Stable levamlodipine composition
A technology of levamlodipine and its composition, which is applied in the field of levamlodipine pharmaceutical composition, can solve the problems of poor stability of levamlodipine and low dissolution in vitro, and achieve good stability, good repeatability, and guaranteed stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The sample dissolution curve of embodiment one The sample dissolution curve of embodiment two The sample dissolution curve of embodiment three
Embodiment 4
[0024] The sample dissolution curve of embodiment four The sample dissolution curve of embodiment five Dissolution curve of the sample of Comparative Example 1
[0025] The present invention will be further described below by taking levamlodipine besylate as an example but not limited to levamlodipine besylate.
[0026] Embodiment one
[0027] prescription:
[0028]
[0029] Process:
[0030] 1) After mixing the levamlodipine besylate and calcium hydrogen phosphate of the prescribed amount, pass through an 80-mesh standard sieve;
[0031] 2) Mix the remaining auxiliary materials with the materials in 1) evenly, and punch out tablets with a diameter of Φ6.0mm.
Embodiment 2
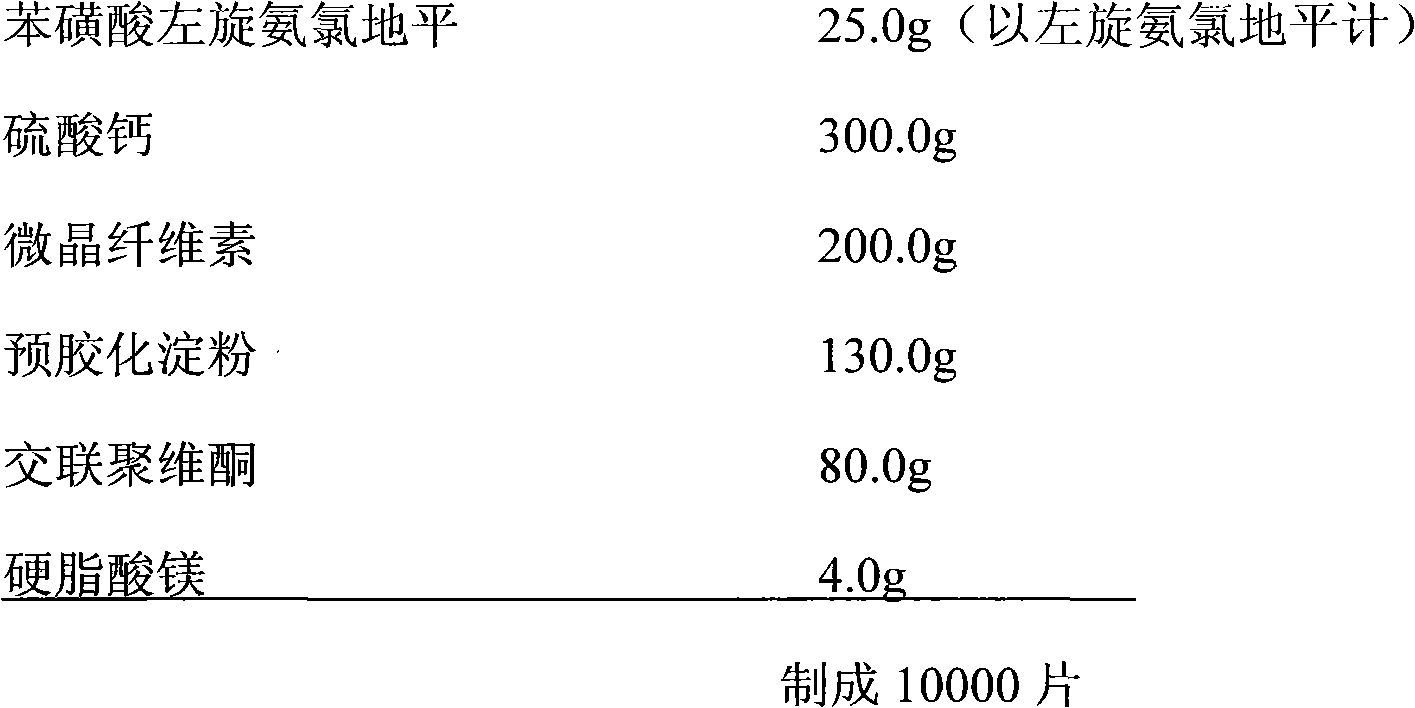
[0033] prescription:
[0034]
[0035] Process:
[0036] 1) cross 80 mesh standard sieves after mixing the levamlodipine besylate and calcium sulfate of prescription quantity;
[0037] 2) Mix the remaining auxiliary materials with the materials in 1) evenly, and punch out tablets with a diameter of Φ6.0mm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com