Universal cancer vaccines and methods of making and using same
A technology of cancer and vaccine composition, applied in breast cancer vaccines, chemical instruments and methods, vaccines, etc., can solve problems such as no vaccines to prevent cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
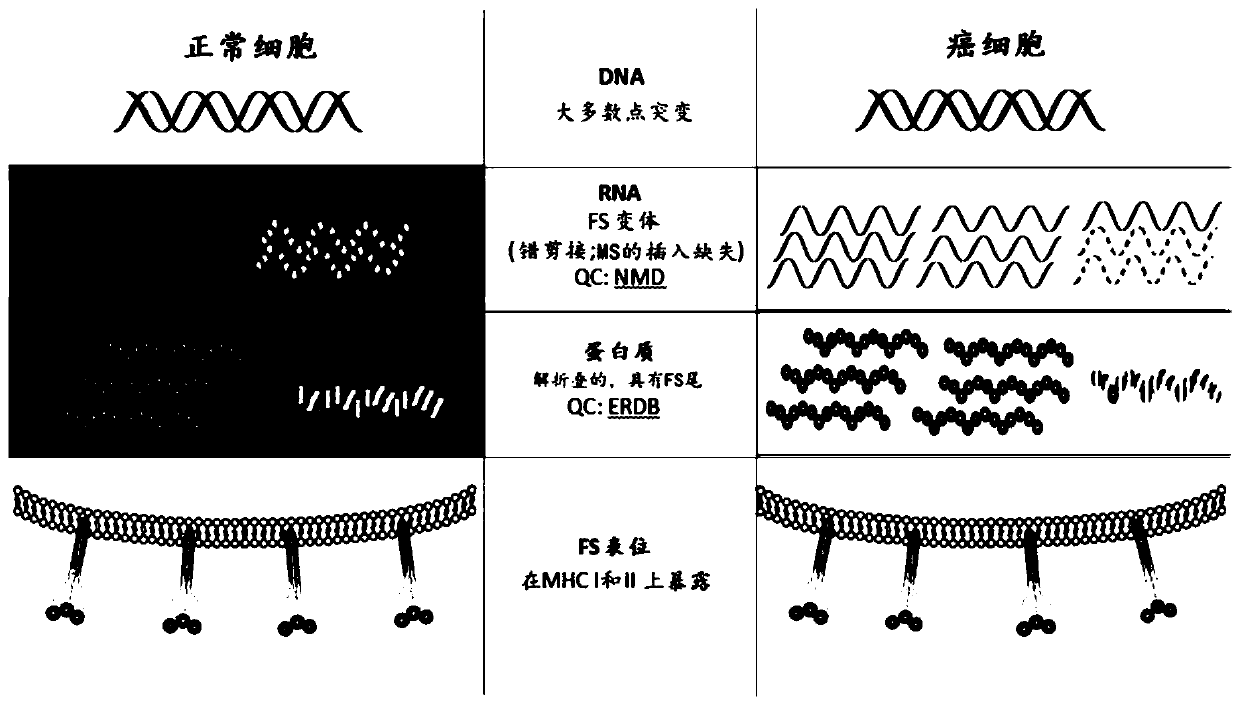
[0113] Example 1: Model as a basis for the development of a universal cancer vaccine
[0114] image 3 A model is presented in which outlines the basis of the universal therapeutic or preventive cancer vaccines herein. As information flows from DNA to RNA to protein, the error rate generally increases. These errors include insertions and deletions (insertions) of one or more nucleotides during the transcription of microsatellites (MS) in exons. These errors will create a background level of frameshift (FS) transcripts, which encode truncated proteins with FS peptides at the C-terminus. The level of FS peptides in normal cells is controlled by protein quality control mechanisms such as nonsense mediator decay (NMD) and ER-related degradation. However, in abnormal and potentially cancerous cells, these errors increase across the board due to chromosomal instability or critical, widely effective variants. Under these conditions, the number of FS peptides produced exceeds the cap...
Embodiment 2
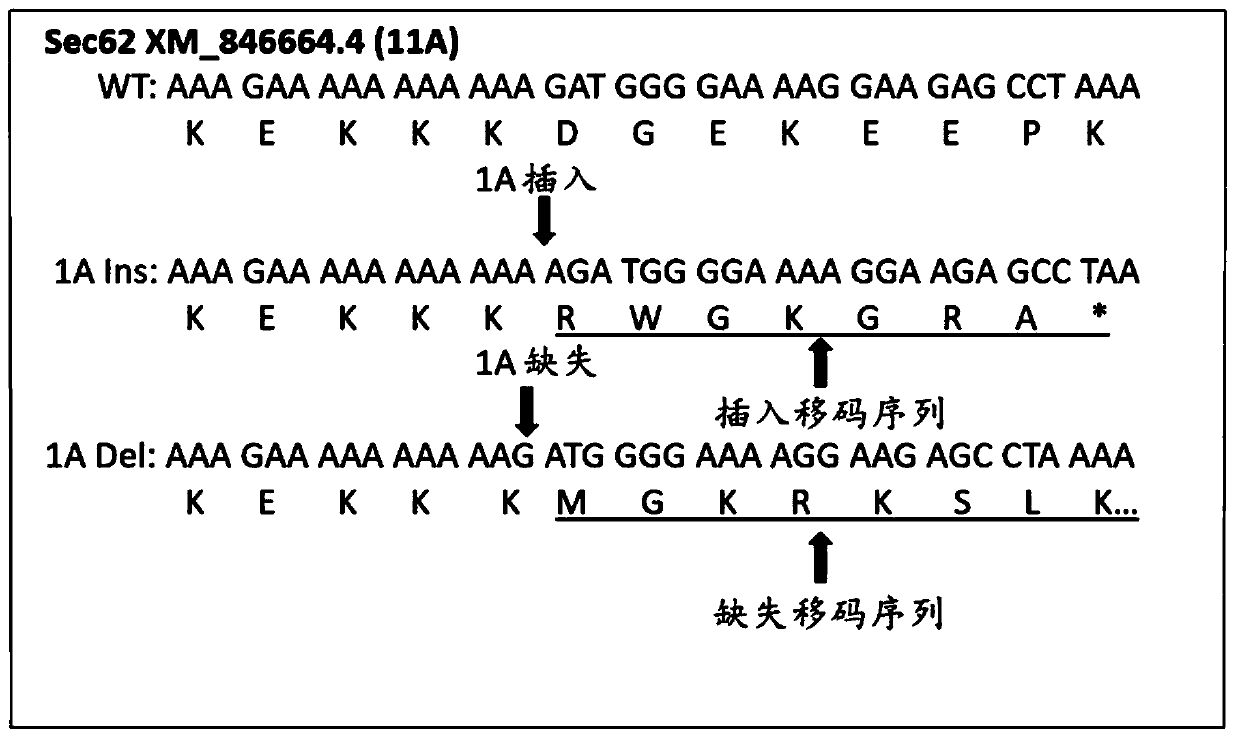
[0115] Example 2: Demonstrate that there are indels in RNA but not in DNA
[0116] To determine whether common FS variants in different cancers are more due to RNA transcription errors than DNA variants, the MS region of Sec62 was examined in both human and dog tumors. For either MS, no insertion or deletion was detected in the DNA. However, for both types of MS, there is a detectable level of an A insertion in the RNA of the same tumor ( Figure 13 ).
[0117] In order to determine whether tumor cells have more FS transcripts than normal cells, MS indel rate analysis was performed using the current human EST database. The results showed that in different cancers, the homopolymer indel rate in the coding region was significantly higher than Indel rate in normal cell transcripts ( Figure 14 ).
Embodiment 3
[0118] Example 3: Immunoreactivity of frameshift peptides
[0119] It can be determined that the increase in abnormal proteins (including proteins with FS) overwhelms the protein quality control system, which triggers an immune response to it. We have developed an array that displays all possible MS FS encoded in the human proteome that meet the previously described length and expression criteria. There are about 3,000 such peptides (and about 220K peptides from missplicing of exons). Figure 4 , Figure 5 , Image 6 with Figure 7 Show that these arrays can be used to construct different types of universal vaccines. Shows a universal vaccine against 6 advanced human tumors and 8 advanced dog tumors. A different set of peptides was selected for the universal protective vaccine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com