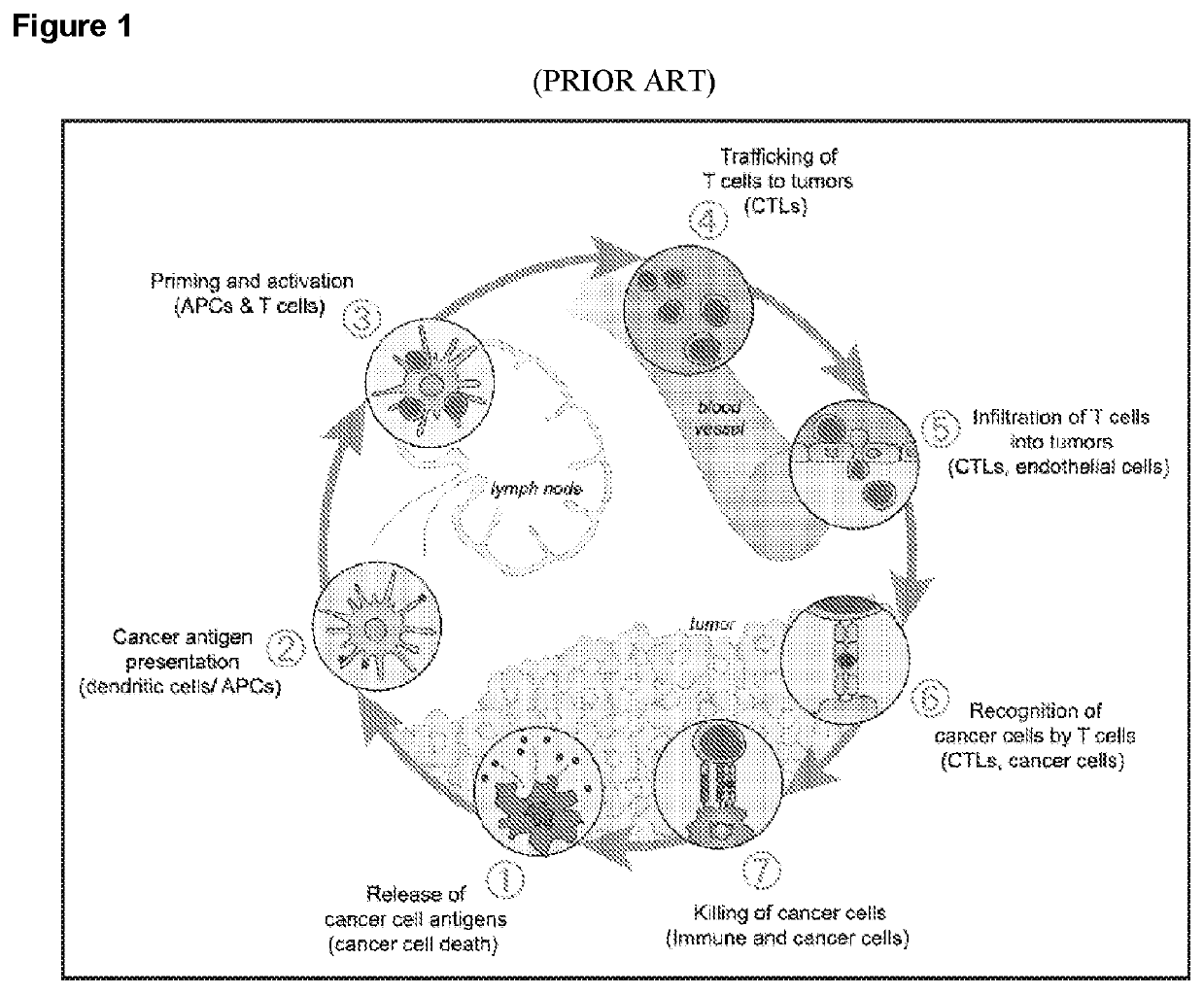
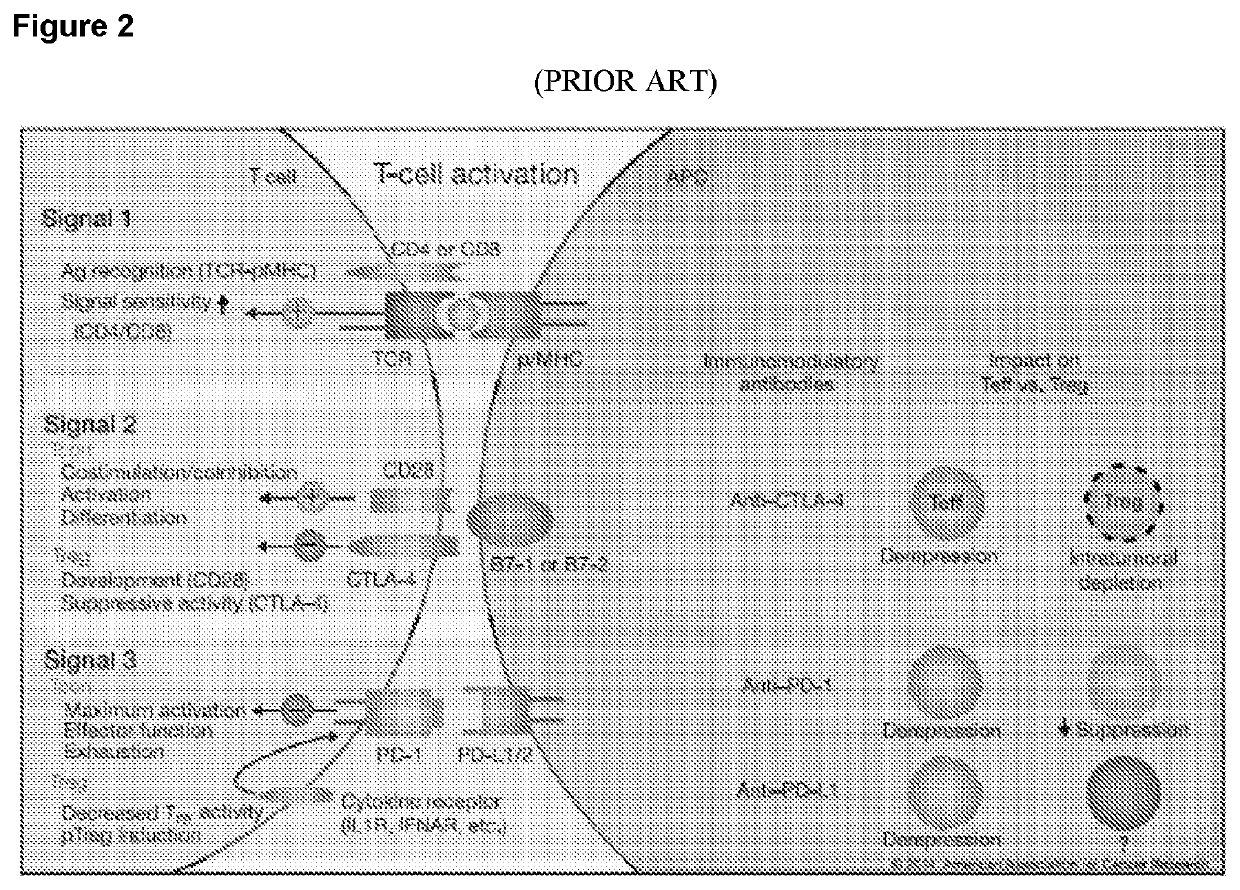
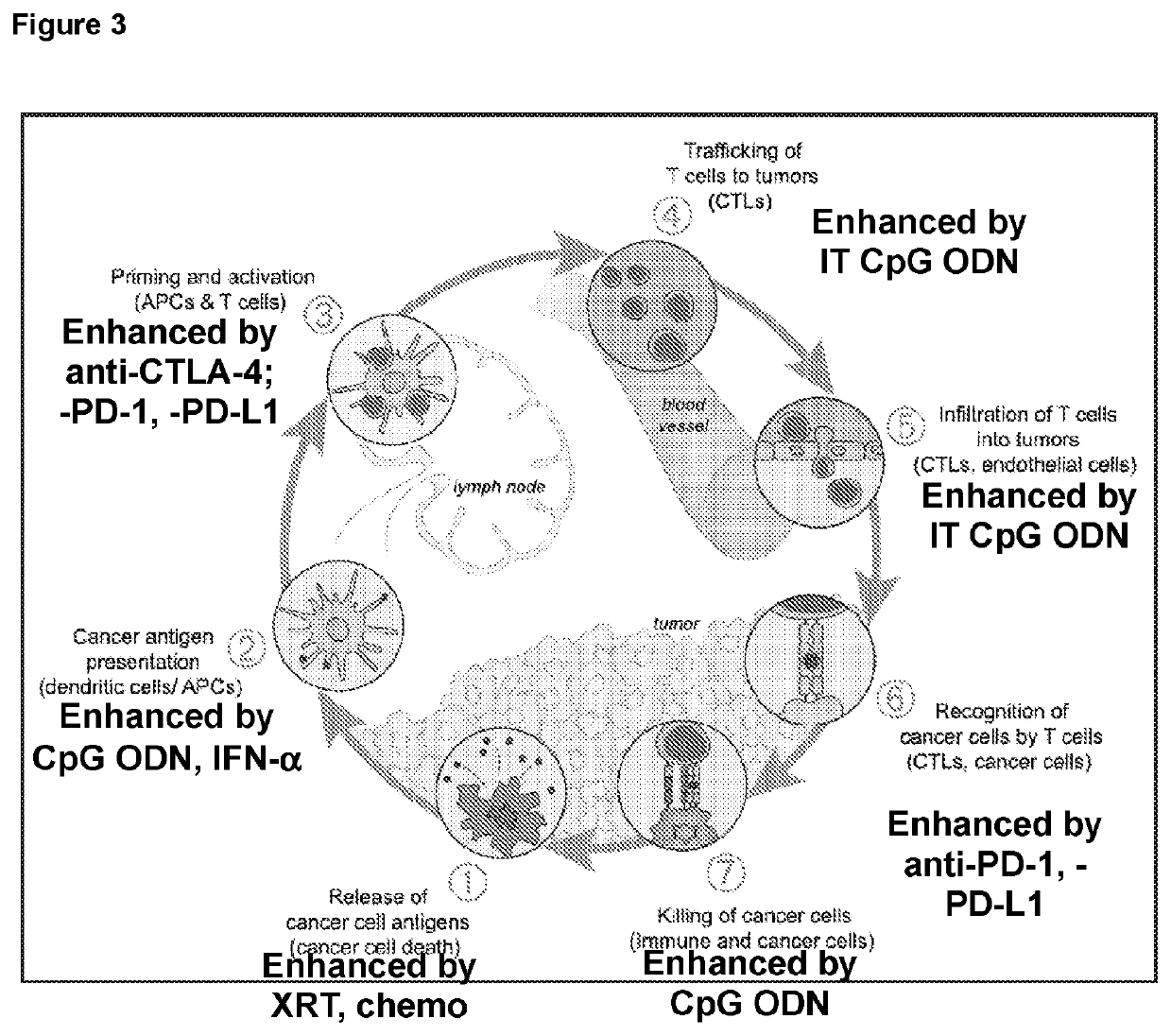
Combination tumor immunotherapy
a tumor immunotherapy and tumor technology, applied in the field of tumor immunotherapy, can solve the problems of insufficient understanding of the immunobiology of cancer, inability to predict which of the many different possible combinations, and rare and limited immune response to immune therapy, so as to facilitate the induction of clinically beneficial anti-tumor immunity, inhibit the immune response to tumors, and enhance the step 2 of the cancer immunity cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0475]In order to achieve optimal synergy for a combination of a CpG ODN and checkpoint inhibitor (+ / −XRT), the CpG ODN should be designed to induce the maximal level of type I IFN possible, with the lowest level of IL-10 possible. Of the CpG ODN classes described above, the closest to this ideal is the A-class. In order to improve the A-class ODN, they can be understood in terms of two semi-independent components: (i) the 5′ and 3′ termini of the A-class CpG ODN, and (ii) the core palindrome. The purpose of the polyG domains in the 5′ and 3′ termini is to form G tetrads that self-assemble into nanoparticles, positioning the palindromes in a favorable way to activate TLR9, and providing a very strong multimerization of TLR9 in the early endosomes, leading to strong IRF3 / 7 activation (and downstream IFN-α secretion) without triggering a more sustained signal that would lead to B cell activation and strong IL-10 production. The G tetrads formed by the polyG domains may also help to st...
example 2
[0494]In vitro experiments were performed to examine the effects of changes in palindrome sequence, number of 5′ and 3′ G, number of 5′ and 3′ phosphorothioate internucleotide linkages, and substitution of 5-iodo-2′-deoxyuridine within the palindromes on IFN-α secretion by human peripheral blood mononuclear cells (PBMCs).
[0495]PBMCs from a normal human donor were cultured in the presence or absence of the indicated ODN in triplicate and results plotted as mean+ / −standard deviation (SD) in FIGS. 4 and 5, for two different human donors. PBMCs were isolated over histopaque-1077 (Sigma) and plated at 1.25×106 / mL, 220 UL / well in RPMI 1640 (10% FBS, glutamine, Pen / Strep) in a 96-well U-bottom tissue culture plate. ODN were added to a final concentration of 5, 1 or 0.2 μg / mL (FIG. 4) or at a lowest concentration of 0.5 pLg / mL (FIG. 5) and cells were incubated for 48 hours. Cells were then spun down and supernatants transferred to new plates and frozen at −20° C. until use. Supernatants wer...
example 3
[0504]In vitro experiments were performed to examine the effects of changes in palindrome sequence, number of 5′ and 3′ phosphorothioate internucleotide linkages, formulation of a native DNA CpG-A ODN in a virus-like particle (VLP), and substitution of 2-O-methyl sugars within the 3′ end of the CpG-A ODN on potency and peak IFN-α secretion by human PBMC.
[0505]Experimental conditions were generally as in Example 2, except that in this case the indicated ODN were cultured with the PBMC in triplicate at the concentrations of 5 μg / mL (concentration or “conc A” in FIGS. 6 and 7); 1 μg / mL (“conc B” in FIGS. 6 and 7) and 0.5 μg / mL (“conc C”) for all of the ODN except for two samples:
[0506]1. The completely PO ODN G10 (labeled as “CYTO003” in FIGS. 6 and 7) was cultured at ODN concentrations of 50 μg / mL (“conc A” in FIGS. 6 and 7), 10 μg / mL (“conc B”) and 2 μg / mL (“conc C”); and
[0507]2. Samples labeled as “CytQbAb” in FIGS. 6 and 7 contained the G10 ODN packaged within a virus-like particle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| equilibrium dissociation constant | aaaaa | aaaaa |
| equilibrium dissociation constant | aaaaa | aaaaa |
| equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com