Preparation method and application of a novel regulatory dendritic cell
A dendritic cell and regulatory technology, applied in the generation of new regulatory dendritic cells and in the field of immunotherapy, to improve the quality of life, remove tumor cells, and enhance the effect of killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Main experimental reagents and consumables
[0073] (1) Main experimental reagents
[0074] Penicillin, streptomycin and trypsin-EDTA were purchased from GIBCO.
[0075] Human lymphocyte separation medium (Ficoll, Tianjin Haoyang Biological Products Co., Ltd.)
[0076] Cytokines rhIL-2, rhIL-12, IL-4, IL-7, GM-CSF, TNF-σ (Peprotech);
[0077] Human recombinant soluble CD40 ligand (rh sCD40-L; Bender Company);
[0078] Antibody: mouse anti-human CD11c, CD80, CD40, CD86, CD29, CD34, CD44, CD73, CD90, CD105, CD106, CD117, Flk-1, HLA-ABC, CD3 monoclonal antibody, CD28 monoclonal antibody and HLA-DR antibody , anti-mouse NK1.1mAb, anti-mouse CD8+T cell mAb, rabbit anti-mouse CD16 / CD56, CD8, CD3, CD19 flow antibody (Becton Dickinson Company, biolegend Company);
[0079] Magnetic bead sorting kit: CD34, CD4, CD8, NK cells (MiltenyiBiotec);
[0080] ELISA kit: IFN-γ (BD Pharmingen);
[0081] CFSE staining solution kit (Beiyuntian Company)
[0082] Cell culture ...
Embodiment 2
[0086] Example 2, In vitro induction method of irf4+irf8+regulatory dendritic cells (irf4+irf8+regDC)
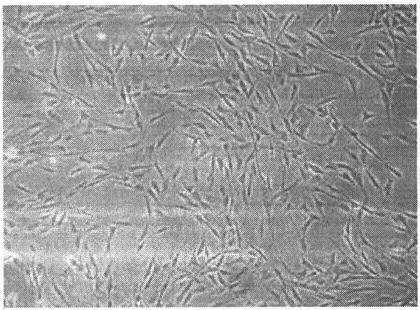
[0087] Step 1. Isolation, cultivation and identification of third-generation mesenchymal stem cells derived from human bone marrow ( Figure 1-2 )
[0088] (1) Separation
[0089] 1. Aseptically collect 5-10ml of bone marrow from healthy volunteers in a sterile heparin tube.
[0090] 2. Take a sterile centrifuge tube and properly dilute the bone marrow with D-Hanks solution.
[0091] 3. Take a new centrifuge tube and add Ficoll which has been brought back to room temperature and the above-mentioned diluted bone marrow respectively. Careful operation should not damage the interface when adding. The ratio of the two is 1:1.
[0092] 4. After balancing the centrifuge tubes above, put them into a desktop centrifuge at room temperature, and centrifuge at 1800 rpm for 20 minutes at 20°C. After the centrifuge tube was taken out, the buffy coat layer was aseptically aspirated ca...
Embodiment 3
[0127] Example 3, Morphology and Identification of irf4+irf8+ Regulatory Dendritic Cells (irf4+irf8+regDC)
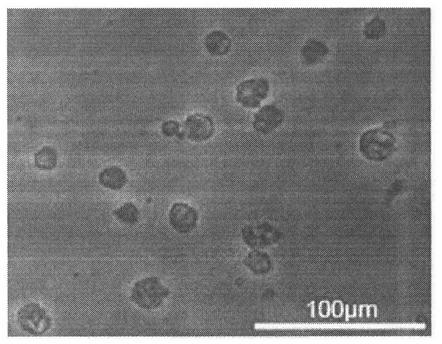
[0128] (1) Form ( Figure 7 )
[0129] Regulatory dendritic cells (regDC) generated from hematopoietic stem progenitor cells induced by mesenchymal stem cells were observed under a light microscope, and synapses could be seen on the cell membrane.
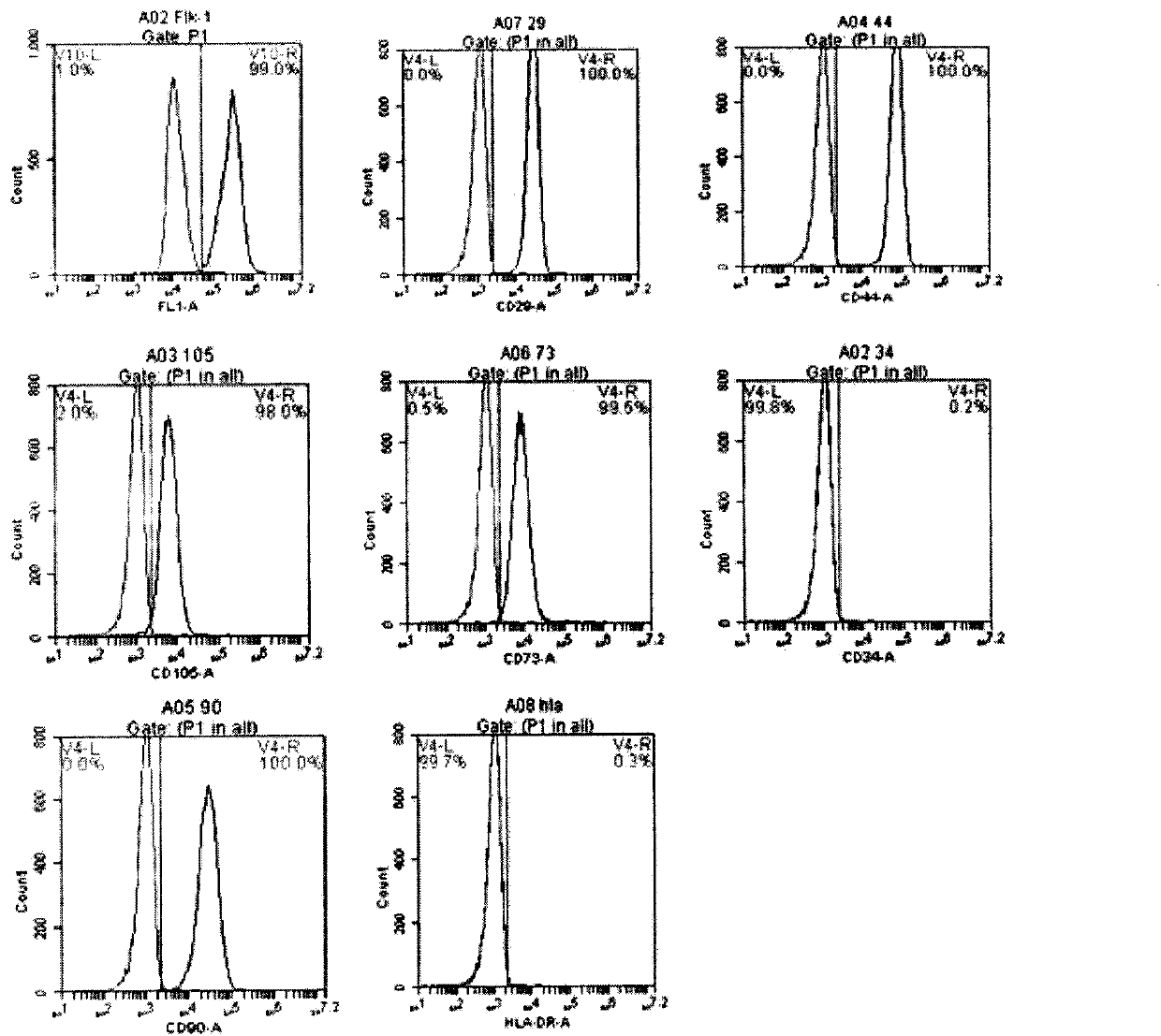
[0130] (2) Identification of surface marker factors ( Figure 8 )
[0131] Detection of the expression of relevant cell surface marker factors and co-stimulatory molecules indicated that the induced new regulatory dendritic cells (regDC) had low expression of CD11c and weak expression of CD80, CD86, and CD40.
[0132] (3) Induce the number of regulatory dendritic cells ( Figure 9 )
[0133] After 7 days of co-culture of mesenchymal stem cells and hematopoietic stem progenitor cells, the suspension cells were harvested. Flow cytometry showed that a single group of cells was obtained, accounting for 56.9% of all suspend...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com