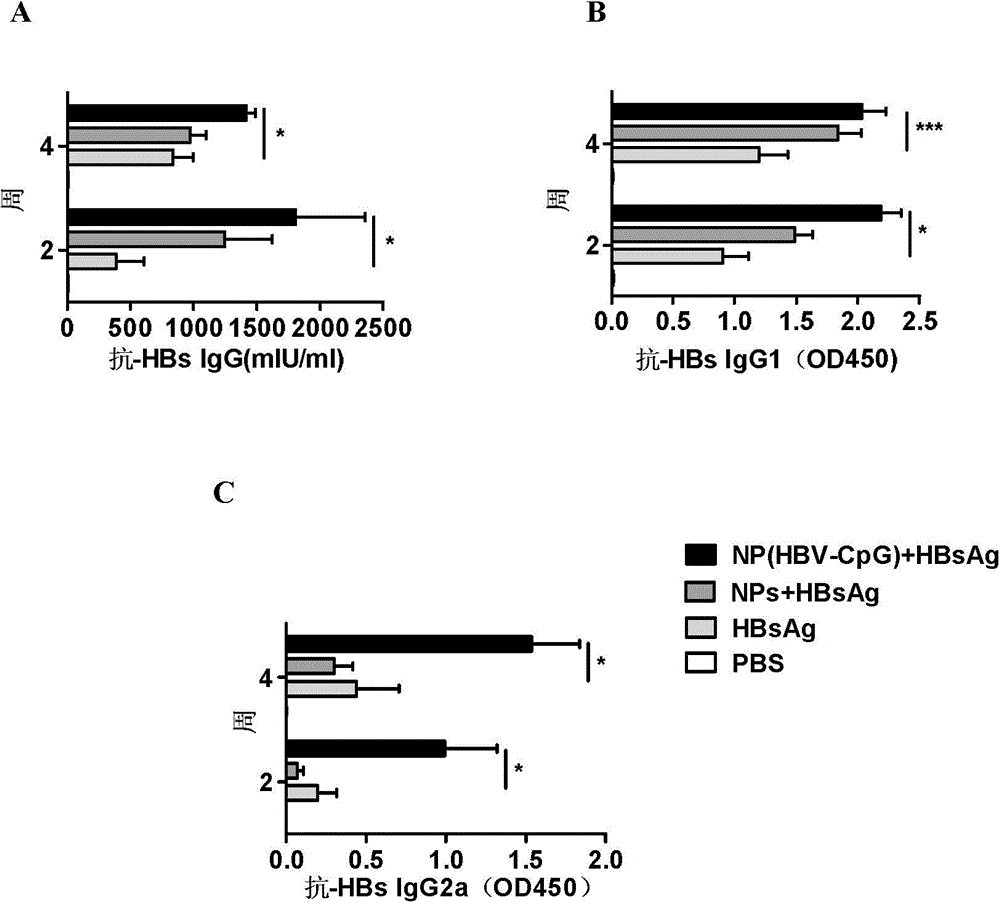
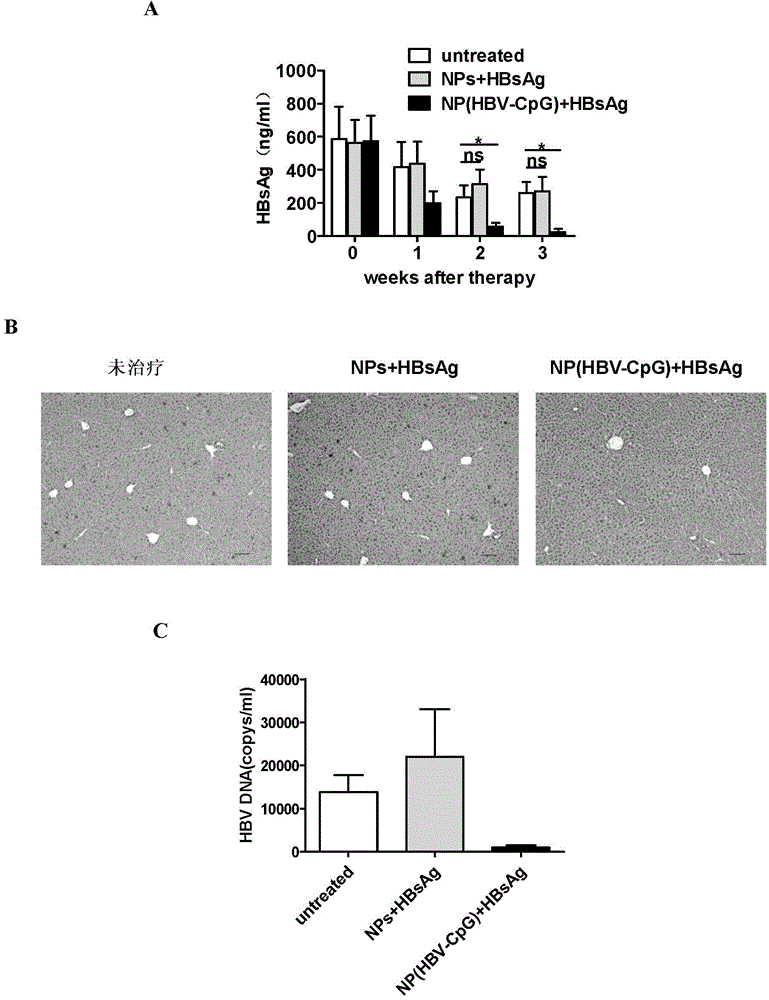
The invention relates to an HBV (Hepatitis B Virus)-CpG (Cytosine Phosphate Guanosine) oligonucleotide sequence capable of inducing alpha-interferon expression, and further relates to a nano-particle prepared by coating the HBV-CpG oligonucleotide sequence by using a nano material PEG-PLA, and relates to an application of the nano-particle in prevention and treatment of hepatitis B, wherein the oligonucleotide sequence is shown in SEQ ID NO: 1 or SEQ ID NO: 2; the nano material PEG-PLA is a segmented copolymer prepared from polyethylene glycol and poly lactic acid; and the nano material PEG-PLA has the general formula of PEGm-PLAn, wherein m represents the number-average molecular weight of a PEG chain segment and can be 2000, 5000 or 10000, and n represents the number-average molecular weight of a PLA chain segment and can be 5000 or 10000. The CpG(HBV-CpG) from HBV gene sources coated by the nano material PEG-PLA not only can be used for preparing a prophylactic vaccine for hepatitis B but also can be used for preparing a therapeutic vaccine for hepatitis B.
Owner:UNIV OF SCI & TECH OF CHINA
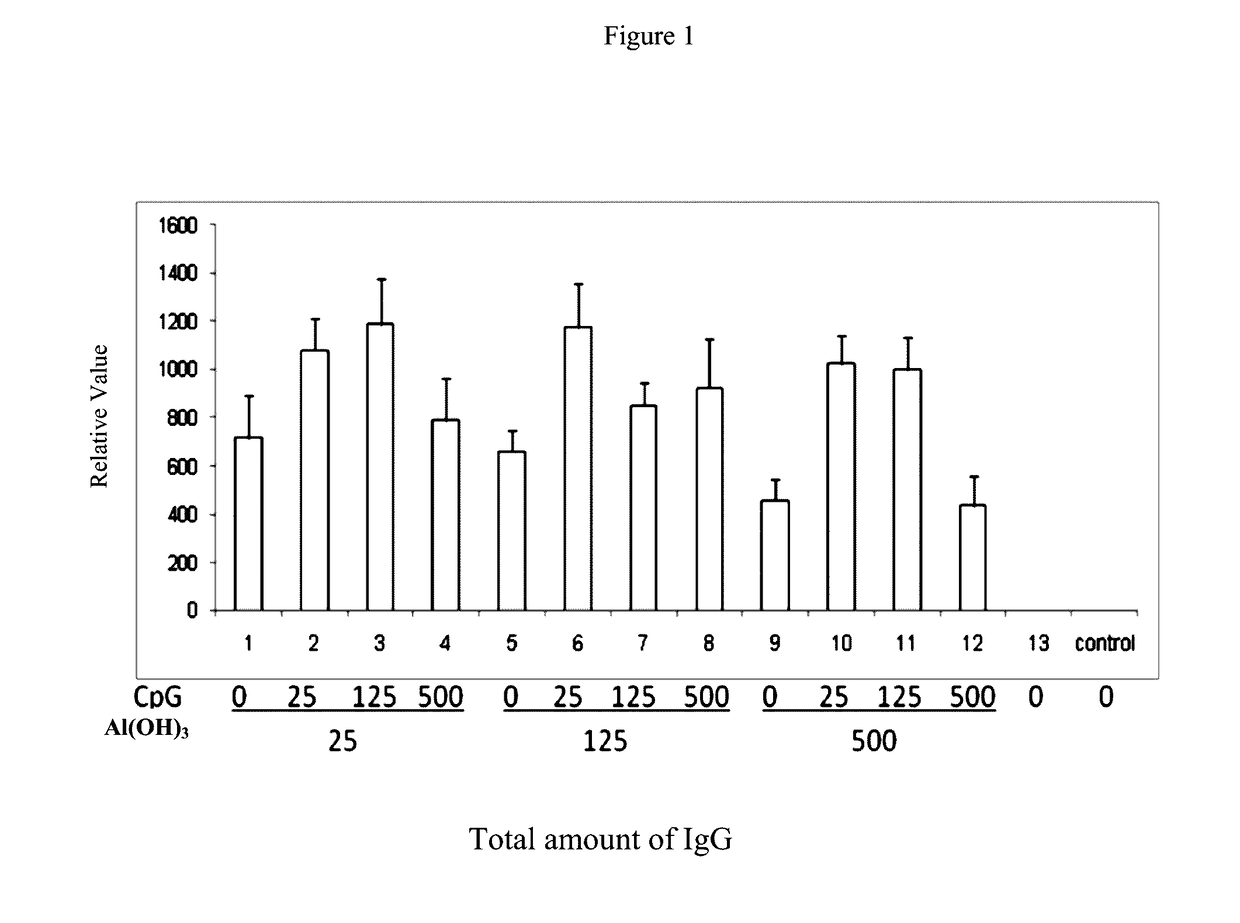
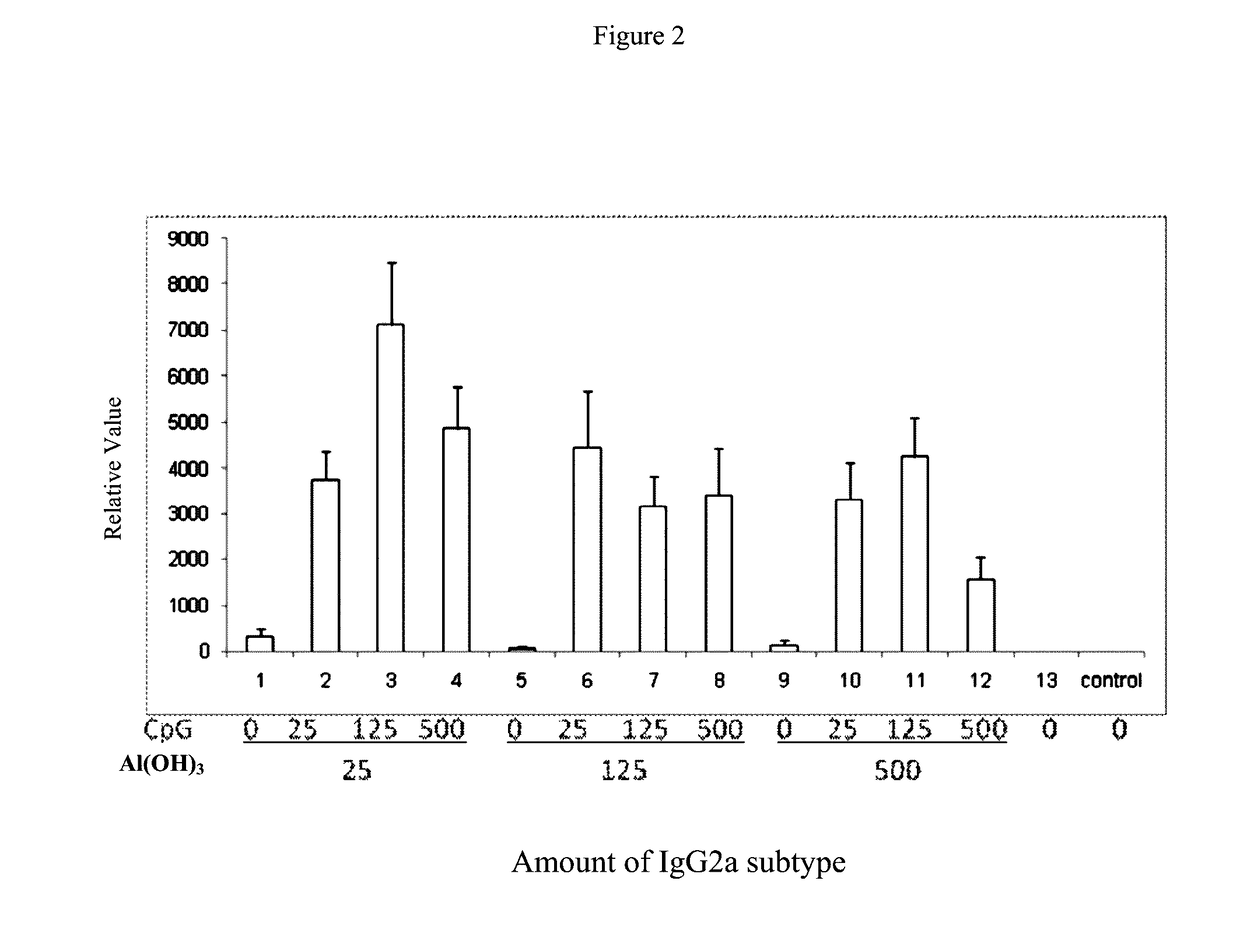
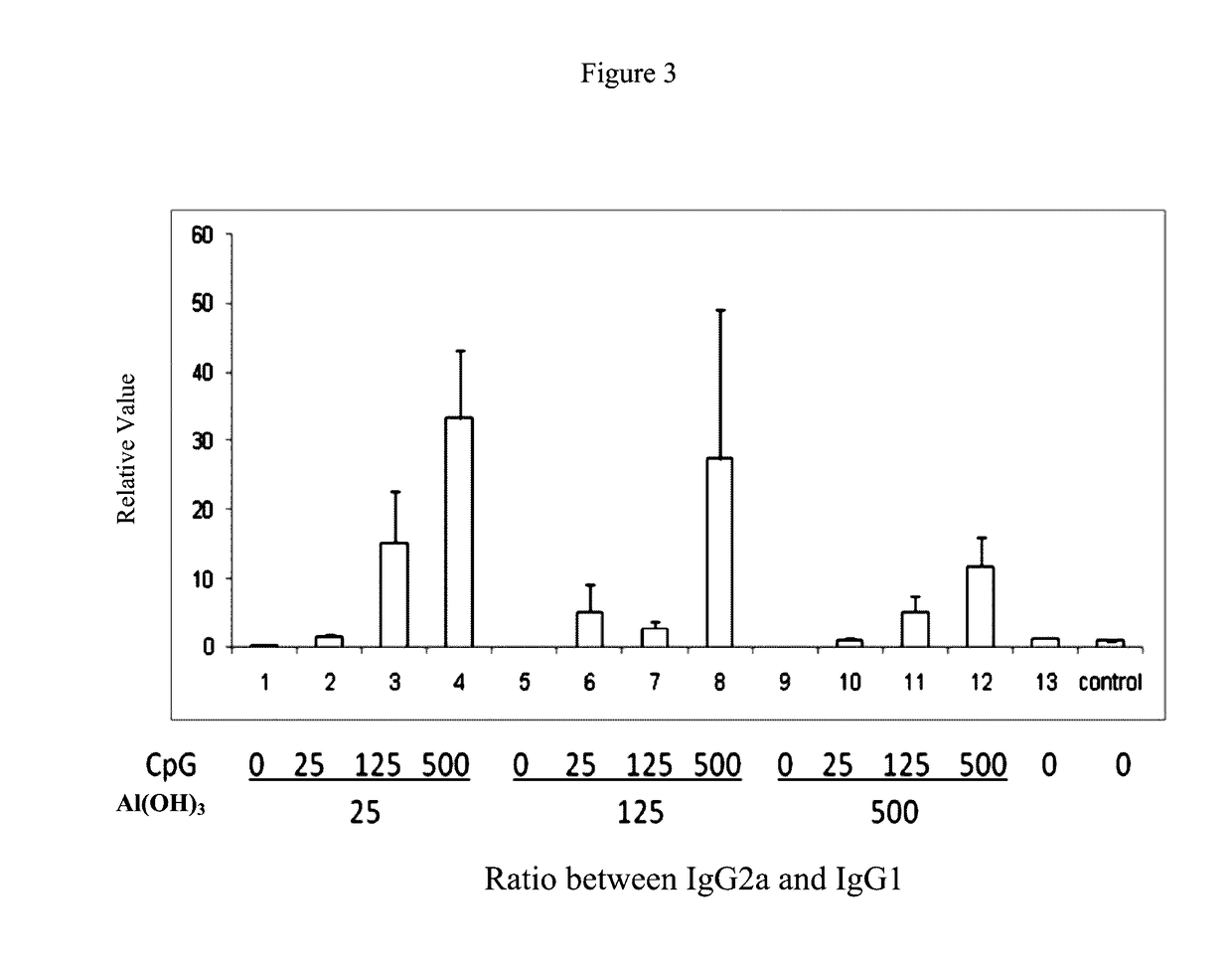
Pharmaceutical Compositions Comprising CpG Oligonucleotides
ActiveUS20170136119A1Protection levelAdministration in decreasedViral antigen ingredientsAntiviralsAntigenAdjuvant
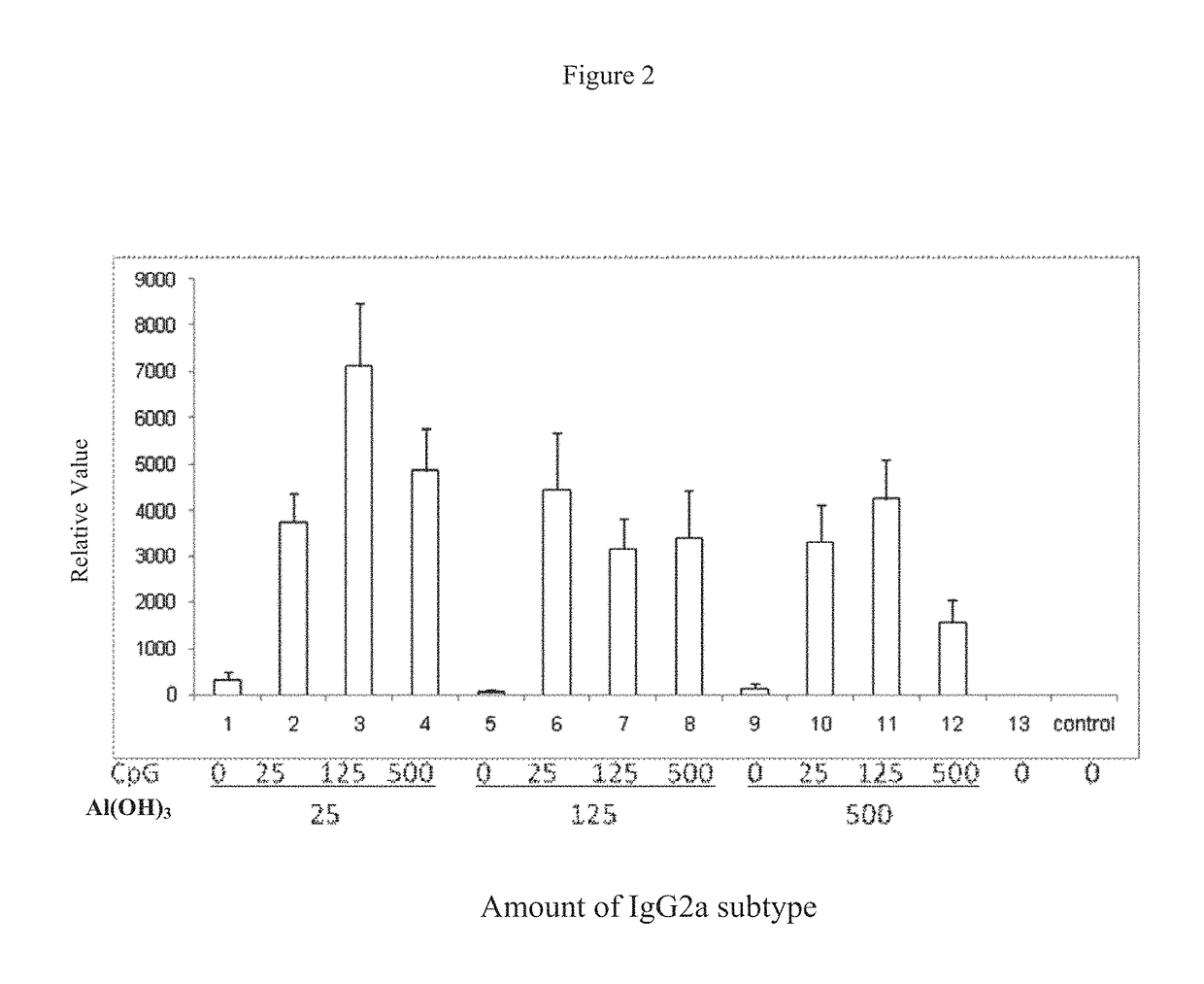
The present invention provides a pharmaceutical composition for inducing immune response in a subject, comprising: (a) an antigen with a concentration ranging from 1 μg / ml to 100 μg / ml; (b) CpG oligonucleotides having a sequence of 5′-tcgacgttcgtcgttcgtcgttc-3′, with a concentration ranging from 25 μg / ml to 500 μg / ml, and (c) an aluminium adjuvant with a concentration ranging from 25 μg / ml to 500 μg / ml. Such pharmaceutical composition can induce or boost immune response against antigen in the subject.
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
MODIFIED CpG OLIGODEOXYNUCLEOTIDE WITH IMPROVED IMMUNOREGULATORY FUNCTION
InactiveUS20090214530A1Enhance immune regulation functionOrganic active ingredientsSugar derivativesAnticarcinogenPeripheral blood mononuclear cell
The present invention relates to a modified CpG oligodeoxynucleotide (ODN) which is prepared by coupling a consecutive sequence of deoxyribothymine (dT) to the 3′-terminus of CpG ODN having immunoregularory function, thereby improving immunoactivity of splenocytes, macrophages and peripheral mononuclear cells, and therefore, can be effectively used as a vaccine adjuvant for preventing and treating hepatitis B or an anticancer agent. Since the phosphorothioate CpG ODN having the consecutive sequence of dT at its 3′-terminus shows high activity inducing Th-1 immune response and does not elicit in vivo toxicity with guaranteeing its safety, it can be effectively used as a vaccine adjuvant.
Owner:YONSEI UNIVERSITY
Pharmaceutical compositions comprising CpG oligonucleotides
ActiveUS10052378B2Protection levelAdministration in decreasedBacterial antigen ingredientsViral antigen ingredientsAntigenAdjuvant
The present invention provides a pharmaceutical composition for inducing immune response in a subject, comprising: (a) an antigen with a concentration ranging from 1 μg / ml to 100 μg / ml; (b) CpG oligonucleotides having a sequence of 5′-tcgacgttcgtcgttcgtcgttc-3′, with a concentration ranging from 25 μg / ml to 500 μg / ml, and (c) an aluminum adjuvant with a concentration ranging from 25 μg / ml to 500 μg / ml. Such pharmaceutical composition can induce or boost immune response against antigen in the subject.
Owner:CHANGCHUN HUAPU BIOTECHNOLOGY CO LTD
Serum-free bone marrow culture medium as well as preparation method and application thereof
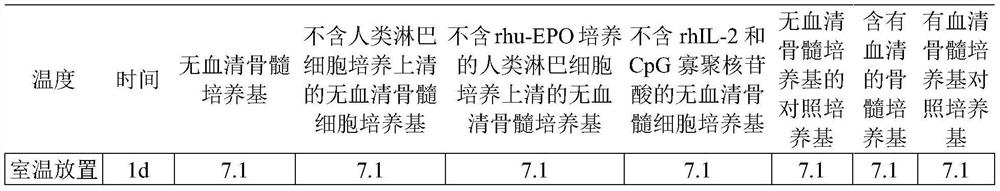
ActiveCN112063579ALow costGood dispersionPreparing sample for investigationCulture processSodium phosphatesSodium glycerophosphate
The invention provides a serum-free bone marrow culture medium as well as a preparation method and application thereof. The culture medium comprises a basal culture medium, and human lymphocyte culture supernatant which is cultured by adding L-ascorbic acid, vitamin E, penicillin, streptomycin, sodium pyruvate, sodium selenite, glutamine, rhIL-2, CpG oligonucleotide, linoleic acid, linolenic acid,stearic acid, BSA, sodium glycerophosphate, HEPES, NaH2PO4, Na2HPO4 and rhu-EPO into the basal culture medium. Quality control between batches is facilitated. The human lymphocyte culture supernatantcultured by the rhu-EPO is used for providing growth factors required by bone marrow growth, so that the production cost is reduced. The sodium glycerophosphate and the like are used as a pH buffer system, the pH value is more stable, and the pH value is stable in the storage process. rhIL-2 and CpG oligonucleotides are adopted to stimulate the activity of B cells, and more karyotypes can be obtained when the oligonucleotides are used for karyotype analysis.
Owner:上海培晖生物科技发展有限公司
Application of PEG (polyethylene glycol)-PLA (Poly Lactic Acid) nano-material-coated HBV (Hepatitis B Virus)-CpG (Cytosine Phosphate Guanosine) in prevention and/or treatment of hepatitis B
ActiveCN103233011BImprove immunityEfficient removalDigestive systemAntiviralsPhosphatePolyethylene glycol
The invention relates to an HBV (Hepatitis B Virus)-CpG (Cytosine Phosphate Guanosine) oligonucleotide sequence capable of inducing alpha-interferon expression, and further relates to a nano-particle prepared by coating the HBV-CpG oligonucleotide sequence by using a nano material PEG-PLA, and relates to an application of the nano-particle in prevention and treatment of hepatitis B, wherein the oligonucleotide sequence is shown in SEQ ID NO: 1 or SEQ ID NO: 2; the nano material PEG-PLA is a segmented copolymer prepared from polyethylene glycol and poly lactic acid; and the nano material PEG-PLA has the general formula of PEGm-PLAn, wherein m represents the number-average molecular weight of a PEG chain segment and can be 2000, 5000 or 10000, and n represents the number-average molecular weight of a PLA chain segment and can be 5000 or 10000. The CpG(HBV-CpG) from HBV gene sources coated by the nano material PEG-PLA not only can be used for preparing a prophylactic vaccine for hepatitis B but also can be used for preparing a therapeutic vaccine for hepatitis B.
Owner:UNIV OF SCI & TECH OF CHINA
Dimeric cpg oligonucleotides for use in modulating immune responses
Pharmaceutical compositions comprising a CpG oligonucleotide, a buffer agent, and one or more salts having a total salt concentration of about 80-130 mM. A majority population of the CpG oligonucleotides in the composition is in dimeric form. Also provided herein are uses of the pharmaceutical compositions for modulating immune responses in subjects in need of the treatment, for example, cancer patient.
Owner:MICROBIO (SHANGHAI) CO LTD
Preparation method and application of a novel regulatory dendritic cell
ActiveCN105886471BIncrease lethalityIncrease the likelihood of cureMammal material medical ingredientsBlood/immune system cellsDendritic cellOligonucleotide
The invention provides a preparation method of a novel regulatory dendritic cell and a DC-CIK combined tumor immunotherapy method. The preparation method comprises the following steps: separating mesenchymal stem cells and hematopoietic stem cells of a mammal; together culturing the mammal's mesenchymal stem cells and hematopoietic stem cells under conditions suitable for cell growth and proliferation; and after culturing for 1-7 days, recovering from a suspension culture medium and identifying so as to obtain the novel regulatory dendritic cells. The invention is characterized in that the novel regulatory dendritic cell is converted to mature regulatory dendritic cells when induced by CpGODN (CpG oligonucleotides) or an inducer with the same induction function, wherein expression level of irf4+ and irf8+ is increased and the cell has an immunoregulation function. The novel regulatory dendritic cell can suppress tumor growth and can be combined with DC-CIK for tumor immunotherapy.
Owner:微能生命科技集团有限公司
A kind of natural immune booster for aquatic products and its application
ActiveCN109430536BImprove immunityImprove stress resistanceAccessory food factorsDNA preparationAquatic animalAstaxanthin
The invention discloses a natural immune enhancer for aquatic products and its application. The natural immune enhancer comprises the following components and parts by weight: 8-10 parts of CpG oligonucleotides, 5 parts of Phaffia yeast powder rich in astaxanthin ‑10 parts, fructooligosaccharides 10‑20 parts, mannan oligosaccharides 10‑20 parts. The natural immune enhancer is applied to the feed of aquatic animals, and its added amount is 0.05-0.3% of the weight of the feed. The beneficial effects are: each component in the natural immune enhancer of the present invention plays a role of mutual gain, improves the immunity and anti-stress ability of aquatic animals, improves the resistance to diseases, promotes the growth of aquatic animals, and has low cost; the natural immune The enhancer is used in the feed of aquatic animals to improve the survival rate of the whole cycle of aquatic animals and make up for the problem of adding antibiotics in the feed.
Owner:浙江皇冠科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com