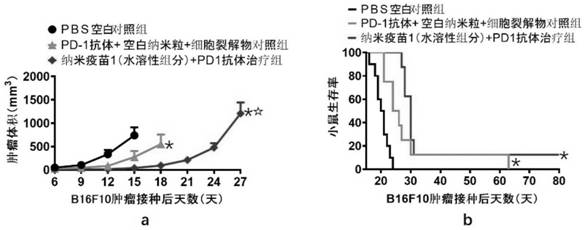
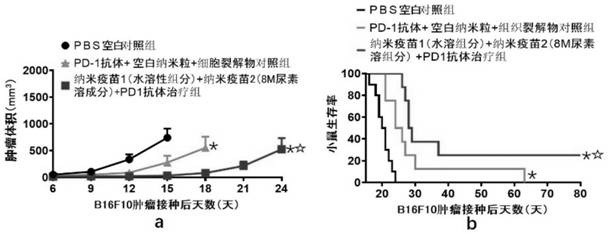
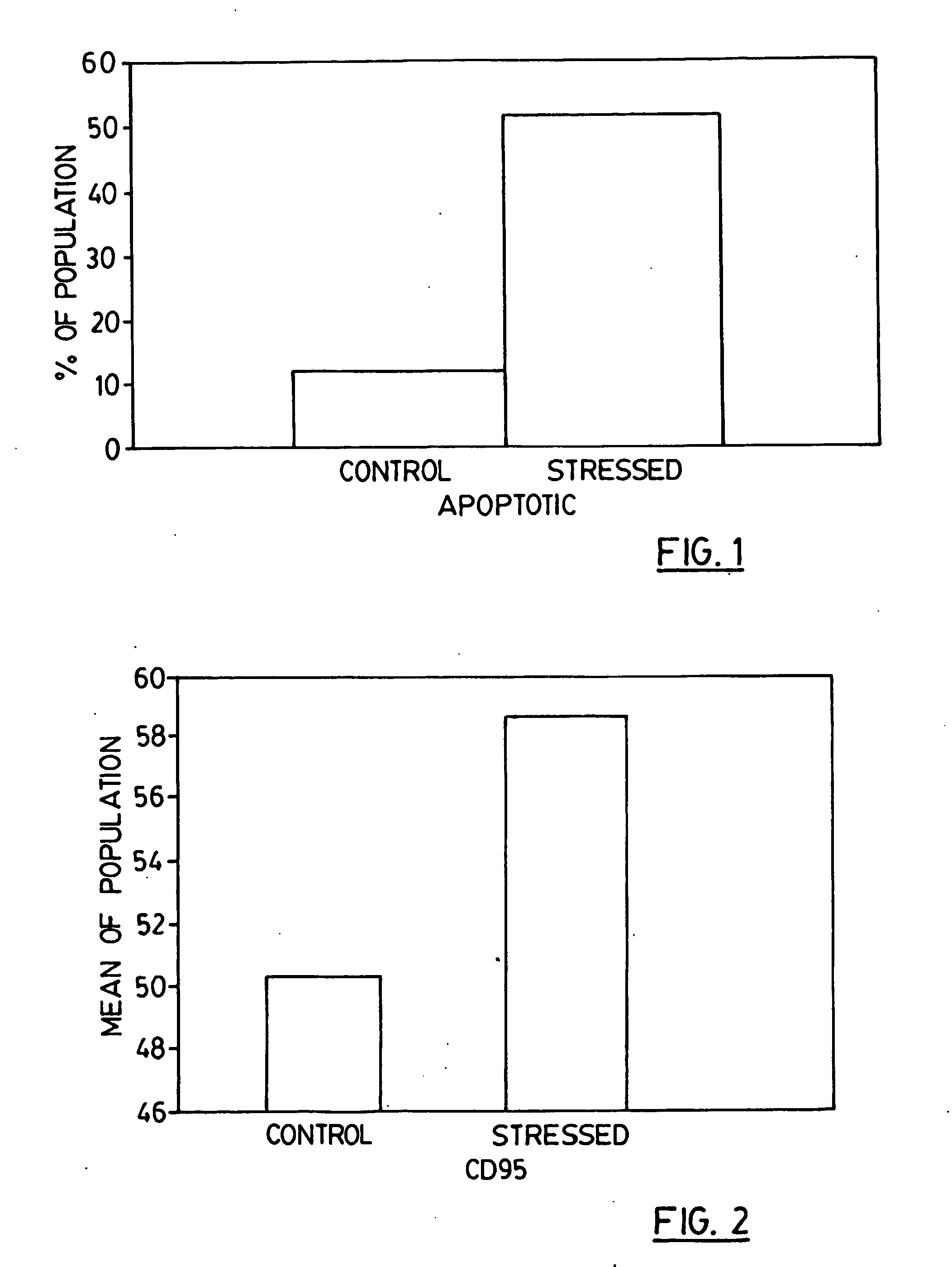
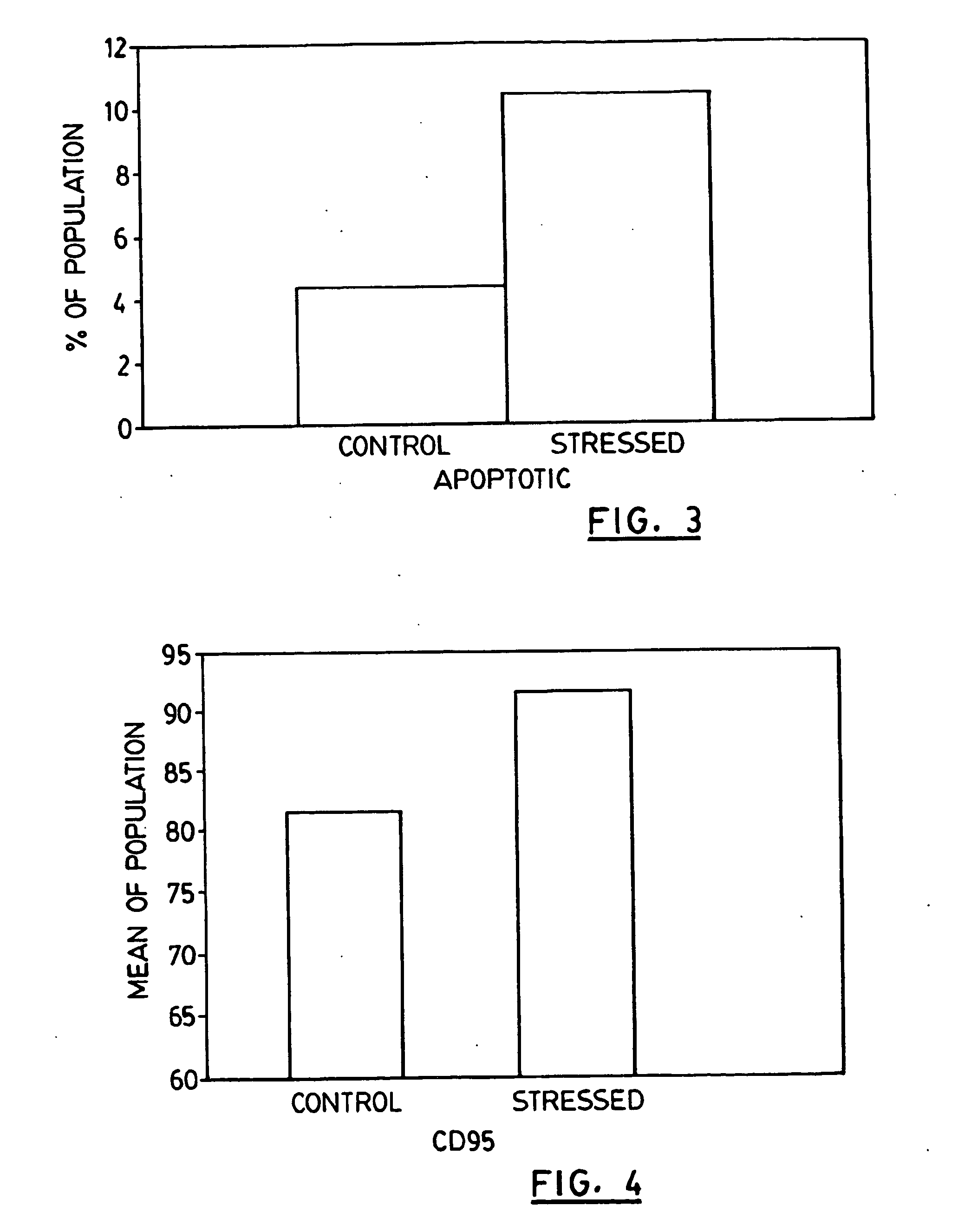
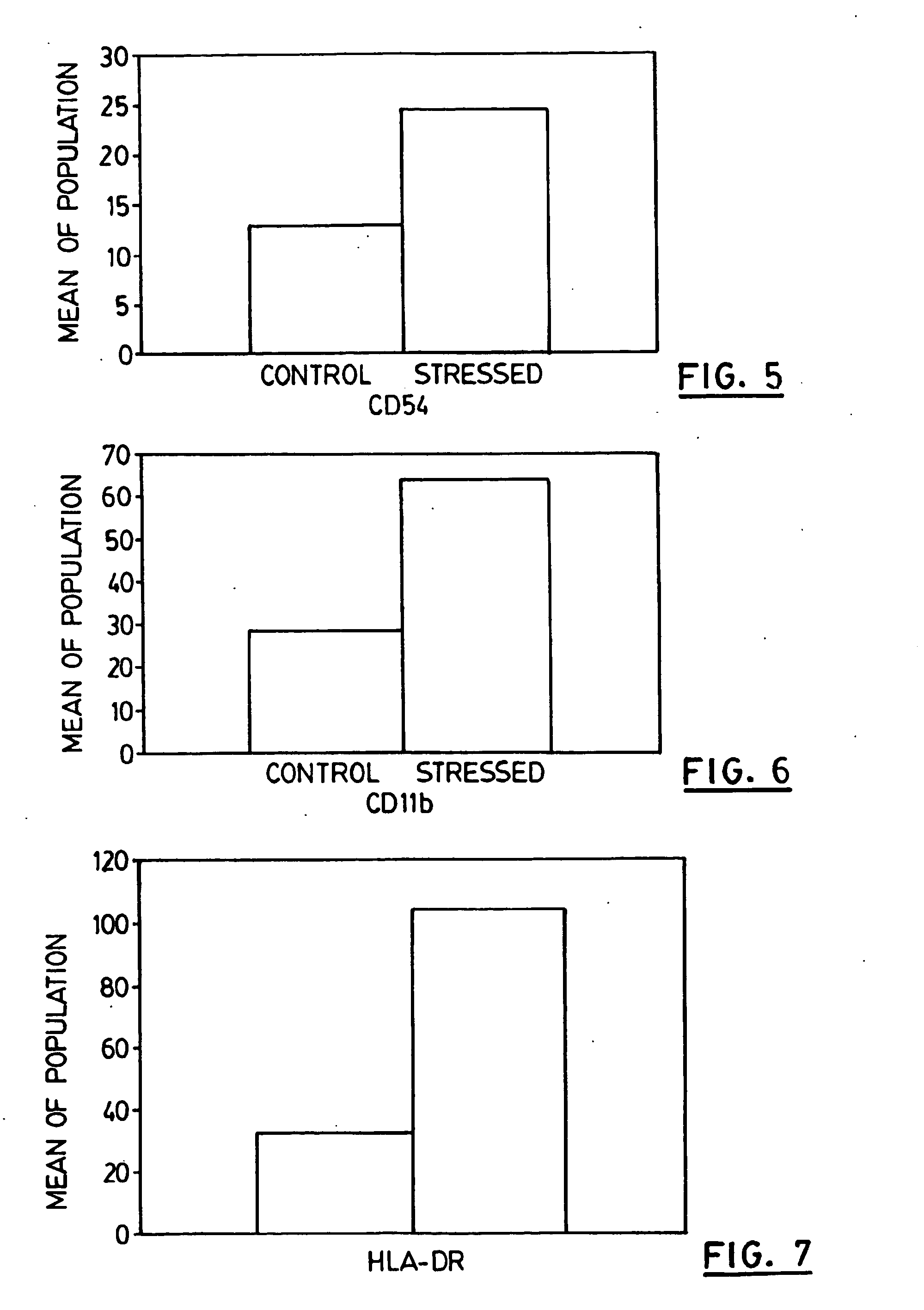
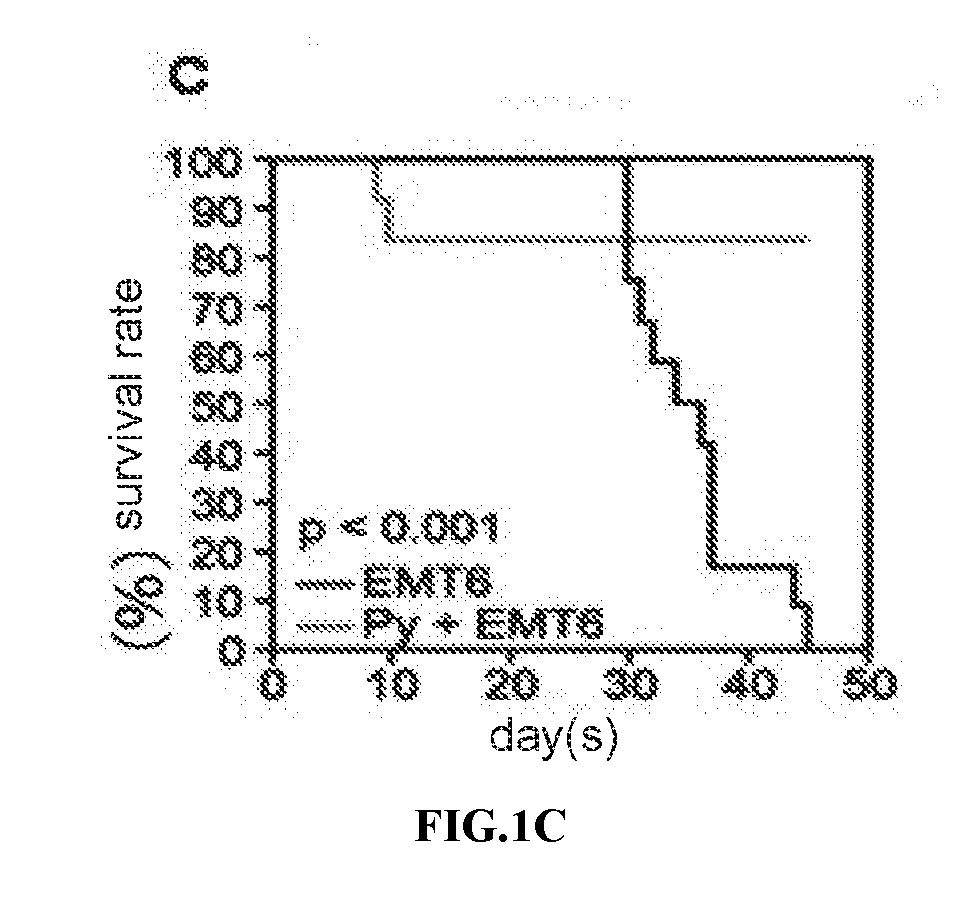
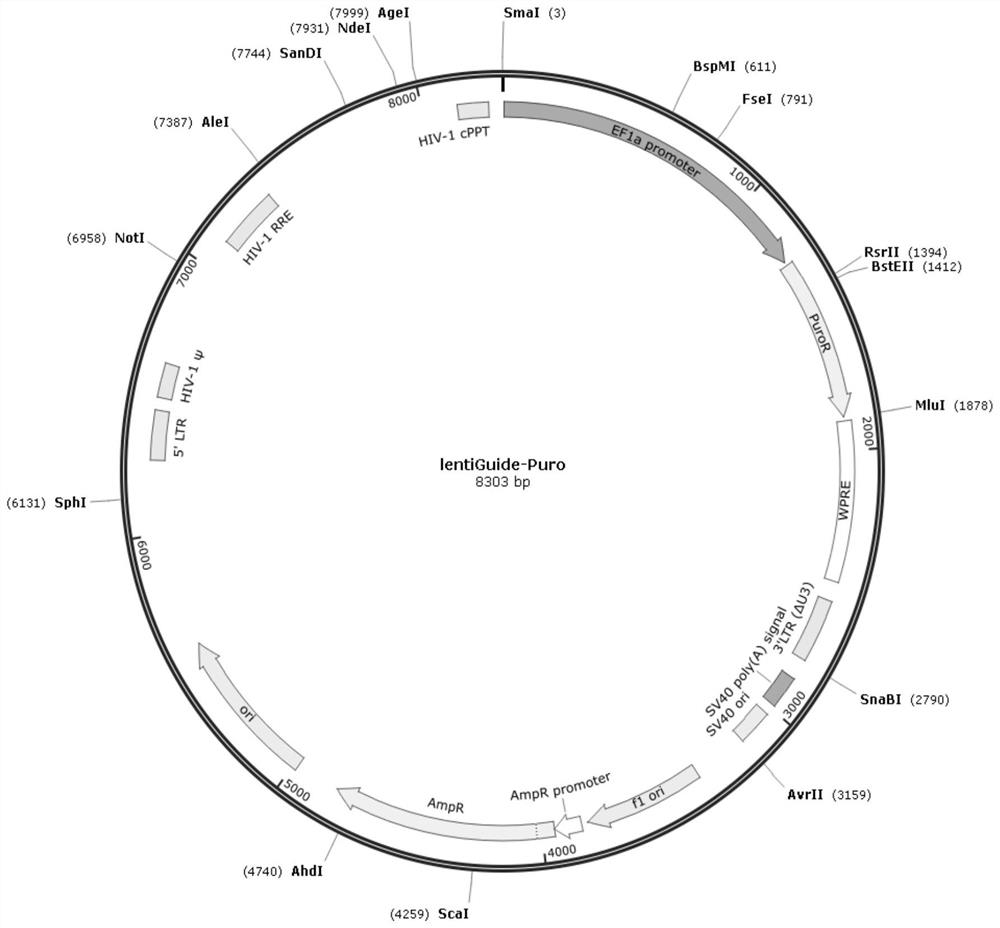
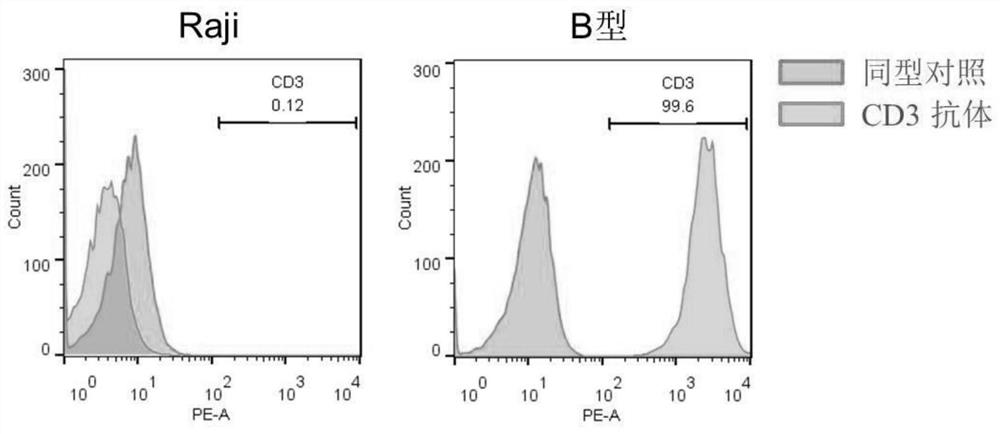
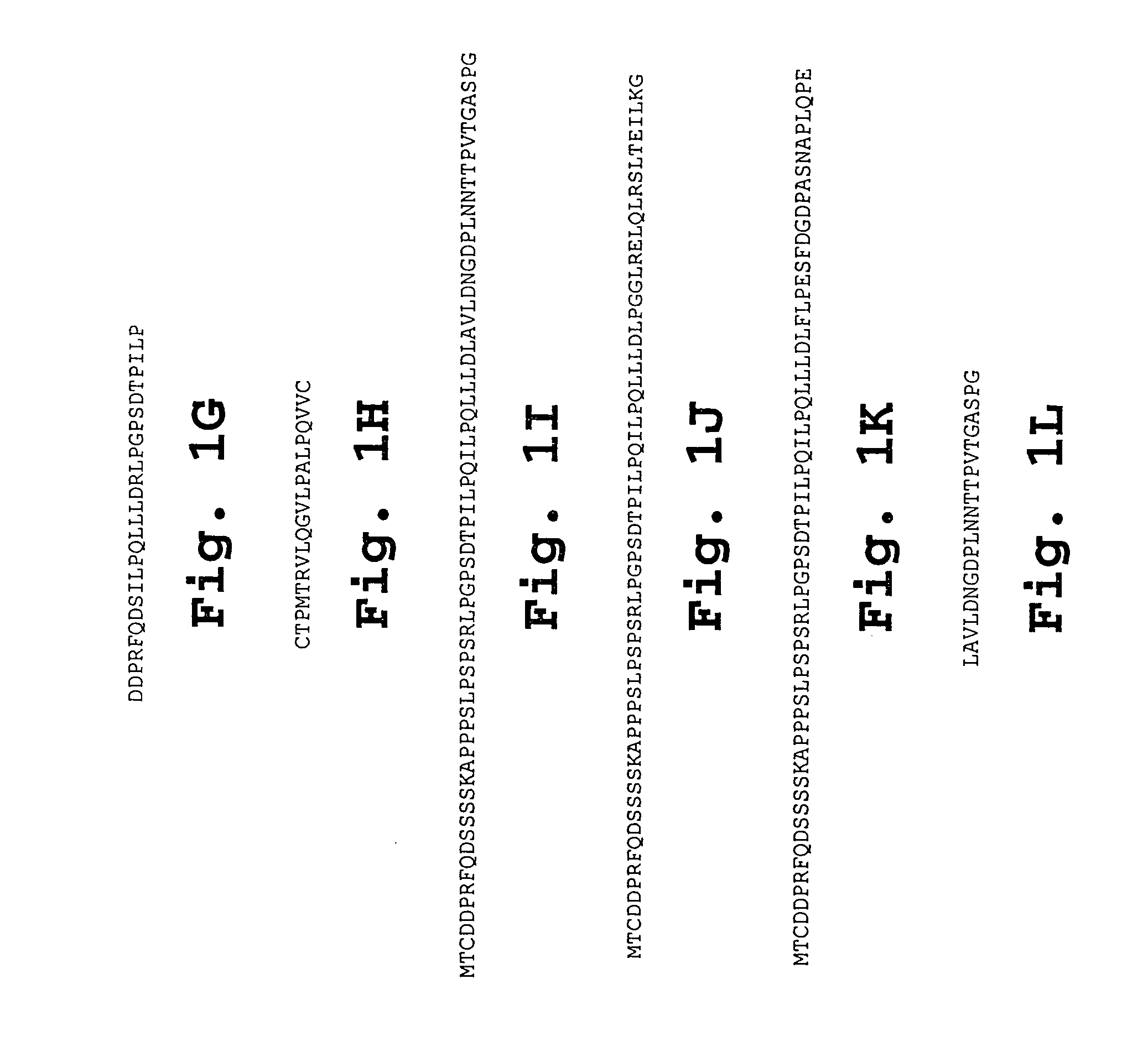
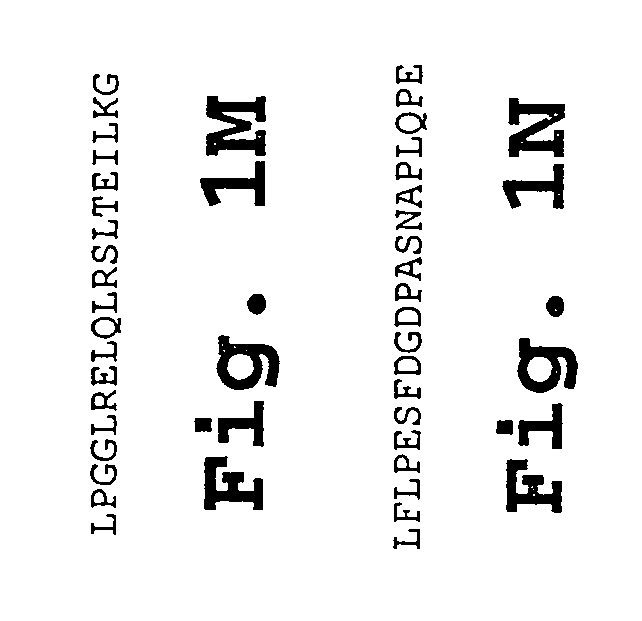
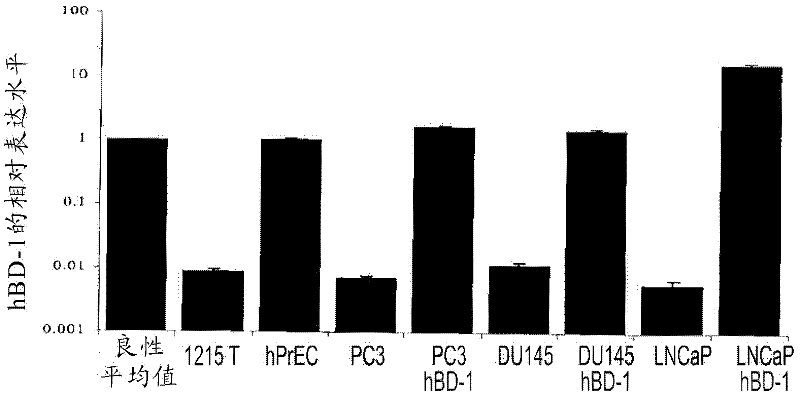Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
141results about "Breast cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
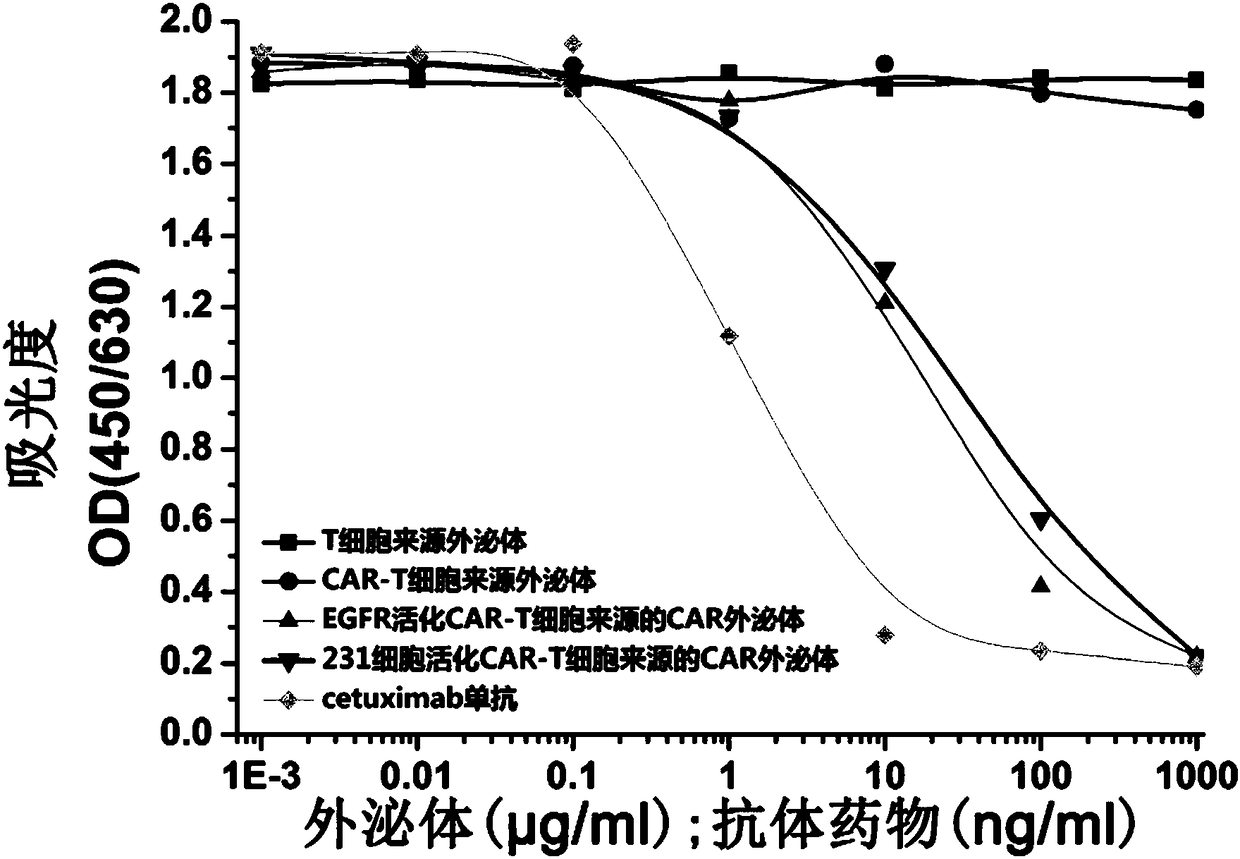
Preparation method of immune cell exosome carrying chimeric antigen receptor and application thereof
ActiveCN108315305AEnhanced tissue penetrationAbility to overcome adverse reactionsOrganic active ingredientsMammal material medical ingredientsAntigenDisease
The invention relates to the field of biological medicine, in particular to a preparation method for obtaining immune cell exosome carrying a CAR through separation. Specifically CAR immune cell is activated through specific antigen, produced exosome is further analyzed, separated, purified and enriched, and finally the immune cell exosome carrying the CAR is obtained. The exosome can be applied to the treatment of various diseases, such as cancer and severe infectious diseases, overcomes adverse reactions, such as immune inflammation storm, during CAR cell therapy, enhances the CAR tissue infiltration capacity, further has the advantages of high convenience in storage and transportation, and provides a novel strategy for the treatment of relevant diseases.
Owner:PHARCHOICE THERAPEUTICS INC
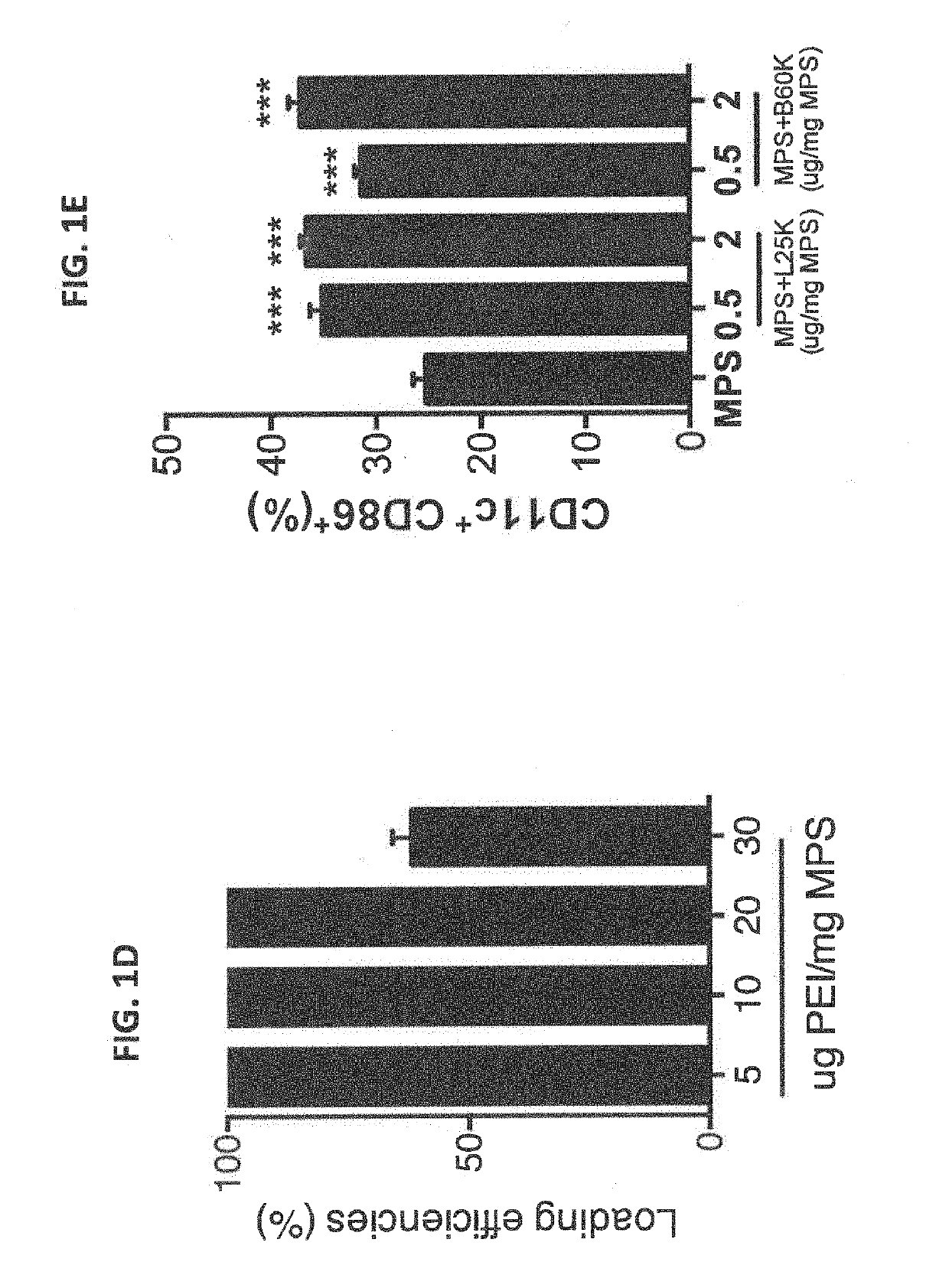
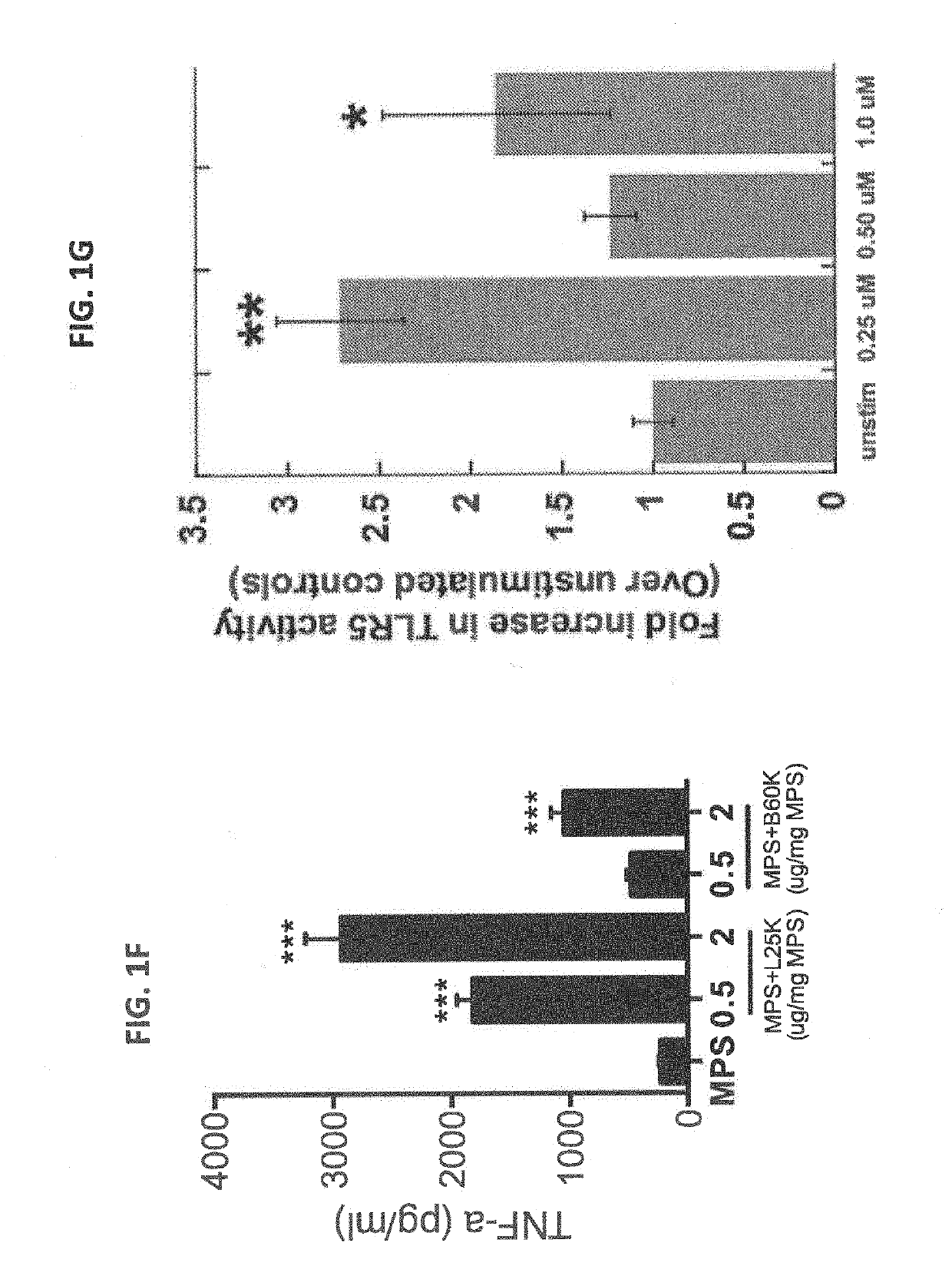
Biomaterials for modulating immune responses
PendingUS20190216910A1Improving immunogenicityReduce the burden onInorganic non-active ingredientsSkin cancer vaccineMicrobiologyBiological organism
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
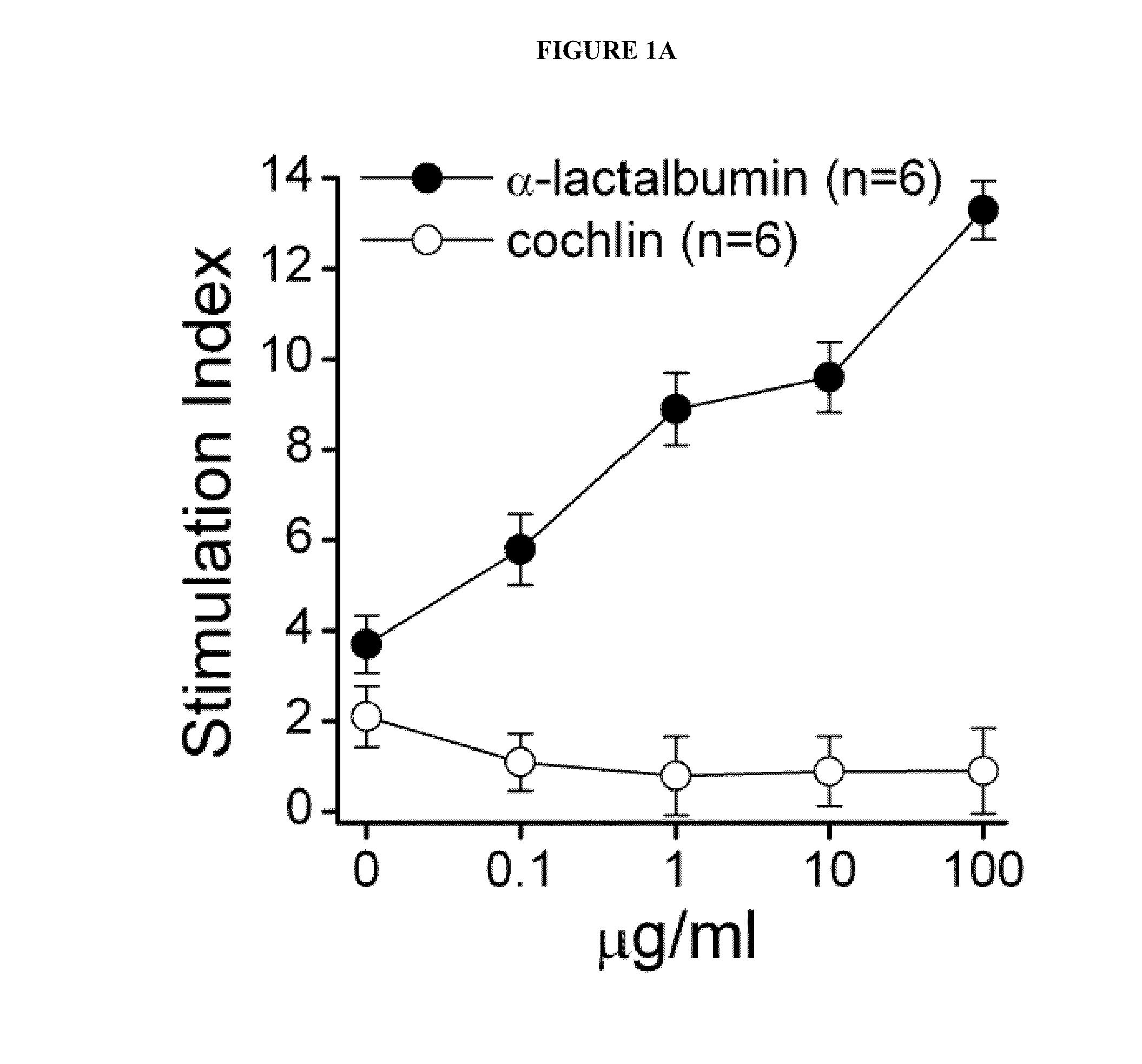
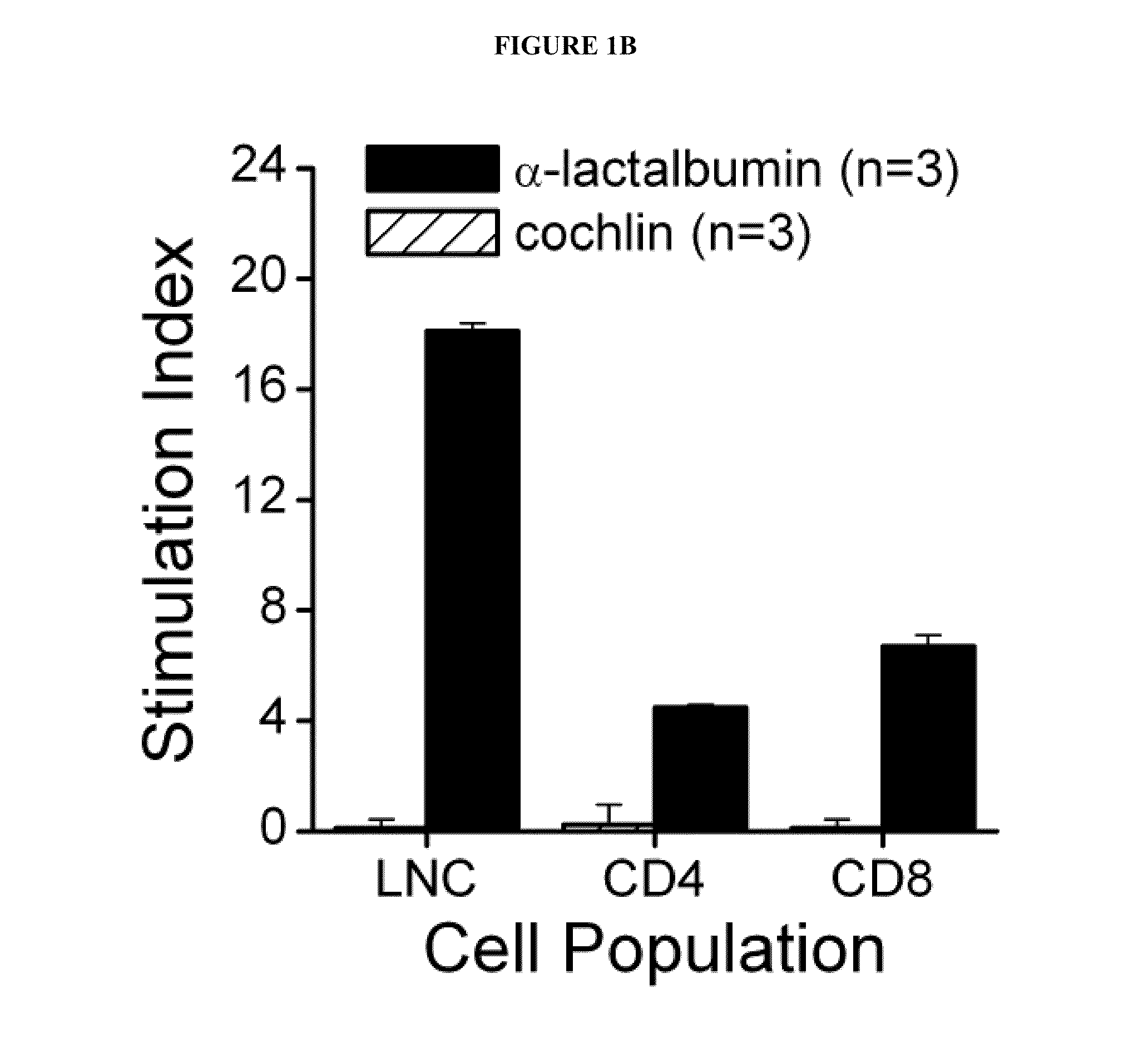
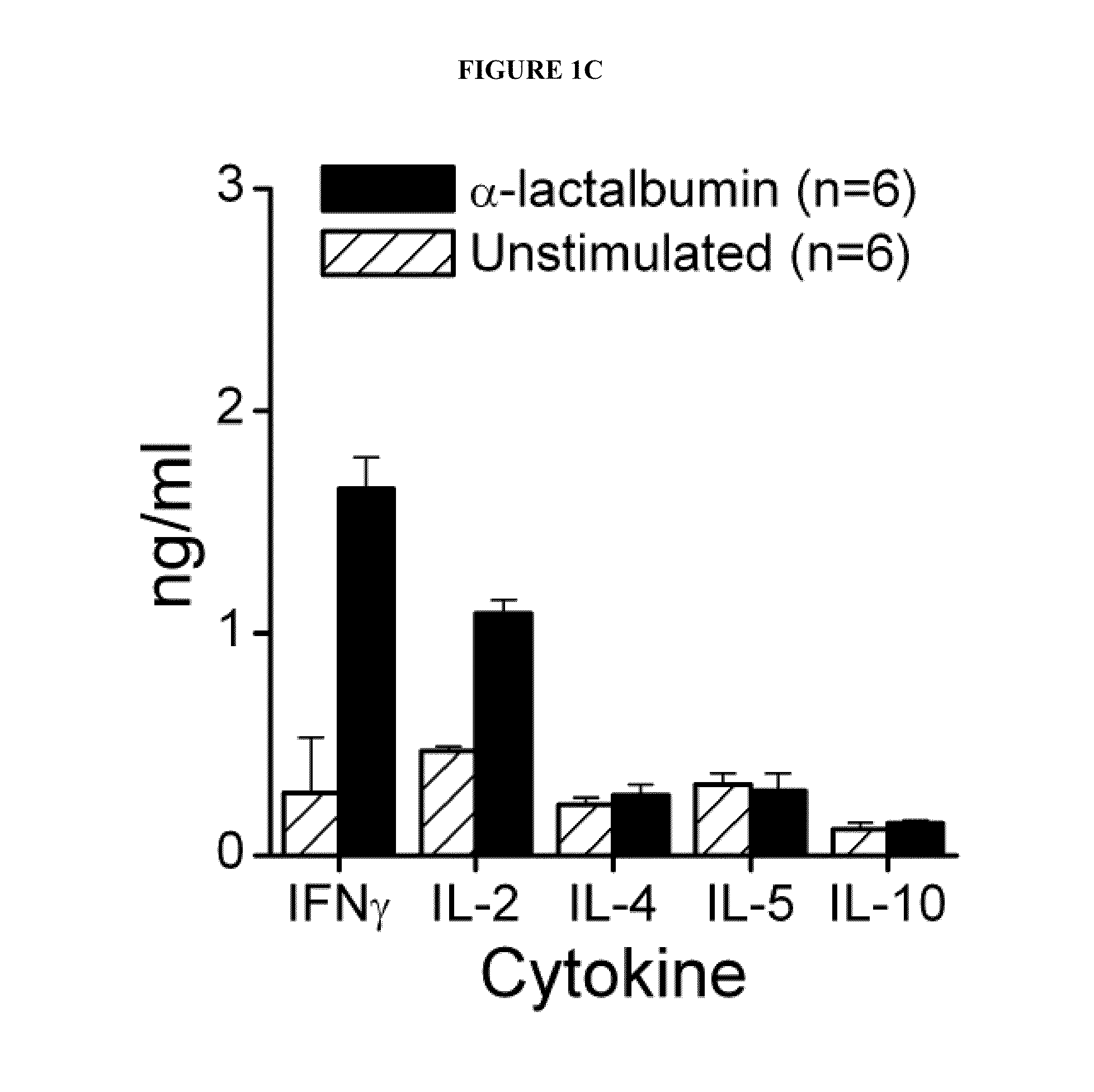
Breast Cancer Vaccine
ActiveUS20120003254A1Safety protectionEasily avoidableArtificial cell constructsCancer antigen ingredientsLactalbuminHuman breast
Compositions and methods for immunization against human breast cancer are disclosed. A breast cancer vaccine comprises an immunogenic polypeptide comprising human α-lactalbumin.
Owner:THE CLEVELAND CLINIC FOUND
Composite superimmunogen for bi-functional vaccine use for the treatment of illnesses associated with a stromal tissue disorder
The invention is relative to novel means of systemic or mucosal vaccinial therapy against some cancers, viral infections and allergy which are provided by the invention under the form of a family of composite superimmunogenic compounds for bifunctional vaccinial use able to induce an immune response raised towards two distinct targets, respectively, the causal pathogenic antigenic structure, on the one hand, and locally produced factors responsible for a subsequent immunotoxic or neoangiogenic stroma disorder, on the other hand.
Owner:NEOVACS SA
Chitosan-Derived Compositions
InactiveUS20190002594A1Organic active ingredientsPhotodynamic therapyMedical disorderKidney Neoplasm
The present invention relates generally to therapeutic compositions comprising chitosan-derived compositions used in connection with methods for treating neoplasms, such as for instance, malignant lung, thyroid and kidney neoplasms, and other types of malignant neoplasms, and other medical disorders.
Owner:IMMUNOPHOTONICS INC
Chimeric antigen receptor, NKG2D CAR-NK cell expressing chimeric antigen receptor, preparation method and application thereof
ActiveCN110028589AImprove anti-tumor effectExcellent anti-tumor performanceVirusesAntibody mimetics/scaffoldsDiseaseNkg2d ligands
The invention provides a chimeric antigen receptor, a NKG2D CAR-NK cell expressing the chimeric antigen receptor, a preparation method and an application thereof. The chimeric antigen receptor comprises an antigen binding domain, a transmembrane domain, and a costimulatory signaling domain, and the antigen binding domain is capable of specifically binding to a tumor specific antigen NKG2D ligand and activating NK cells through the transmembrane domain and the costimulatory signaling domain. The CAR-NK cell uses the NKG2D ligand as a target antigen and specifically uses the NKG2D CAR-NK cells to kill tumor cells. The cell can be used as a therapeutic drug for tumor diseases for the treatment of tumors with high expression of the ligand of NKG2D molecules, and provides a novel method for tumor prevention and treatment.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
Humanized anti-Periostin monoclonal antibody as well as preparation method and application of humanized anti-Periostin monoclonal antibody
InactiveCN110551214ASmall toxicityBiologically activeImmunoglobulins against animals/humansAntibody ingredientsComplementarity determining regionHeavy chain
The invention discloses a humanized anti-Periostin monoclonal antibody as well as a preparation method and application of the humanized anti-Periostin monoclonal antibody, and belongs to the technicalfield of medicines. The humanized anti-Periostin monoclonal antibody comprises a light chain complementary determination zone and a heavy chain complementary determination zone, wherein an amino acidsequence of the light chain complementary determination zone is shown in SEQ ID NO.1-3, and an amino acid sequence of the heavy chain complementary determination zone is shown in SEQ ID NO.4-6. The invention also discloses the preparation method and the application of the humanized anti-Periostin monoclonal antibody. Besides the specificity being combined with Periostin proteins and Periostin positive tumor cells, the humanized anti-Periostin monoclonal antibody provided by the invention also has the humanization property and also can reduce toxic side effects of human application.
Owner:杨澜
Whole-cell component conveying system and application thereof
The application belongs to the field of immunotherapy and discloses a conveying system for conveying water-soluble components and non-water-soluble components of whole-cell components by using nano-scale or micron-scale particles and application of the conveying system in preparation of vaccines for preventing and treating cancers. The whole-cell component conveying system is composed of the nano-scale or micron-scale particles and the whole-cell components loaded by the particles, wherein the whole-cell components are water-soluble components and non-water-soluble components of whole cells in cells or tissues. The water-soluble components and the non-water-soluble components are loaded in the nano particles or the micron particles, so that variant proteins or polypeptides generated by the cancers in the cell components are loaded in the nano particles or the micron particles. Substances with immunogenicity, which are generated due to disease mutation, in the whole-cell components can be used for preventing and treating the cancers. The whole-cell component conveying system can be used for preparing the vaccines for preventing and / or treating the cancers.
Owner:SUZHOU ERSHENG BIOPHARMACEUTICAL CO LTD
Chitosan-Derived Compositions
The present invention relates generally to therapeutic compositions comprising chitosan-derived compositions used in connection with methods for treating neoplasms, such as for instance, malignant lung, thyroid and kidney neoplasms, and other types of malignant neoplasms, and other medical disorders.
Owner:IMMUNOPHOTONICS INC
Compositions and methods for selected tumour treatment
InactiveUS20050063995A1Reduce decreaseShorten the progressEnergy modified materialsColon cancer vaccineAbnormal tissue growthMelanoma
Disclosed are novel compositions, methods and vaccines, which upon administration to a patient suffering from a melanoma, colon carcinoma tumor or breast cancer, postpone and / or reduce the need for chemotherapy treatment, slow the progression of or eliminate the tumor and / or alleviate the symptoms of the tumor. The compositions comprise stressed colon carcinoma, melanoma or breast cancer cells, preferably autologous such cells.
Owner:VASOGEN IRELAND LTD
A Composition, A Treatment Method and An Application Thereof
InactiveUS20190015458A1Good effectImprove efficiencyColon cancer vaccineProtozoa material medical ingredientsMelanomaTreatment field
The present invention relates to the field of treatment of tumor, and especially to a composition comprising a plasmodium, a treatment method and an application thereof. The composition of the present invention has therapeutic effects on colorectal carcinoma, lung carcinoma, breast carcinoma, gastric carcinoma and hepatic carcinoma etc., can inhibit the growth of tumor and prolong the life of the tumor patients, whereas has no therapeutic effect on melanoma and lymphoma; meanwhile, the present invention describes that the long-term plasmodium infection has better therapeutic effect on tumors, and the plasmodium immunotherapy of the present invention does not take the fever time as a course standard when treating tumors, but should be used to extend the duration of plasmodium infection as much as possible until the progression of tumors can be controlled under the premise of protecting the organ functions and life safety of the patients.
Owner:BLUE ELEGANT BIOTECH CO LTD
Specific chimeric antigen receptor T cell targeting nkg2dl, preparation method and application thereof
ActiveCN109803983BEffective targeted attackHigh kill rateVirusesAntibody mimetics/scaffoldsAbnormal tissue growthAntigen receptors
A specific chimeric antigen receptor targeting NKG2DL, its coding sequence, and modified immune response cells, as well as their preparation method and application. The modified immune response cells can effectively target and attack various tumor cells, especially positive tumor cells expressing NKG2DL, and can be used to prepare preparations for treating tumors.
Owner:NANJING KAEDI BIOTHERAPEUTICS LTD
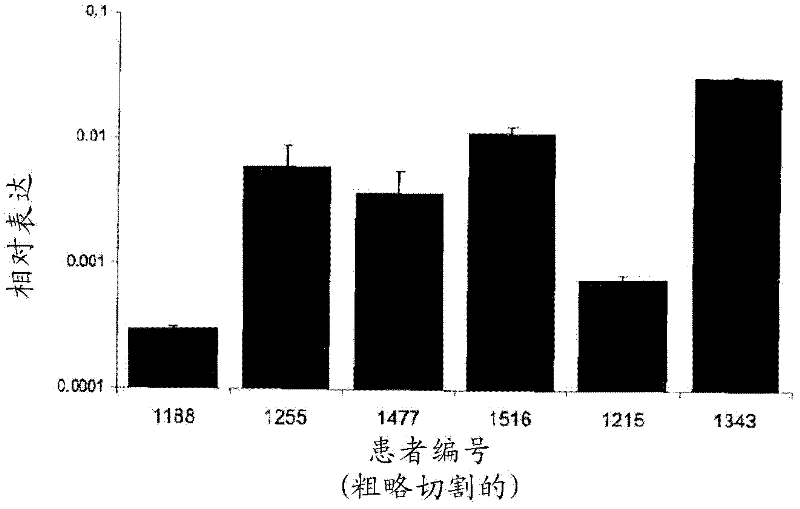
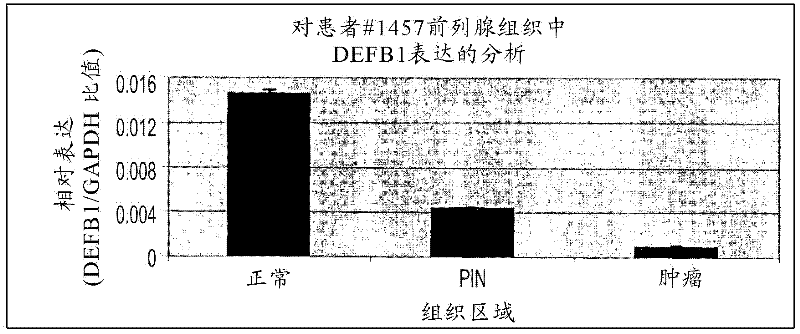
Targeting pax2 for the treatment of breast cancer
The present application provides methods of prevention and / or treatment of breast cancer in a subject by inhibiting expression of PAX2. In the certain embodiments, the method of inhibiting expression of PAX2 is to administrate the subject a nucleic acid encoding an siRNA for PAX2. A method of treating cancer in a subject by administering DEFB1 or by increasing expression of DEFB1 is also provided.
Owner:PHIGENIX INC
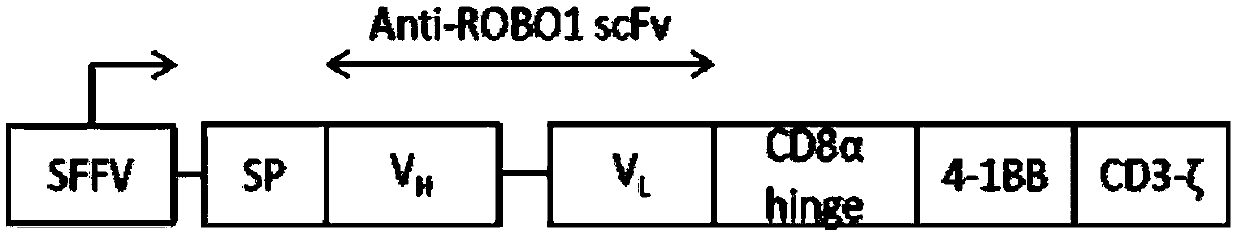
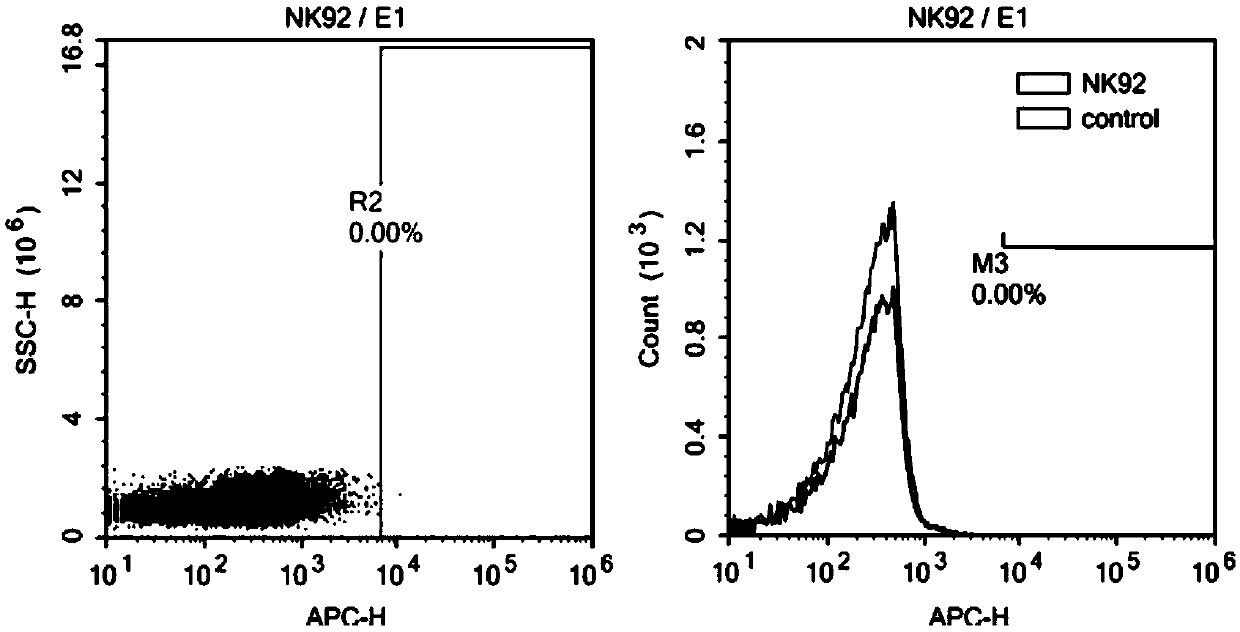
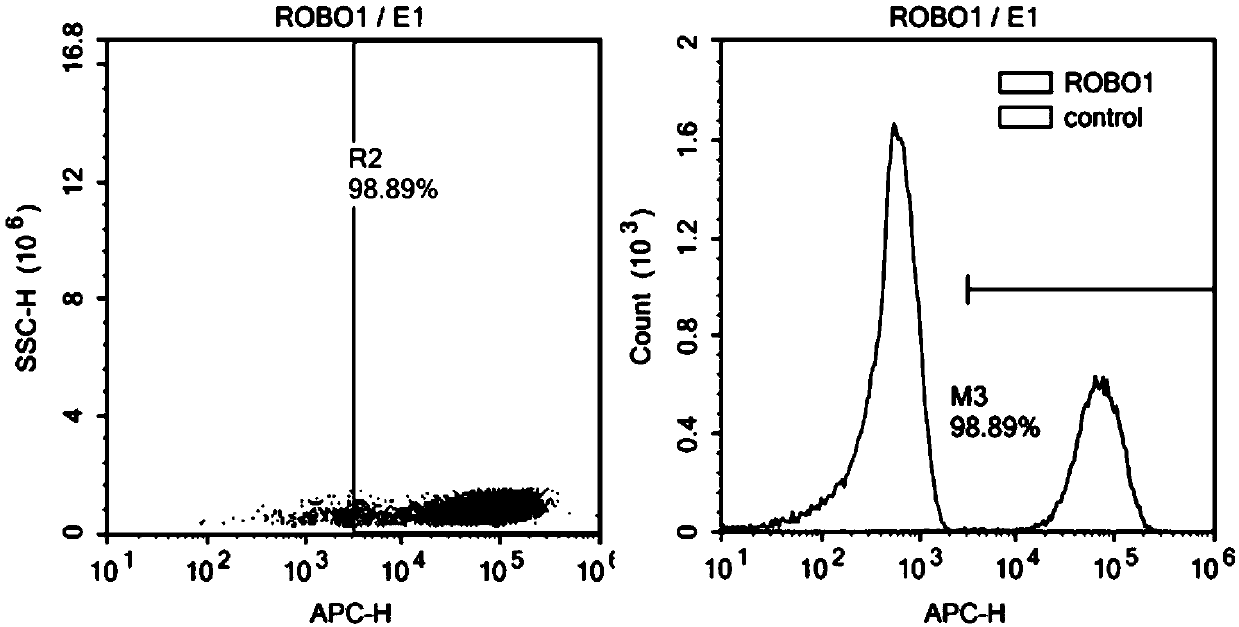
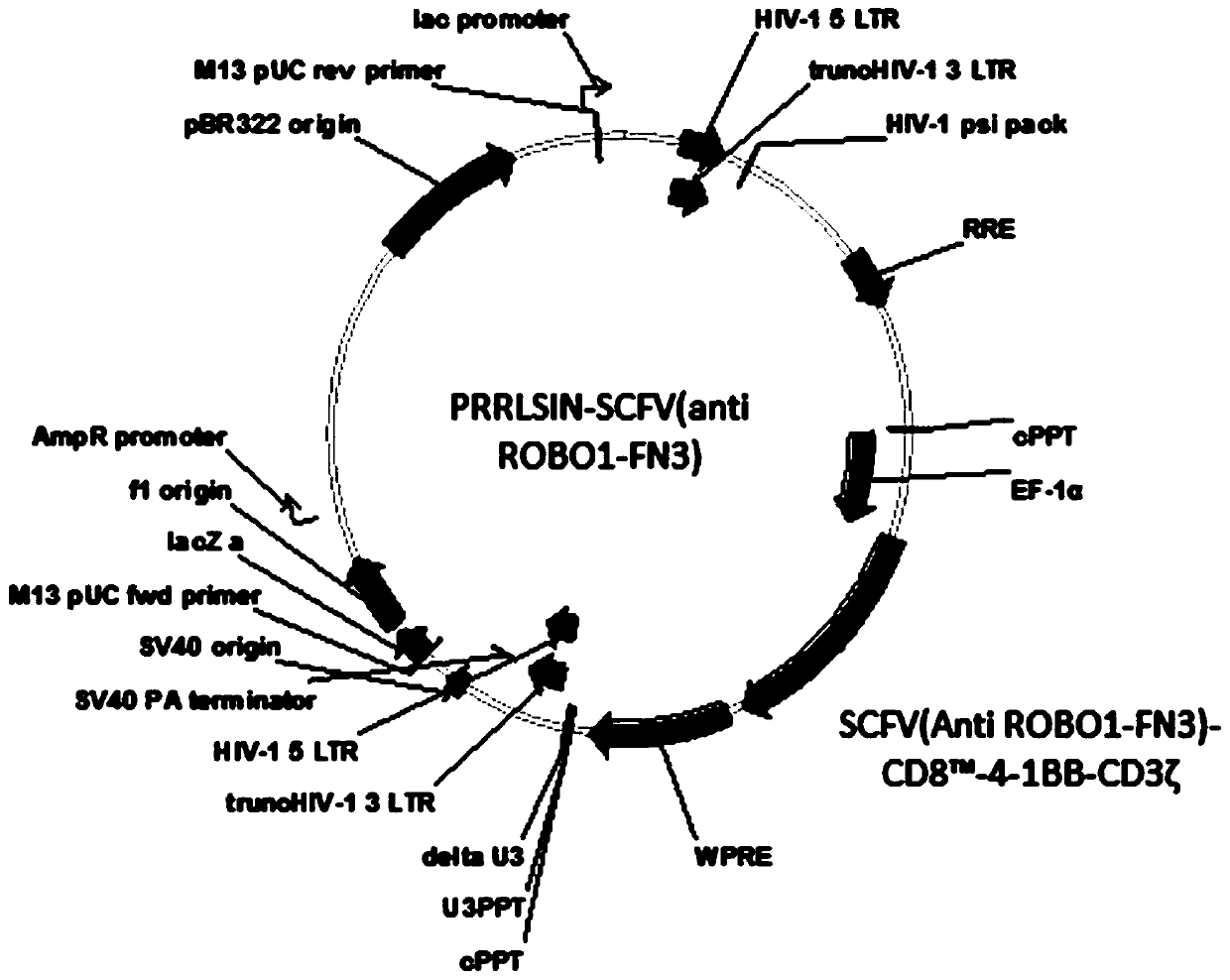
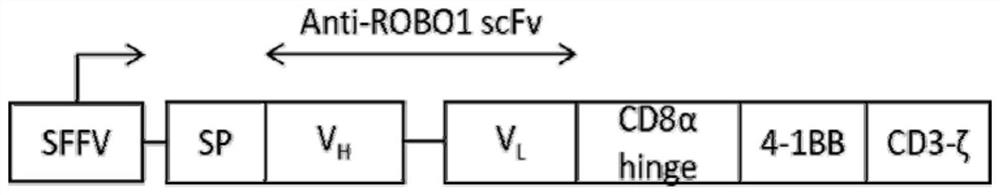
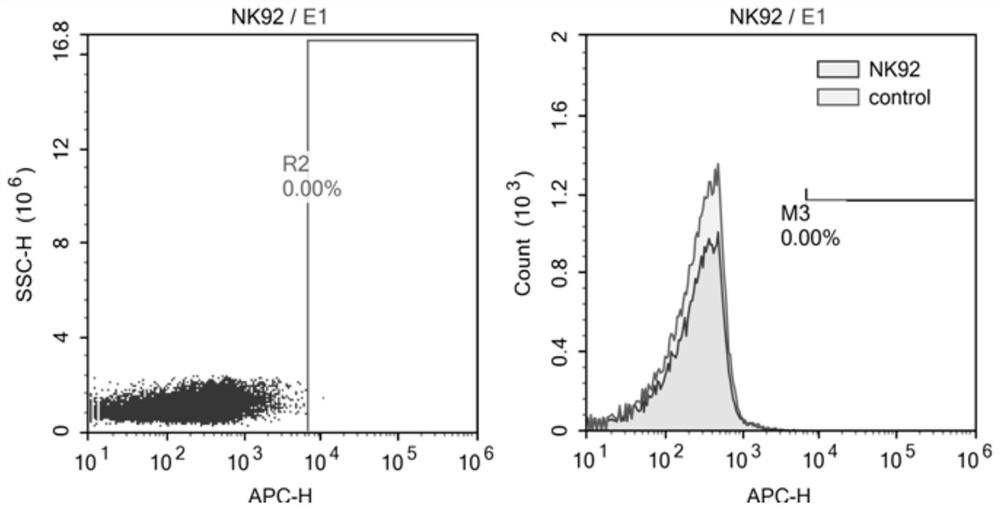
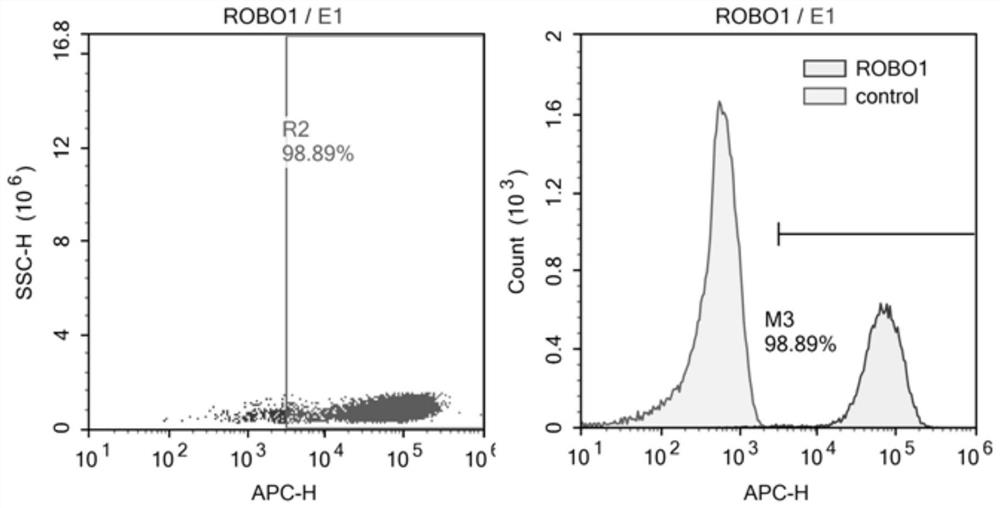
Nucleotide sequence encoding CAR (Chimeric Antigen Receptor), ROBO1 CAR-NK cell expressing CAR as well as preparation and application of ROBO1 CAR-NK cell
ActiveCN109810995AGood killing effectImprove securityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseSide effect
The invention provides a nucleotide sequence encoding a CAR (Chimeric Antigen Receptor) and an ROBO1 CAR-NK cell expressing the CAR. The ROBO1 CAR-NK cell provided by the invention specifically killstumor cells by applying an ROBO1 antibody to the construction of a CAR-NK cell which takes an ROBO1 molecule as a target antigen. The ROBO1 CAR-NK cell can be taken as a medicament for treating tumordiseases, is used for treating tumors with high expression of the ROBO1 molecules, is free from undesirable phenomena such as cytokine storm, and provides a novel treatment method for tumors which cannot be treated with traditional surgery, chemotherapy and radiotherapy; moreover, the ROBO1 CAR-NK cell has the advantages of small toxic and side effects, high safety and better killing effect compared with an ROBO1 CAR-T cell.
Owner:四川阿思科力生物科技有限公司
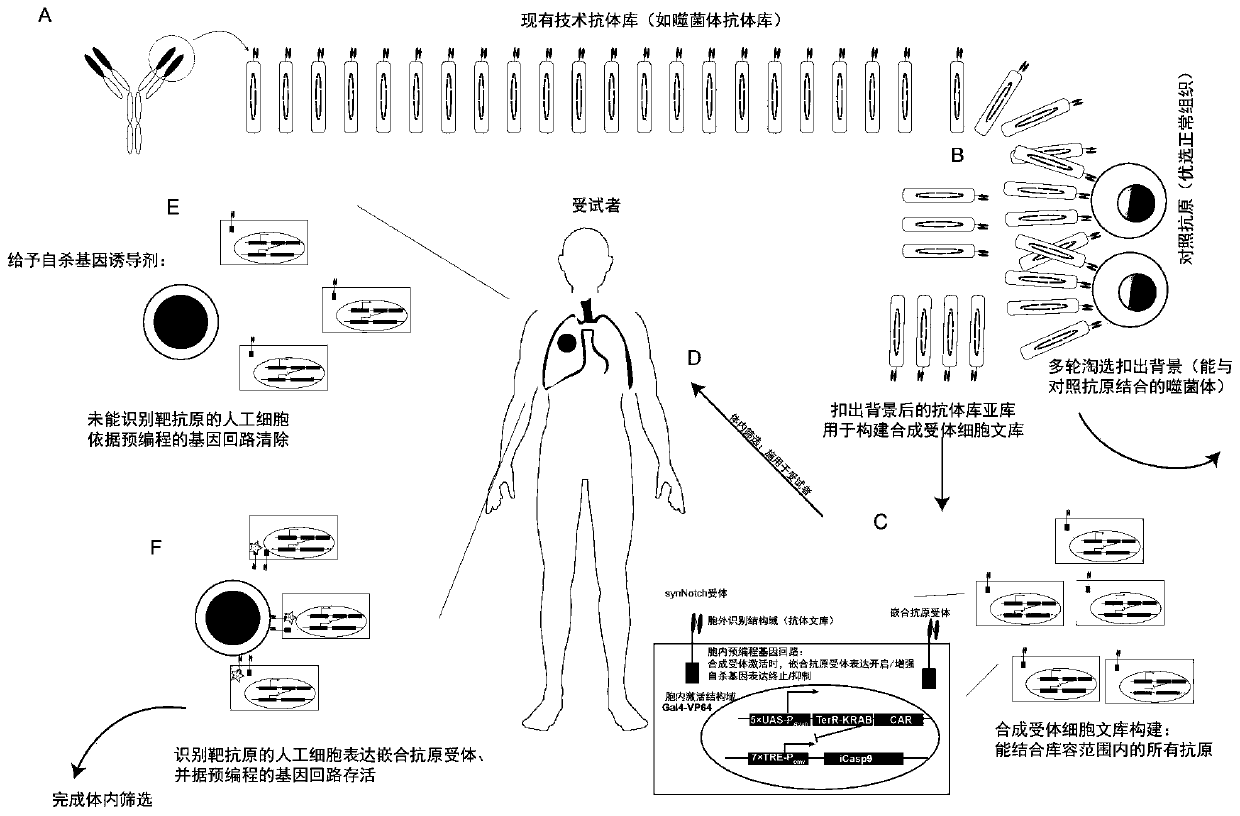
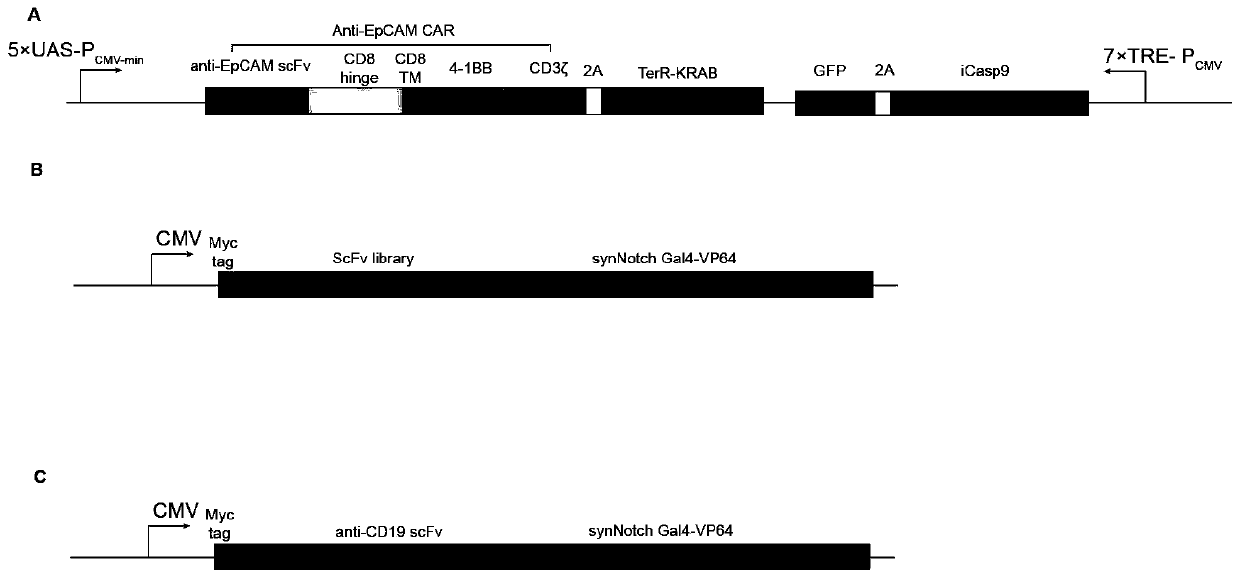
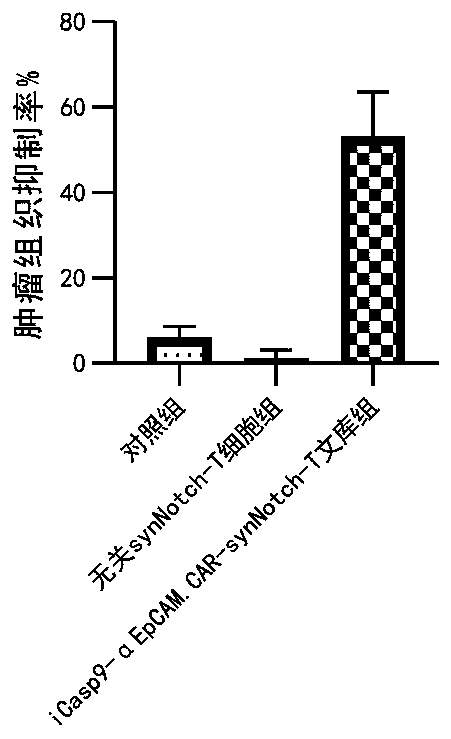
Carrier assembly carrying gene element combination, recipient cell library, preparation and screening methods and application
The invention provides a carrier assembly carrying a gene element combination, a recipient cell library, a preparation and screening method and an application, the recipient cell library is formed byfusing cells and the carrier assembly, and the carrier assembly at least carries three gene elements, respectively a plurality of first gene elements encoding one or more idiotypic synNotch receptors,respectively; a second gene element carrying one or more gene loops; a third gene element encoding one or more idiotypic chimeric antigen receptors. Wherein, when the first genetic element encodes one idiotypic synNotch receptor, the third genetic element must encode at least three idiotypic chimeric antigen receptors, and when the third genetic element encodes one idiotypic chimeric antigen receptor, the first genetic element must encode three idiotypic synNotch receptors. The gene loop is pre-programmed, a regulatory homeopathic factor is combined with a transcription factor, upon activation of the synNotch receptor encoded by the first gene element, the chimeric antigen receptor encoded by the third gene element is controllably expressed.
Owner:PHARCHOICE THERAPEUTICS INC
Biomaterials for modulating immune responses
PendingCN110418651AInorganic non-active ingredientsSkin cancer vaccineMicrobiologyBiological organism
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Universal cancer vaccines and methods of making and using same
InactiveCN110996990APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer preventionOncology
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA
Application of chimeric antigen receptor taking CD99 as target in combination with anti-tumor drugs
ActiveCN111956795AThe tumor killing effect is remarkableImprove survival rateOrganic active ingredientsVirusesAntigen receptorsTherapeutic effect
The invention provides a combined medication method for treating tumors and an application of the combined medication method. The combined medication method combines chimeric antigen receptor immune cell therapy and chemotherapy and has a stronger anti-tumor effect than single chemotherapy or single immune cell therapy. Meanwhile, the dosage of chemotherapeutic drugs can be reduced, and toxic andside effects generated by the chemotherapeutic drugs are reduced. The problems that off-target is caused by high heterogeneity of tumors in a process of treating the tumors with CAR-T cells, and the treatment effect is not ideal due to singleness of a target are solved.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Double-targeting chimeric antigen receptor, encoding gene and recombinant expression vector
PendingCN113248619AHigh precisionGood treatment effectVirusesAntibody mimetics/scaffoldsSingle-Chain AntibodiesAntigen binding
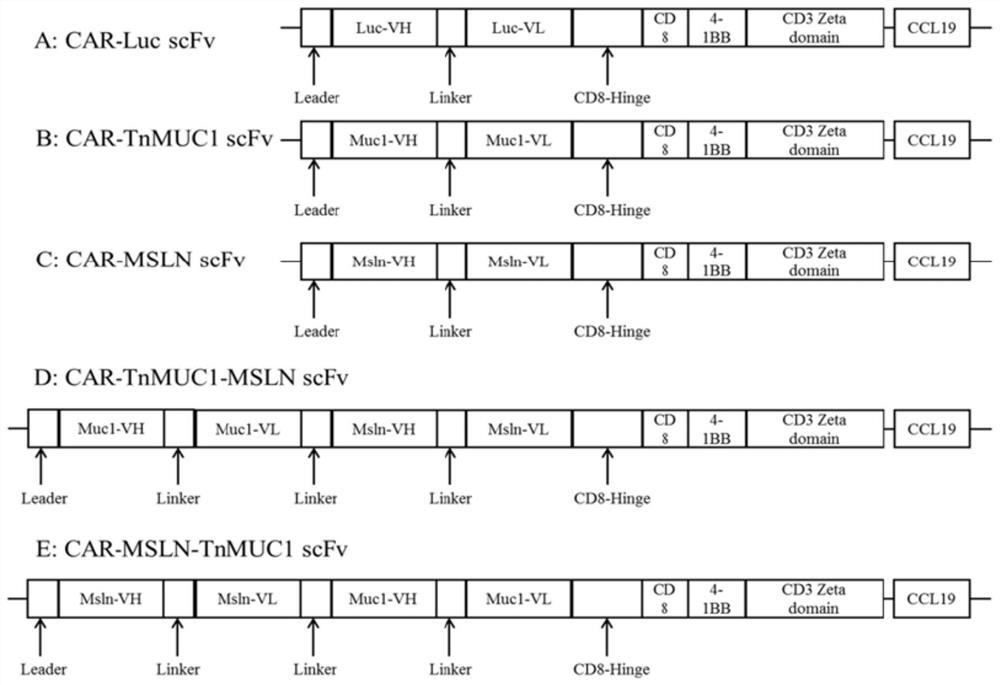
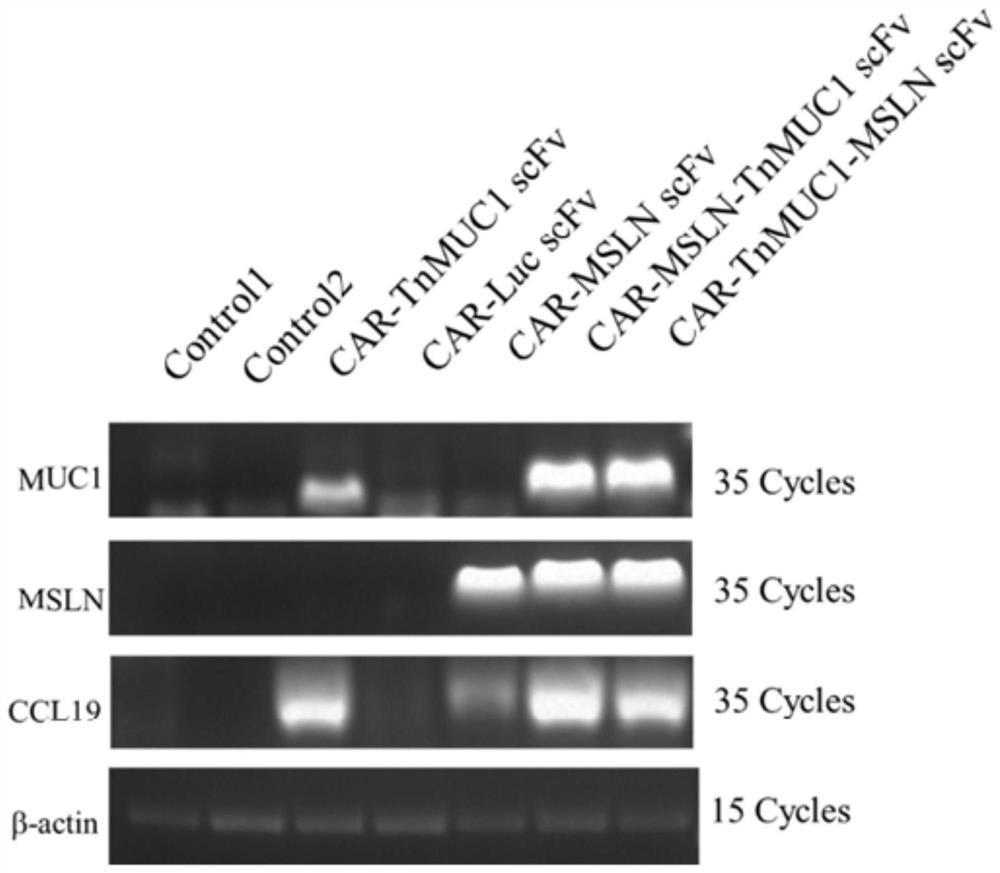
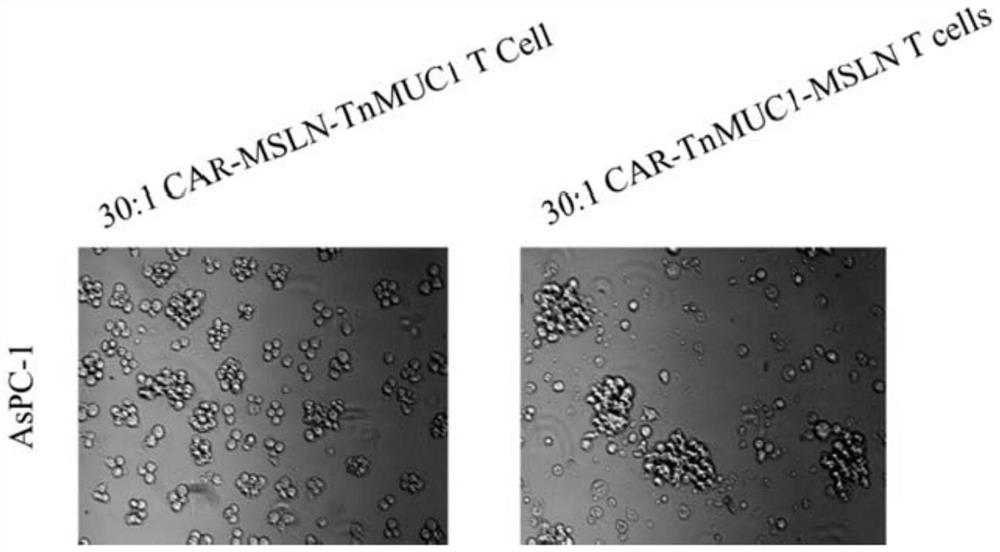
The invention discloses a double-targeting chimeric antigen receptor, which comprises a double-antigen binding region, a first hinge region, a chimeric antigen receptor T cell activation domain and a protein co-expression self-cleavage domain which are sequentially connected in series, and the double-antigen binding region comprises a heavy chain VH and a light chain VL of an MSLN and MUC1 single-chain antibody which are connected in series, and a second hinge region connected with the MSLN and the MUC1 single-chain antibody. The double-targeting CAR-T cell can kill tumor cells expressing double antigens, killing is more accurate and efficient, meanwhile, the off-target effect is reduced or avoided, the side effect of non-specific killing is reduced, and the anti-tumor effect is enhanced.
Owner:上海帝鹤思医疗科技有限公司
Double-chimeric antigen receptor, T cell and construction method and application thereof
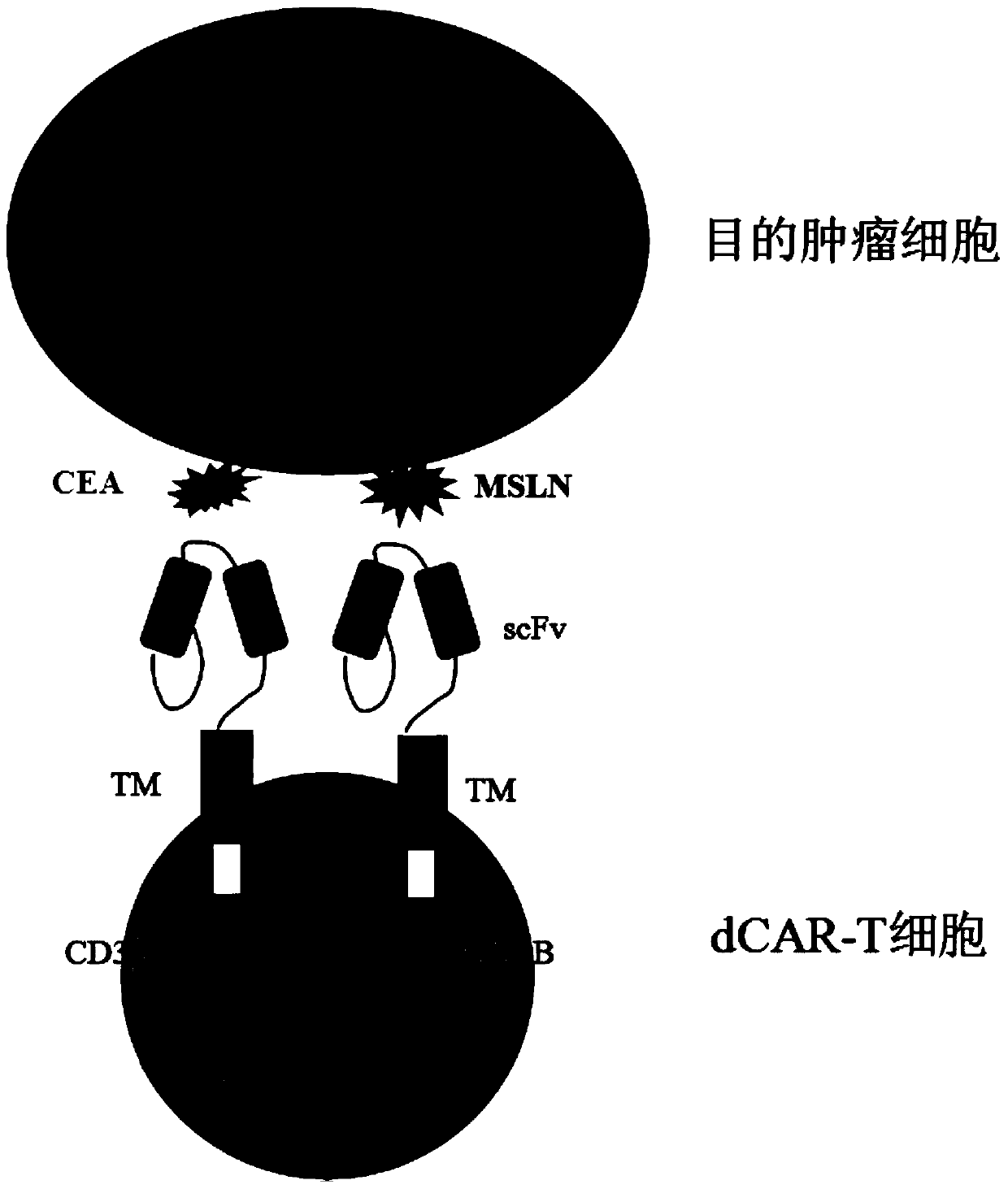
PendingCN110669138AToxicityImprove securityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsTumor antigenSialyl tn
The invention discloses a double-chimeric antigen receptor, a T cell and a construction method and an application thereof, which belong to the field of cellular immunotherapy of tumors. The inventionspecifically relates to a specific structure and a construction method of the double chimeric antigen receptor T cell (dCAR-T cell), and preliminarily discusses the in-vivo and in-vitro activity of the dCAR-T cell. The selected tumor-associated antigens are mesothelin and carcino-embryonic antigens, and researches show that the two tumor antigens can be simultaneously expressed on the surface of asolid tumor, such as pancreatic cancer. The invention discloses an antigen receptor. The in-vitro and in-vivo tests prove that the constructed dCAR-T cell can be permanently and effectively activatedonly under the condition that two antigens are simultaneously recognized, and has efficient anti-tumor activity, so that a specific tumor killing function can be exerted, and the application of CAR-Tcell immunotherapy is improved.
Owner:CHINA PHARM UNIV
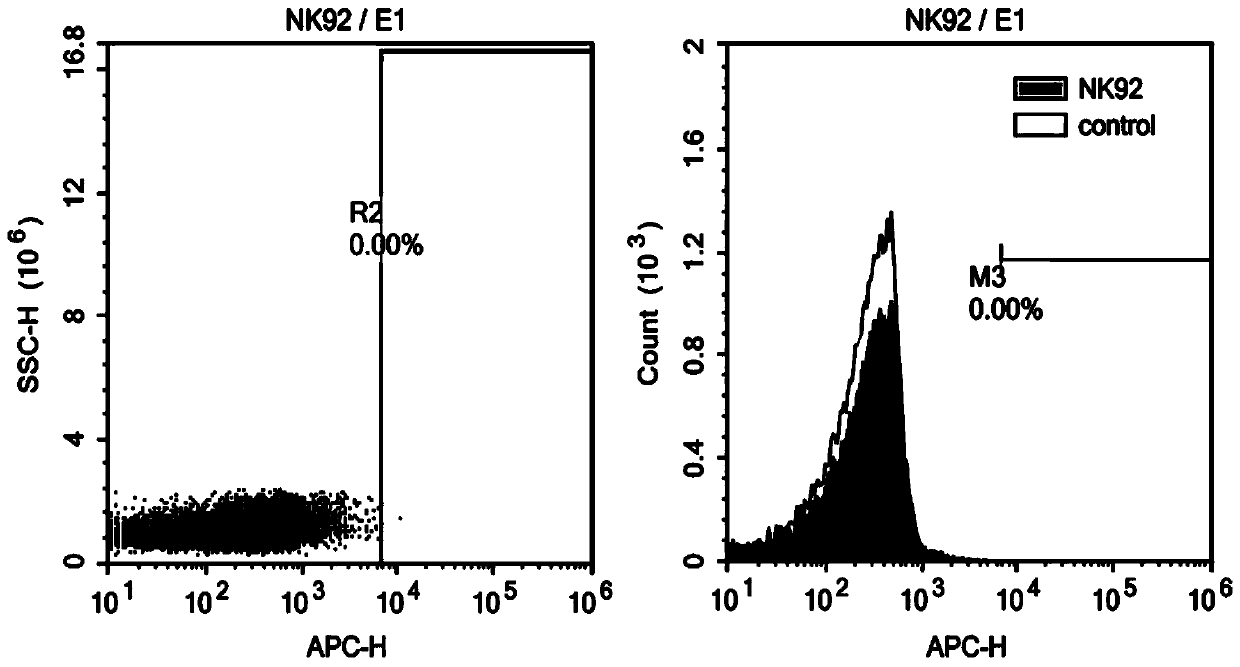
ROBO1 CAR-NK cell carrying suicide gene as well as preparation method and application of ROBO1 CAR-NK cell
PendingCN111269925ASmall side effectsGood killing effectVirusesHydrolasesNatural Killer Cell Inhibitory ReceptorsROBO1
The invention discloses an ROBO1 CAR-NK cell carrying a suicide gene as well as a preparation method and application of the ROBO1 CAR-NK cell. In order to improve the safety and controllability of theCAR-NK therapy, a suicide gene switch element is integrated into a genome through a lentiviral transfection technology on the basis of the current ROBO1 CAR-NK cell to form the CAR-NK with the suicide gene. By adding the suicide gene, the CAR-NK cells can be better controlled, and the clinical safety is further improved.
Owner:ASCLEPIUS SUZHOU TECH CO GRP CO LTD
Vaccine
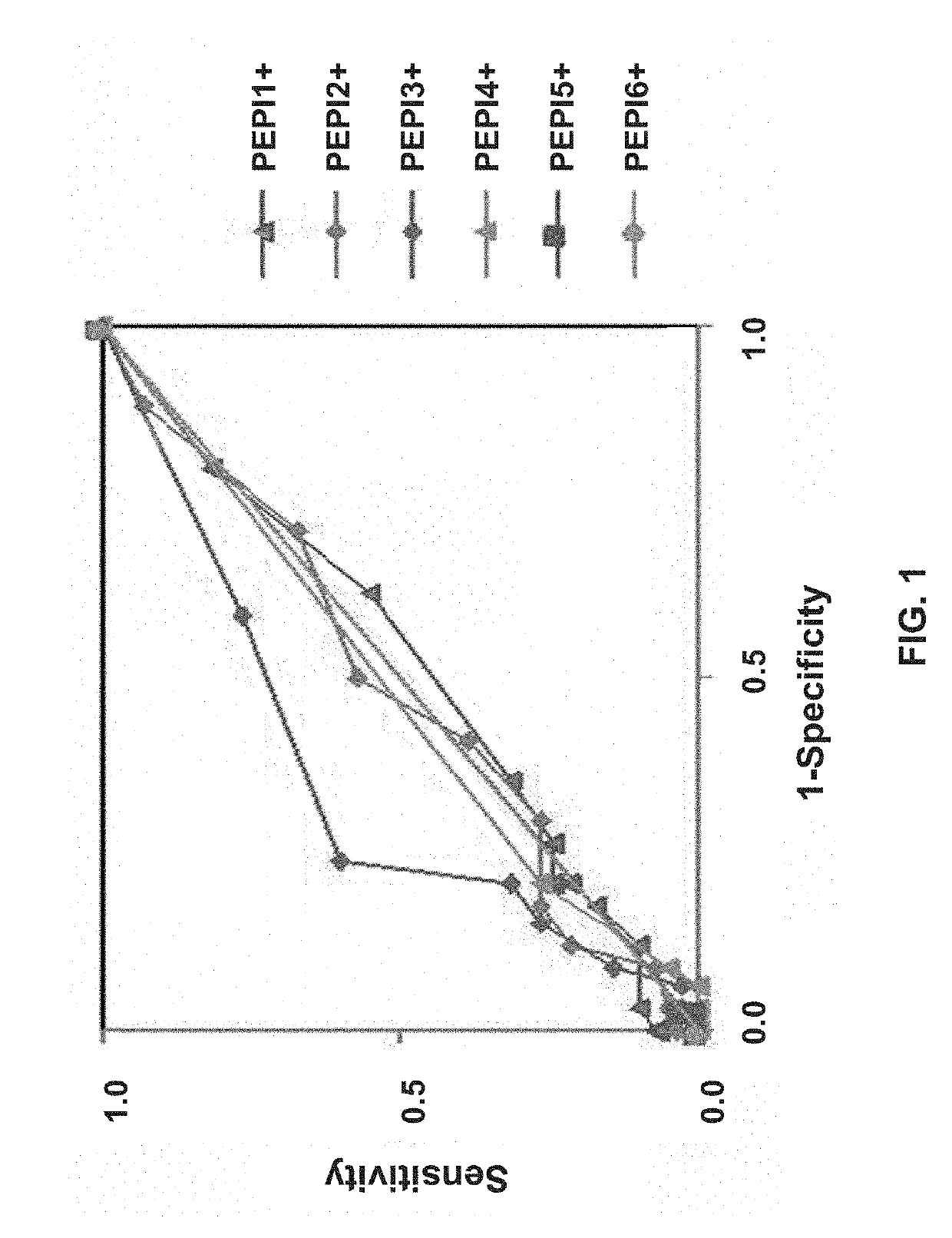
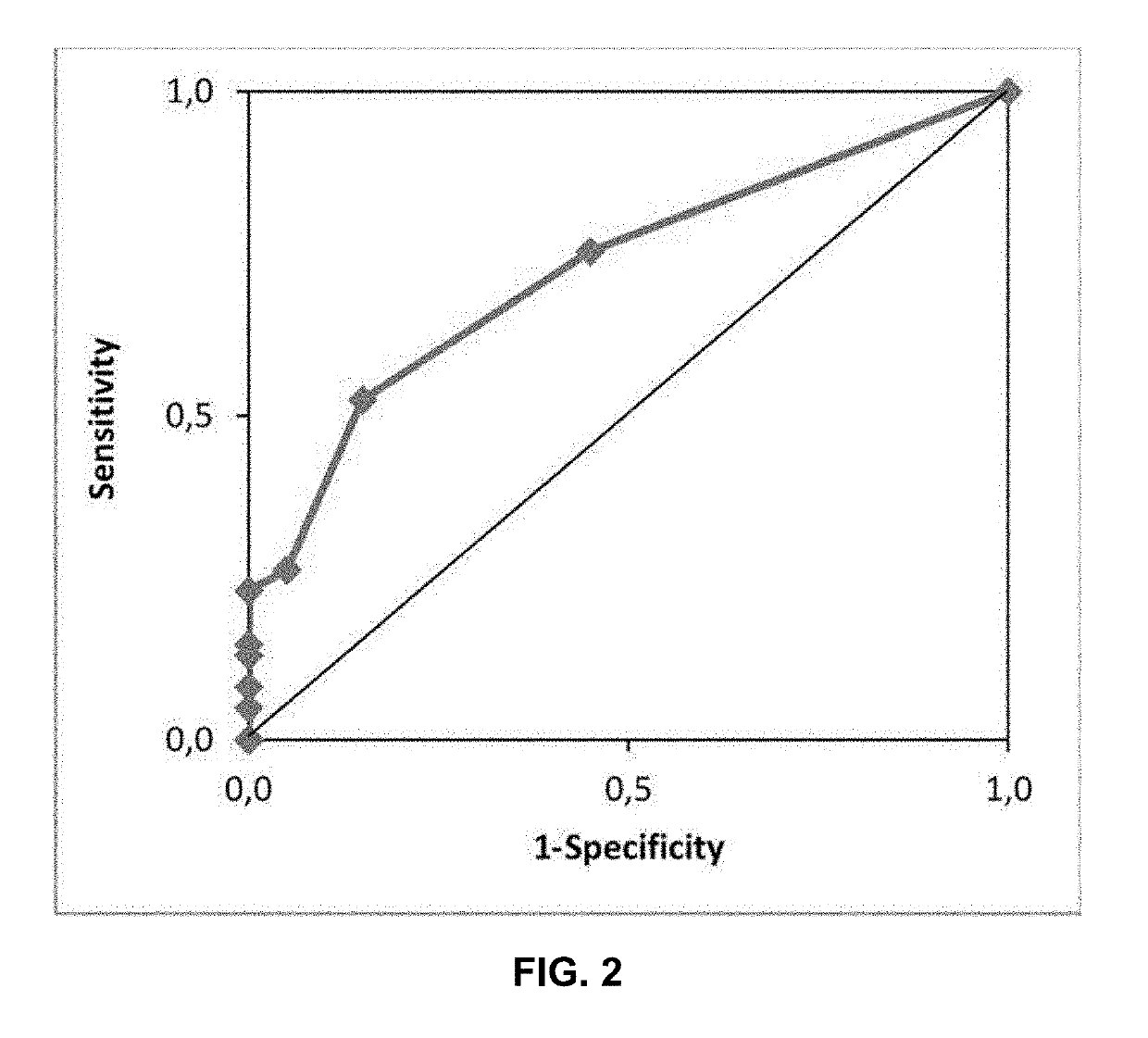
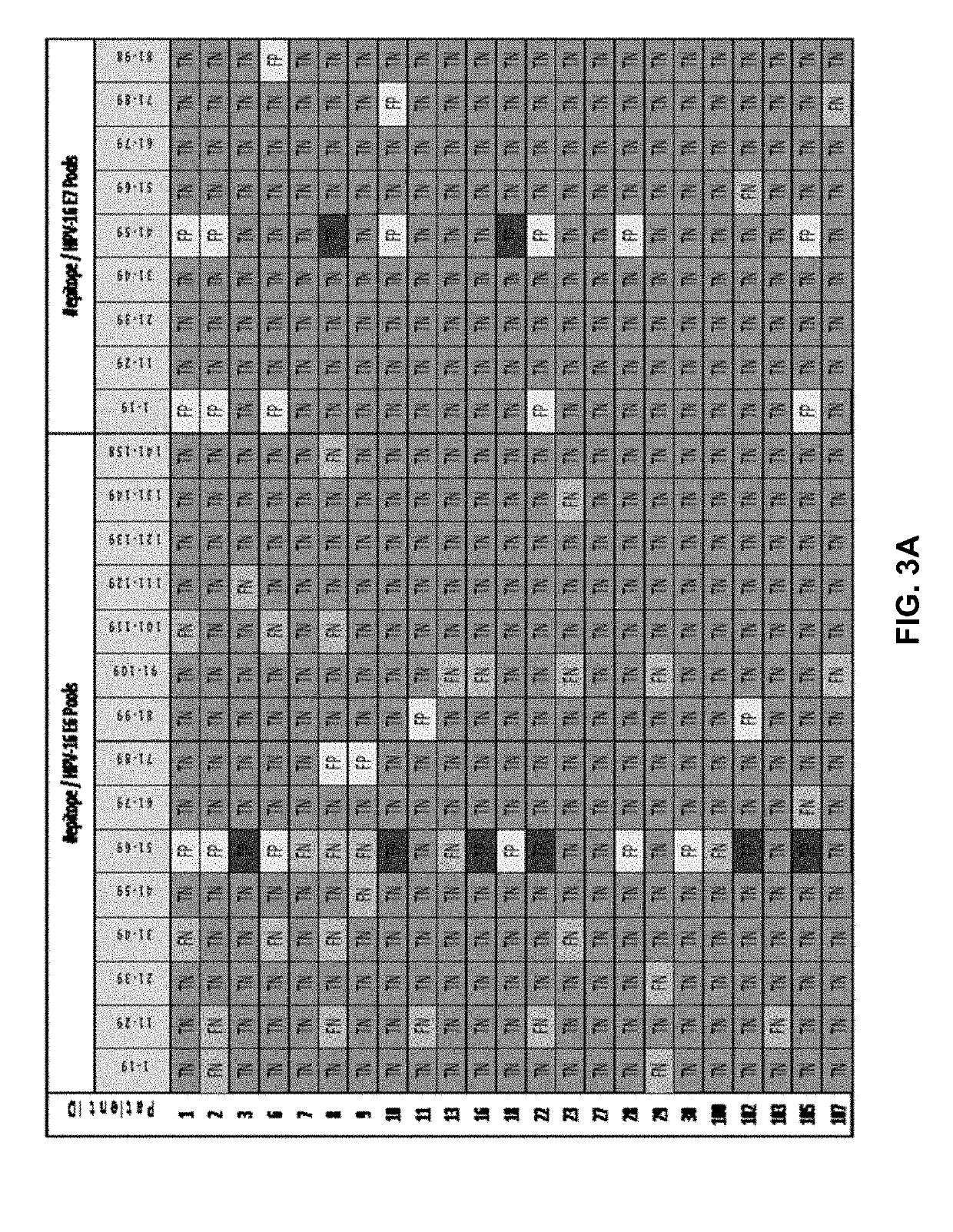
The disclosure relates to polypeptides and pharmaceutical compositions comprising polypeptides that find use in the prevention or treatment of cancer, in particular breast cancer, ovarian cancer and colorectal cancer. The disclosure also relates to methods of inducing a cytotoxic T cell response in a subject or treating cancer by administering pharmaceutical compositions comprising the peptides, and companion diagnostic methods of identifying subjects for treatment. The peptides comprise T cell epitopes that are immunogenic in a high percentage of patients.
Owner:TREOS BIO LTD
Monocyte and macrophage for expressing chemokine receptor with solid tumor directional chemotactic ability, and preparation and application thereof
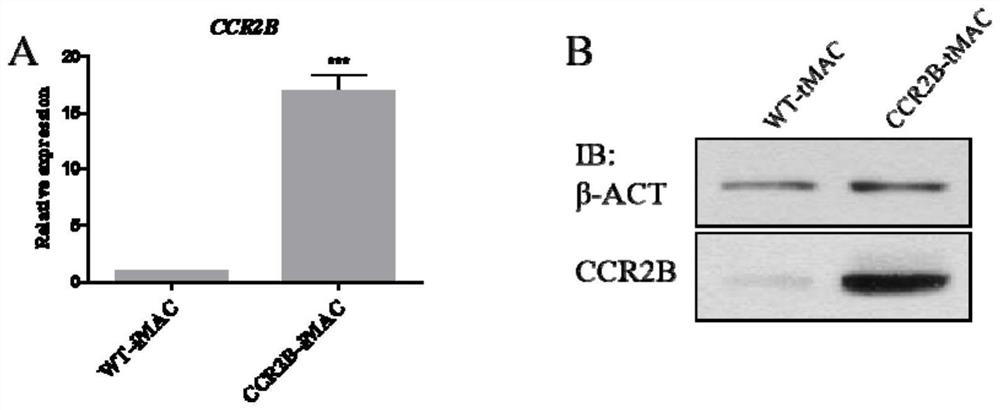
ActiveCN113322238ADownregulation of expression levelReduce non-specific immune attackPeptidesBlood/immune system cellsPluripotential stem cellLentivirus
The invention provides a monocyte and a macrophage for expressing a chemokine receptor with solid tumor directional chemotactic ability, and preparation and application thereof, and relates to the field of biotechnology. The invention provides a macrophage, the macrophage overexpresses the chemokine receptor, the macrophage has the ability of chemotactic migration to a solid tumor and remarkable effects of chemotactic migration to the tumor and infiltration, the specific killing efficiency and utilization rate of adoptively transplanted macrophage can be improved, and non-specific immune attack to normal tissues is reduced. The invention provides a preparation method of macrophages, which comprises the following steps: constructing a lentivirus expression system containing a chemokine receptor gene, integrating the chemokine receptor gene into pluripotent stem cells or mononuclear macrophages by using the lentivirus expression system, performing induced differentiation to obtain the macrophages, and the macrophages can stably overexpress the chemokine receptor for a long time.
Owner:ZHEJIANG UNIV
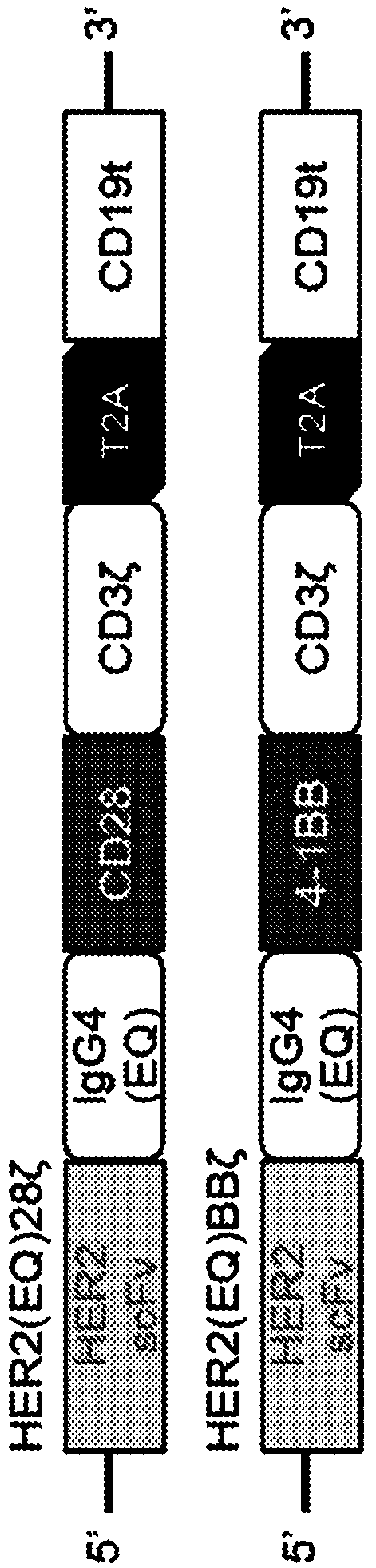
Chimeric antigen receptors targeting her2
ActiveCN108779174AAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen receptorImmune receptor
Chimeric transmembrane immunoreceptors (CAR) which include an extracellular domain targeted to HER2, a transmembrane region, a costimulatory domain and an intracellular signaling domain are described.
Owner:CITY OF HOPE
Chimeric antigen receptor of cell for targeted expression of Claudin 18.2 and application of chimeric antigen receptor
ActiveCN113354739AEfficient killingStrong killing and cytokine release functionVirusesAntibody mimetics/scaffoldsAntigenSingle-Chain Antibodies
The invention discloses a chimeric antigen receptor of a cell for targeted expression of Claudin 18.2 (CLDN 18.2), and particularly discloses a chimeric antigen receptor with an amino acid sequence as shown in SEQ ID NO.14. The chimeric antigen receptor comprises a Claudin 18.2-targeted single-chain antibody, a hinge region, a transmembrane structural domain and an intracellular signal structural domain. The Claudin 18.2-targeted chimeric antigen receptor disclosed by the invention can achieve effective and specific targeted expression of malignant cells (such as tumor cells) of the Claudin 18.2 surface antigen, so that a more efficient method with fewer side effects and adverse reactions is provided for treating some tumors expressing the Claudin 18.2 surface antigen.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
Composition of DC vaccine and NKG2A antagonist and application of composition in anti-breast cancer or liver cancer
PendingCN110575537AInhibit and eliminate proliferative growthInhibition and Elimination of DiffusionLiver cancer vaccineCancer antigen ingredientsSide effectTreatment effect
The invention provides a composition of a DC vaccine and an NKG2A antagonist and application of the composition in anti-breast cancer or liver cancer. The DC vaccine in the composition is different from the early traditional tumor cell full-antigen-loaded DC vaccine, has specific targeting antigens, can more accurately treat tumors and reduce the killing side effects on normal cells; and secondly,the composition further contains a novel immune checkpoint inhibitor NKG2A antagonist which enables tumor cells with CD94-NKG2A / HLA-Ereceptor-ligand on the surface to be recognized and killed by NK cells and T cells, and an antigen presentation function of dendritic cells is enhanced, so that some cells which are not subjected to presentation and antigen by the DC vaccine are captured by an immune system again, the treatment effect on breast and liver cancer is enhanced, effect is faster than the conventional DC vaccine, the time of duration is longer, and the immunotherapy process is promoted.
Owner:刘慧宁
Chorionic gonadotropin DNA vaccines and methods
InactiveUS20060121010A1Elicit immune responseInhibit progressOrganic active ingredientsBiocideEpitopeChorionic gonadotropins
The invention relates to immunotherapy of a mammalian subject by exposing the immune response cells of the subject to a nucleic acid construct encoding at least one hCG immunogenic epitope or precursor thereof such that the nucleic acid construct is taken up and processed by the immune response cells. The invention further relates to compositions comprising such hCG-encoding nucleic acid constructs.
Owner:AVI BIOPHARMA
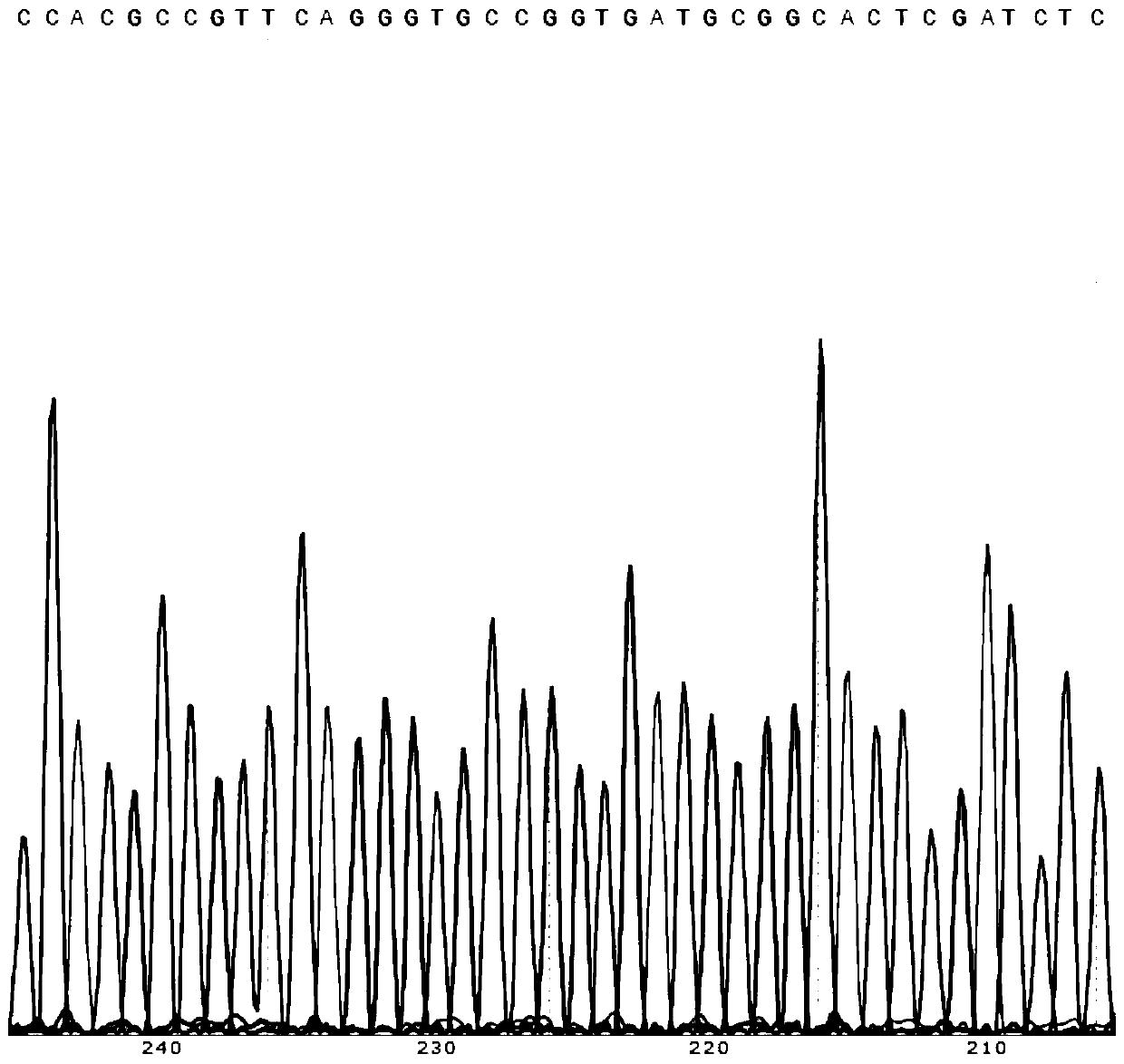
Nucleotide sequence encoding car, robo1 CAR-NK cell expressing the car and its preparation and application
ActiveCN109810995BGood killing effectImprove securityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseSide effect
The present invention provides a nucleotide sequence encoding CAR and ROBO1 CAR-NK cells expressing the CAR. The ROBO1 CAR-NK cells provided by the present invention use ROBO1 antibodies to construct CAR-NK cells, which use ROBO1 molecules as target antigens, and use ROBO1 CAR-NK cells to specifically kill tumor cells. It can be used as a therapeutic drug for tumor diseases, for the treatment of tumors with high expression of ROBO1 molecules, without adverse phenomena such as cytokine storm, and provides a new treatment method for tumors that are ineffective for traditional surgery, chemotherapy and radiotherapy; and with Compared with ROBO1 CAR‑T cells, it has less toxic side effects, higher safety, and better killing effect.
Owner:四川阿思科力生物科技有限公司
Prognostic indicator
InactiveCN1554026AOrganic active ingredientsImmunoglobulins against animals/humansLymphatic SpreadOncology
Owner:UNIV OF LIVERPOOL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com