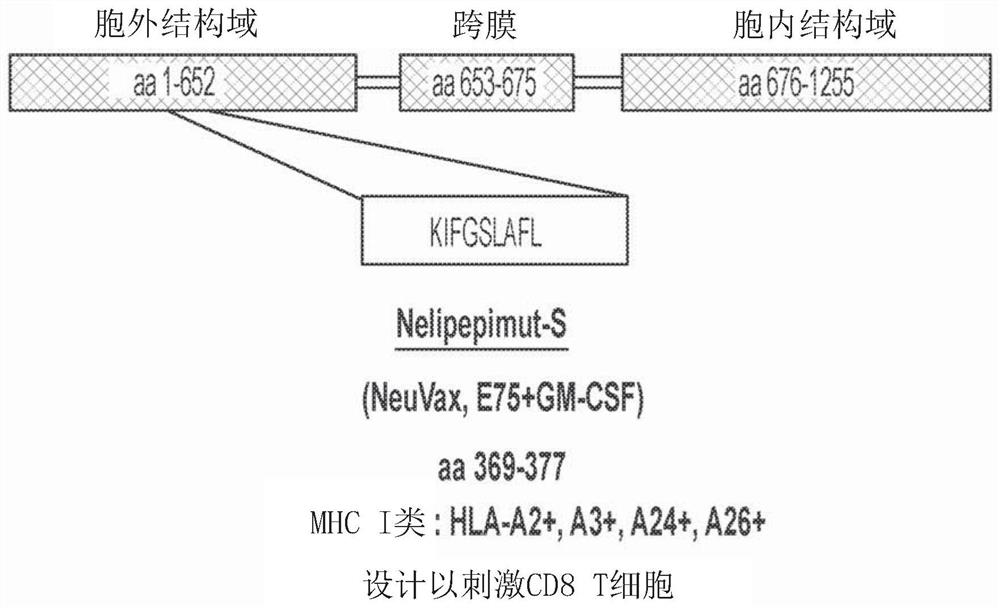
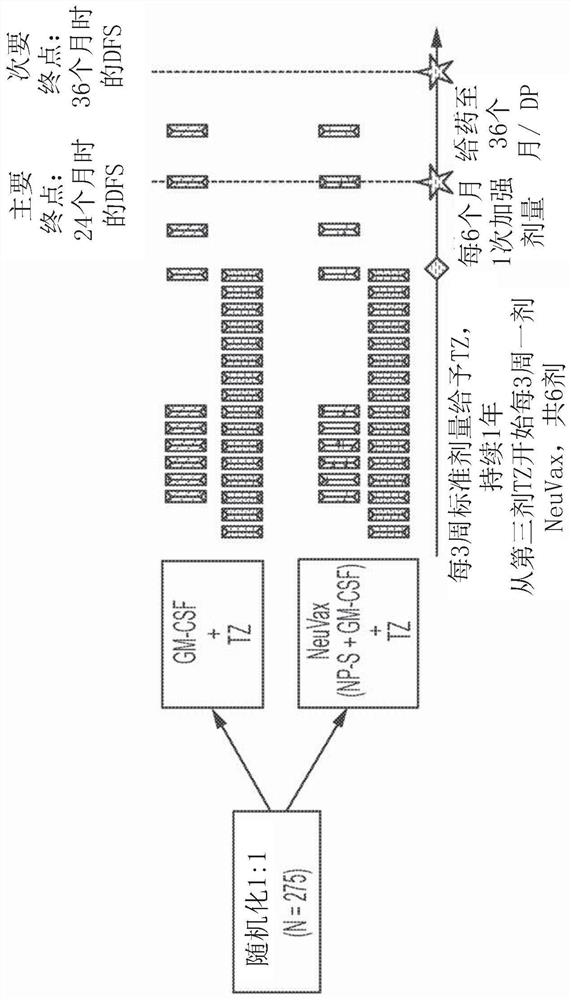
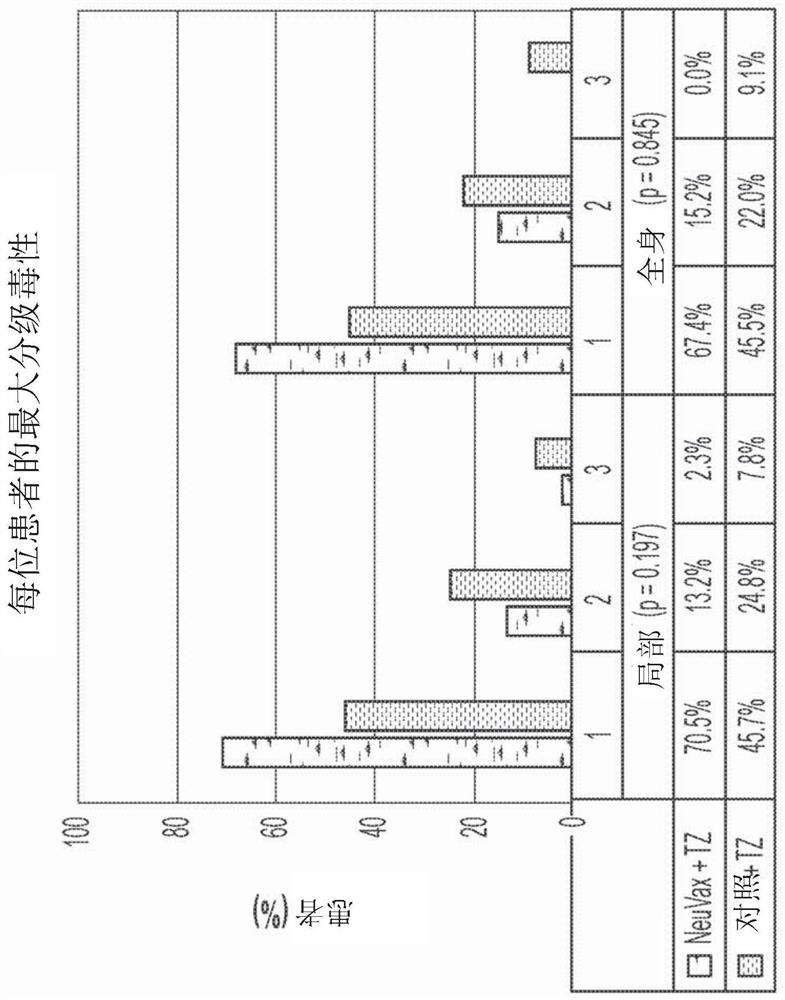
Combination immunotherapy for treatment of triple-negative breast cancer
A triple-negative breast cancer and therapeutic agent technology, applied in breast cancer vaccines, immunoglobulins, anti-animal/human immunoglobulins, etc., can solve problems such as no proven effective targeted therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] Example 1 - Trastuzumab plus Nelipepimut-S (NEUVAX) versus Trastuzumab for the prevention of relapse Prespecified interim analysis comparison of randomized phase 2b trial showing benefit in triple negative (HER2 low expressing) breast cancer patients place .
[0172] Breast cancer with low expression of human epidermal growth factor receptor 2 (HER2) (immunohistochemistry (IHC) 1-2+) is not suitable for adjuvant trastuzumab (TZ) therapy. NSABP B-47 demonstrated that trastuzumab does not improve prognosis in breast cancer with low HER2 expression (Fehrenbacher et al., SABCS, 2017, Abstract GS1-02). These patients currently do not have access to HER2-targeted therapies. TZ is the best known example of successful targeted therapy of cancer. 15-20% of BC patients are eligible to receive TZ because they are HER2 overexpressed (amplified by IHC3+ or FISH).
[0173] Efforts to expand the use of trastuzumab have been undertaken previously. More recently, the NSABP B-47...
Embodiment 2
[0218] Example 2—Activity of NPS in HLA-A24+ triple negative (HER2 low expression) breast cancer patients
[0219] In patients in the triple-negative breast cancer (TNBC) cohort (n=97) of this study (Example 1), aggressive treatment (NPS plus trastuzumab) benefited all HLA types (see Figure 8 , showing a sample plot of hazard ratios for patients of various HLA subtypes in the trial, where the overall hazard ratio [H.R.] for the TNBC cohort—across all HLA types—was an impressive 0.29). However, subgroup analysis of this panel showed that the H.R. was lowest in HLA-A24+ patients with a striking H.R. value of 0.08 and a p-value of 0.003. HLA-A24 positivity was particularly associated with Asian / Pacific Basin populations.
[0220] exist Figure 9 In , DFS of HLA-A24+TNBC patients is shown. In a later subgroup of TNBC patients, the NPS plus trastuzumab combination caused relapse at 24 months relative to the control group (trastuzumab only) in the 24-month DFS landmark analysi...
Embodiment approach 1
[0247] Embodiment 1. A method for treating triple negative breast cancer (TNBC) in an individual comprising administering to the individual: (a) an effective amount of trastuzumab or a derivative thereof; and (b) An effective amount of nelipepimut-S or a variant thereof.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com