Application of PEG (polyethylene glycol)-PLA (Poly Lactic Acid) nano-material-coated HBV (Hepatitis B Virus)-CpG (Cytosine Phosphate Guanosine) in prevention and/or treatment of hepatitis B
A technology of PEG-PLA and nanomaterials, which is applied in the application field of HBV-CpG coated with PEG-PLA nanomaterials in the prevention and/or treatment of hepatitis B, and can solve the problems of toxic and side effects limiting applications, application limitations, and unfavorable hydrophobicity Insoluble drug release and other issues, to achieve the effect of clearing hepatitis B virus and improving immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
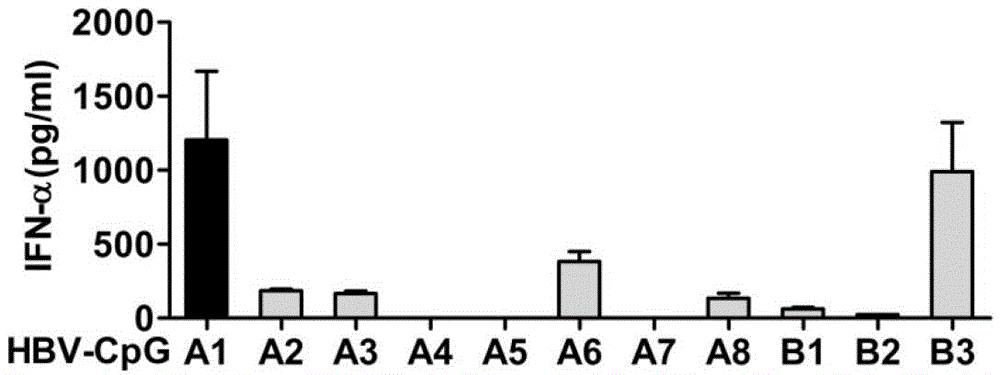
[0050] Embodiment 1: Design and screening of HBV-CpG
[0051] 1. Design of HBV-CpG: According to the characteristics of GenBank HBV gene sequence, a series of CpG-containing oligonucleotide sequences derived from HBV gene were designed and synthesized: A1, A2, A3, A5, A6, A7, A8, B1, B2 and B3, as shown in Table 1, where the capital letters of nucleotides are modified by phosphorothioate bonds. The above sequences were all synthesized by Shanghai Sangon Biosynthesis Co., Ltd.
[0052] Table 1. Design of HBV-CpG
[0053]
[0054] 2. Screening of HBV-CpG:
[0055] (1) Preparation of human peripheral blood mononuclear cells (PBMC):
[0056] Peripheral blood buffy coat was taken from healthy persons (with informed consent) in Hefei Blood Center, Anhui Province, diluted with an equal amount of 1×PBS, and subjected to density gradient centrifugation by Ficoll-Hypaque method, and the mononuclear cells at the interface of the upper and middle layers were absorbed. The white clo...
Embodiment 2
[0062] Embodiment 2: Nanomaterial PEG-PLA coats HBV-CpG
[0063] Weigh 10.0mg of PEG 5000 -PLA 10000 (This laboratory designed and synthesized, and the synthesis method is detailed in references Yang XZ, Dou S, Sun TM, Mao CQ, Wang HX, Wang J. Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. Journal of Controlled Release .2011; 156: 203-11.), supplemented with 1.0 mg cationic lipid BHEM-Chol (designed and synthesized in this laboratory, the synthesis method is detailed in references Yang XZ, Dou S, Sun TM, Mao CQ, Wang HX, Wang J.Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy.Journal of Controlled Release.2011; 156:203-11.) was dissolved in 0.5mL chloroform, and 0.2mg of HBV-CpG was added to the PBS solution ( The volume is 25 μL), ultrasonicated in a probe-type ultrasonic breaker (VC130 type, Sonics, the United States), the output power is 80 watts, and the frequency of ultras...
Embodiment 3
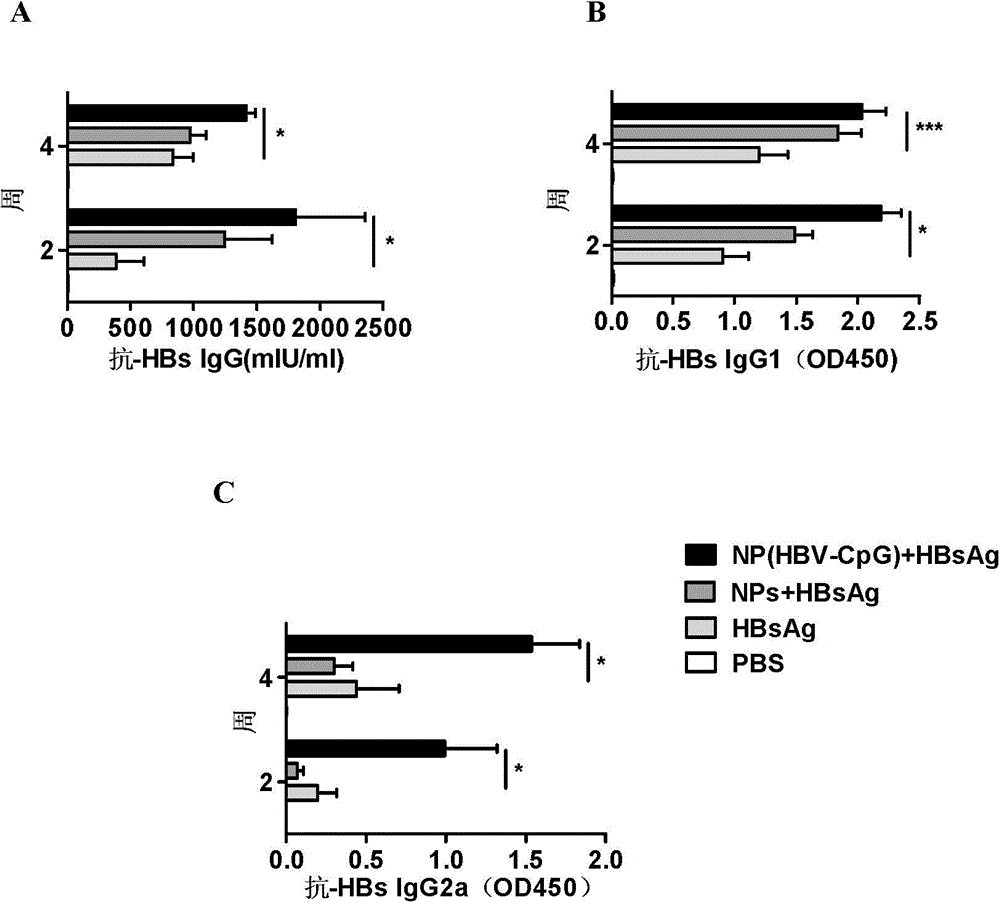
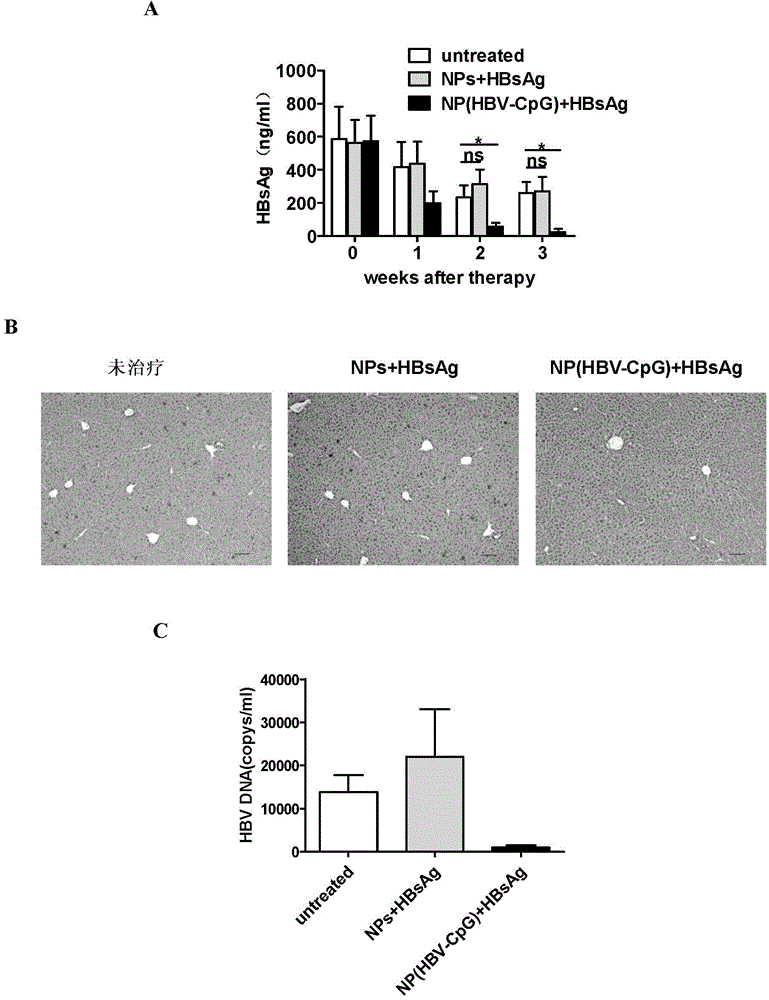
[0064] Embodiment 3: NP (HBV-CpG) synergistically enhances HBsAg vaccine antibody response
[0065] 1. Immunization of experimental animals
[0066]Male BALB / C mice aged 6 to 8 weeks, 20 to 22 g (purchased from Shanghai Slack Experimental Animal Co., Ltd.), were randomly divided into four groups, 6 mice in each group. Group I: PBS control; Group II: HBsAg vaccine alone group: each mouse was immunized with recombinant HBsAg vaccine (purchased from Shenzhen Kangtai Biological Products Co., Ltd.) (2 μg per mouse per injection); Group III: empty nano Carrier combined with HBsAg vaccine group: 500ug PEG-PLA nanomaterial empty carrier (NPs) was mixed with 2 μg of HBsAg to make a homogeneous suspension, and each mouse was immunized; Group IV: NP (HBV-CpG) combined with HBsAg vaccine group of the present invention: 500ugPEG-PLA was coated with 10μg HBV-CpG to form nanoparticle NP (HBV-CpG), and then mixed with 2μg HBsAg to make a homogeneous suspension to immunize each mouse. The mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com