Method for preparing antigen composition capable of targeting glioma cells and glioma stem cells, and vaccine containing the antigen composition
A technology for glioma stem cells and glioma cells, which is applied in the application field of medicines, can solve the problems that antigen-presenting cells cannot quickly identify and activate effector cells, cannot effectively simulate the antigen-presenting process, and cannot obtain antigens, etc. To achieve the effect of improving the utilization rate in vivo, inhibiting the growth of glioma, and enhancing the strength of immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
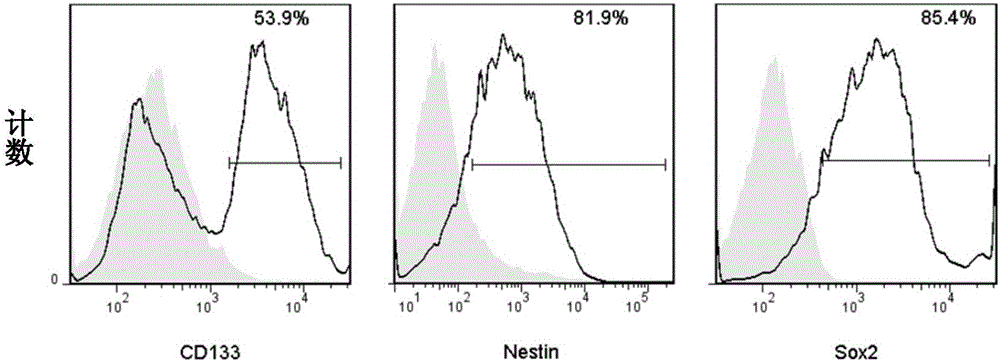
[0021] Example 1: Preparation of antigen composition containing glioma stem cell lysate and glioma cell lysate
[0022] (1) Obtaining a cell population containing glioma stem cells and glioma cells
[0023]Glioma tissues were obtained from patients with grade IV gliomas. After surgical resection of the tumor tissue, the glioma tissue was quickly placed in Neurobasal (NB) medium containing 5 μg / ml gentamicin, 0.9 μg / ml levomycin, 50 ng / ml EGF and 50 ng / ml FGF, It was then placed in a cryopreservation box and quickly transferred to a GMP laboratory for subsequent processing. In a biosafety cabinet, transfer the tumor tissue to new Neurobasal (NB) medium containing 5 μg / ml gentamicin, 0.9 μg / ml levomycin, 50 ng / ml EGF, and 50 ng / ml FGF, and the tumor Cut the tissue into small pieces, remove the hoof tissue and necrotic tissue, and then further mince the tumor tissue into about 1mm 3 Add sterile saline to the obtained fragmented tissue to form a tissue cell suspension, remove l...
Embodiment 2
[0030] Example 2: Using mouse glioma cell line GL261 to prepare antigen composition containing mouse glioma stem cell lysate and mouse glioma cell lysate
[0031] Resuscitate the mouse glioma cancer cell line GL261 frozen in our laboratory (gifted by Professor Chen Wei, University of Minnesota, USA), culture to the logarithmic growth phase, obtain cell suspension, and wash thoroughly with sterile phosphate buffer saline 2 times, count the cells, resuspend the cells with the complete medium of mouse glioma stem cells, and adjust the cell density to 0.5-1×10 6 / ml, transfer every 10-15ml to a 10cm sterile suspension cell culture dish at 37°C, 5% CO 2 and 5%O 2 Culture in a three-gas incubator and change the medium every 2 days. After 10 days of culture, the medium was changed every 3 days. According to the above culture method, tumor spheres were obtained. When changing the medium, when the diameter of the tumor spheres is greater than 100 μm, the tumor spheres can be blown ...
Embodiment 3
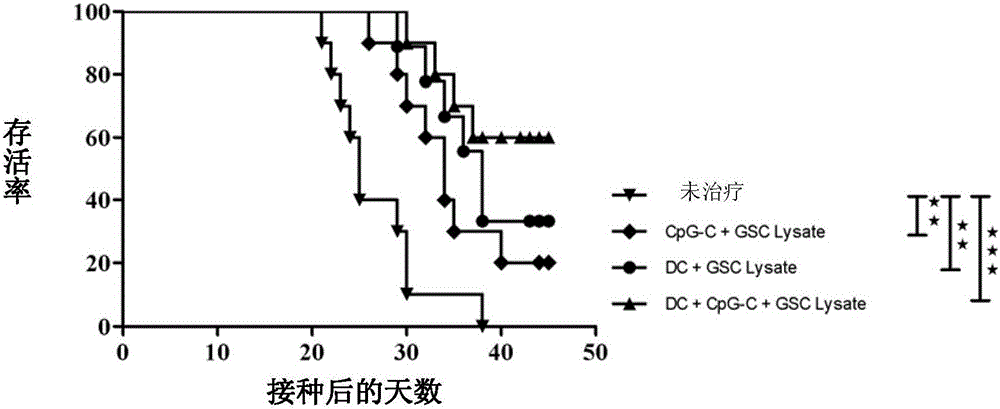
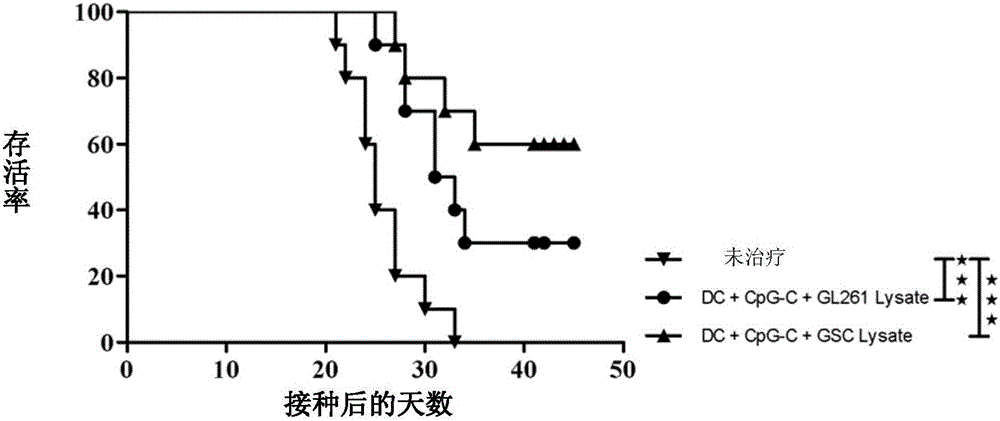
[0039] Embodiment 3: In vivo efficacy detection of the vaccine of the present invention
[0040] In this example, the antigen composition containing glioma stem cell lysates and glioma cell lysates prepared in Example 2, dendritic cells and C-type CpG oligonucleotides (CpG2395, purchased from Invivogen) vaccine was tested for in vivo efficacy; wherein, dendritic cells were isolated from C57BL / 6 mouse bone marrow cells, induced by interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor (GM-CSF), lipopolysaccharide Dendritic cells obtained after (LPS) activation. The vaccine used in this example contains 1000 μg of the antigen composition prepared in Example 2 per milliliter, 4×10 6 dendritic cells and 200 μg C-type CpG oligonucleotides. Each tumor-bearing mouse was injected with 250 μl of vaccine, and 125 μl of vaccine was subcutaneously injected into the shoulders of both sides.
[0041] 50 healthy 6-week-old female SPF-grade C57BL / 6 mice (purchased from B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com