CpG nucleic acid medicine conveying system with pH response and preparation method thereof
A nucleic acid drug and delivery system technology, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and drug combinations, etc. Effects of uptake, enhanced immune response, controlled release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
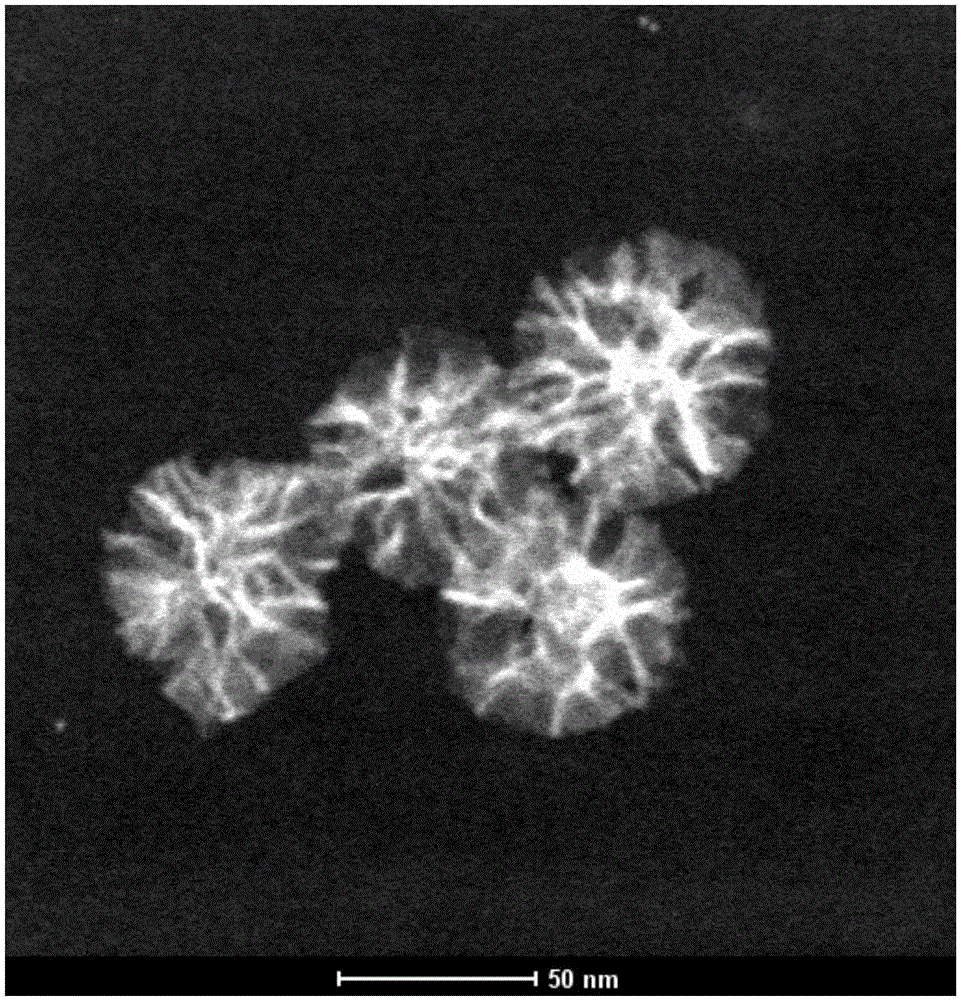
[0040] The first embodiment is a preparation experiment of the pH-responsive CpG nucleic acid drug delivery system provided by the present invention.
[0041] In this embodiment, the CpG nucleic acid drug used is a CpG oligodeoxynucleotide modified with a 5' terminal amino group containing 72 base pairs, and its sequence is:
[0042] 5'-NH2-(CH2) 6 - TCAGAGAGTTAGAGAGTTAGAGAGTCAGAGAGTTAGAGAGTTAGAGAGTCAGAGAGTTAGAGAGTTAGAGAG-3'.
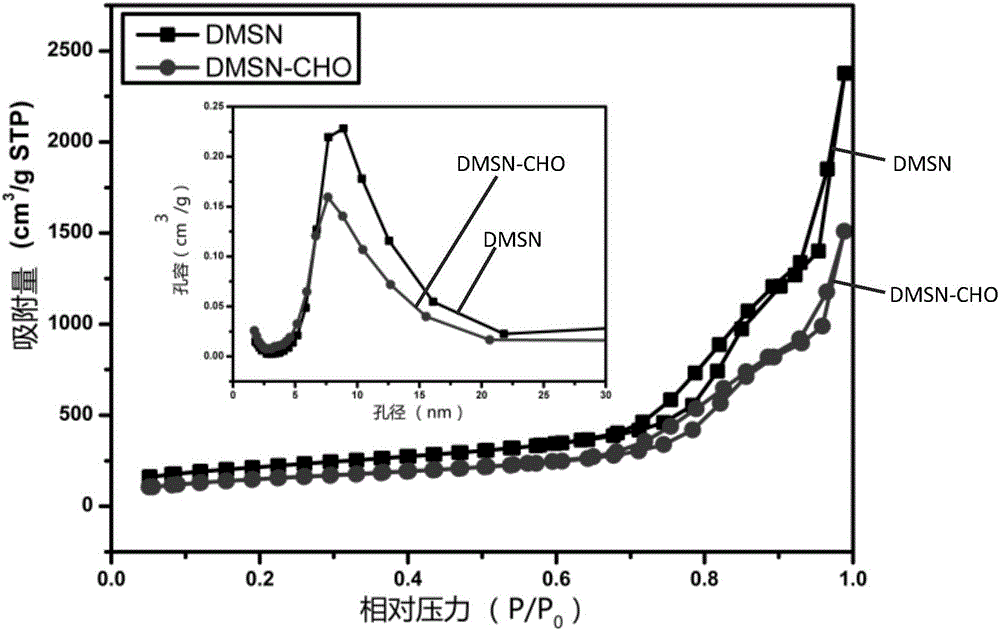
[0043] In this embodiment, the aldehyde-based silane coupling agent used is triethoxysilyl n-butyraldehyde silane, the silicon source is tetraethyl orthosilicate, and the surfactant is cetyltrimethylammonium bromide. The cosolvent is triethanolamine, and the buffer is phosphate buffer at pH 8.1.
[0044] The preparation steps of the pH-responsive CpG nucleic acid drug delivery system provided by the present invention are as follows:
[0045] Step 1: Add 1g of cetyltrimethylammonium bromide and 0.18g of triethanolamine into 60mL of deionized water, ke...
Embodiment 2
[0063] The carrier particles prepared in Example 1 were used for cytotoxicity measurement, and the standard CellCounting Kit-8 method was used for the measurement method. In this example, the macrophage cell line RAW264.7 was purchased from Riken, Japan, and cultured according to the method provided by the supplier.
[0064] The specific experimental process is as follows:
[0065] The carrier particles prepared in Example 1 were dispersed in MEM medium and formulated into a suspension with a concentration of 1 mg / mL. After the RAW264.7 cells were seeded in a 96-well plate (the cell density was 5000 cells / well), the above-mentioned Suspensions of carrier particles were added to 96-well plates at final concentrations of 0, 25, 50, 75, 100 and 200 μg / mL, respectively, and the solution volume was 100 μL.
[0066] Five different concentrations of carrier particle suspensions and the blank control group without carrier particles were co-cultured with the cells for 24 hours, and th...
Embodiment 3
[0072] In this example, a fluorescent labeling method was used to conduct a cell uptake experiment on the CpG nucleic acid drug delivery system prepared according to the method provided in Example 1.
[0073] In this example, the fluorescent marker FITC is used to modify the CpG nucleic acid drug in Example 1 to obtain the FITC-modified CpG nucleic acid drug. Then use the FITC-modified CpG nucleic acid drug as a CpG nucleic acid drug, and prepare a FITC-modified CpG nucleic acid drug delivery system according to the preparation method provided in Example 1.
[0074] will be 1.5×10 4 RAW264.7 cells were seeded in a 35mm glass-bottom culture dish, and after 24 hours of culture, the FITC-modified CpG nucleic acid drug delivery system was added to the culture dish with a final concentration of 100 μg / mL. After 4 hours, the cells were washed twice with buffer and fixed with 4% (mass percent) formaldehyde solution for 10 minutes; Cells were treated at room temperature for 10 minut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com