Compound of atorvastatin and levorotatory amlodipine and preparing method thereof
A technology of levamlodipine and atorvastatin, which is applied in the field of preparation of pharmaceutical compositions, can solve the problems of instability, amorphous atorvastatin is easy to absorb moisture and the like, and achieves good stability, various indicators and dissolution rates. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
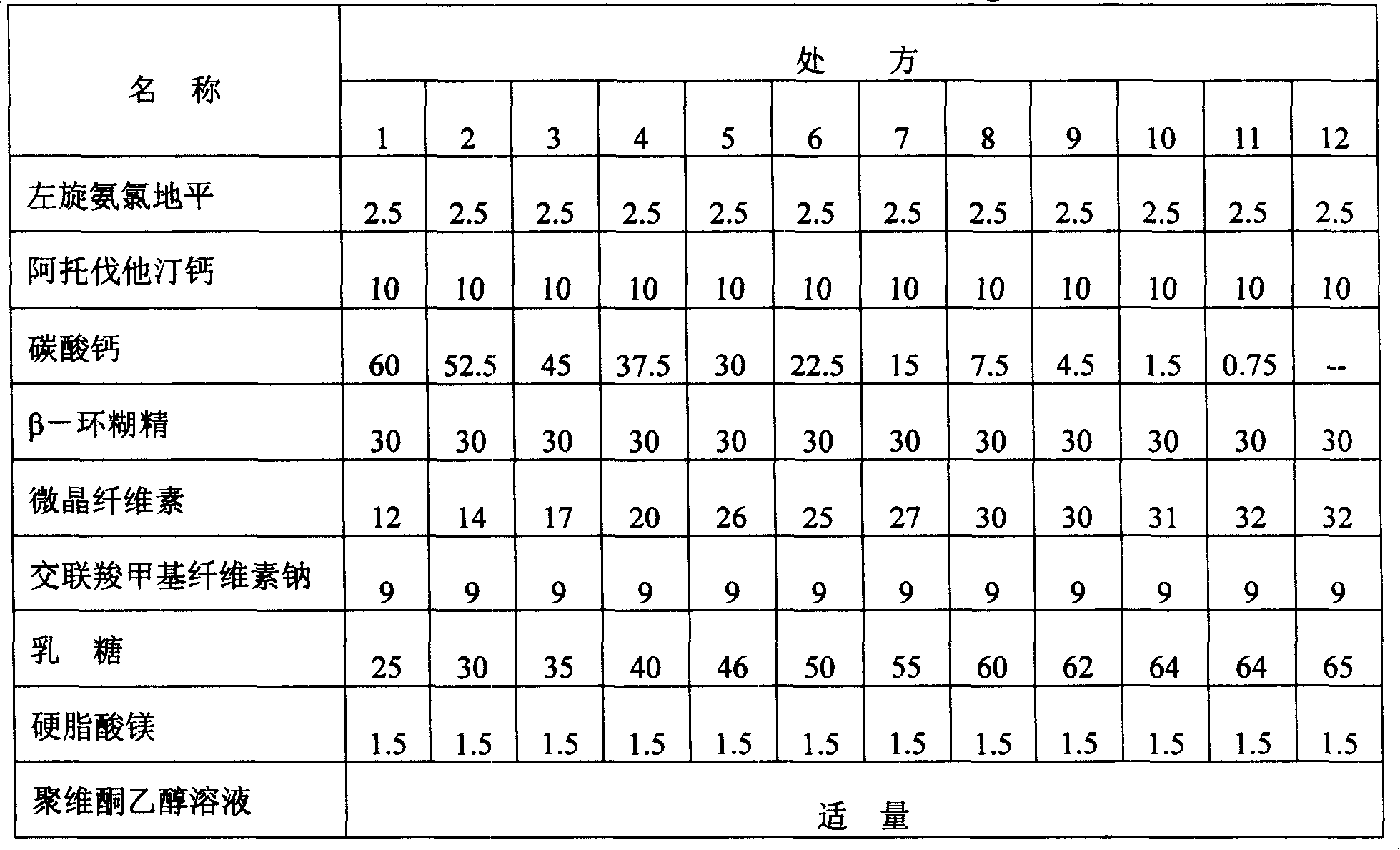
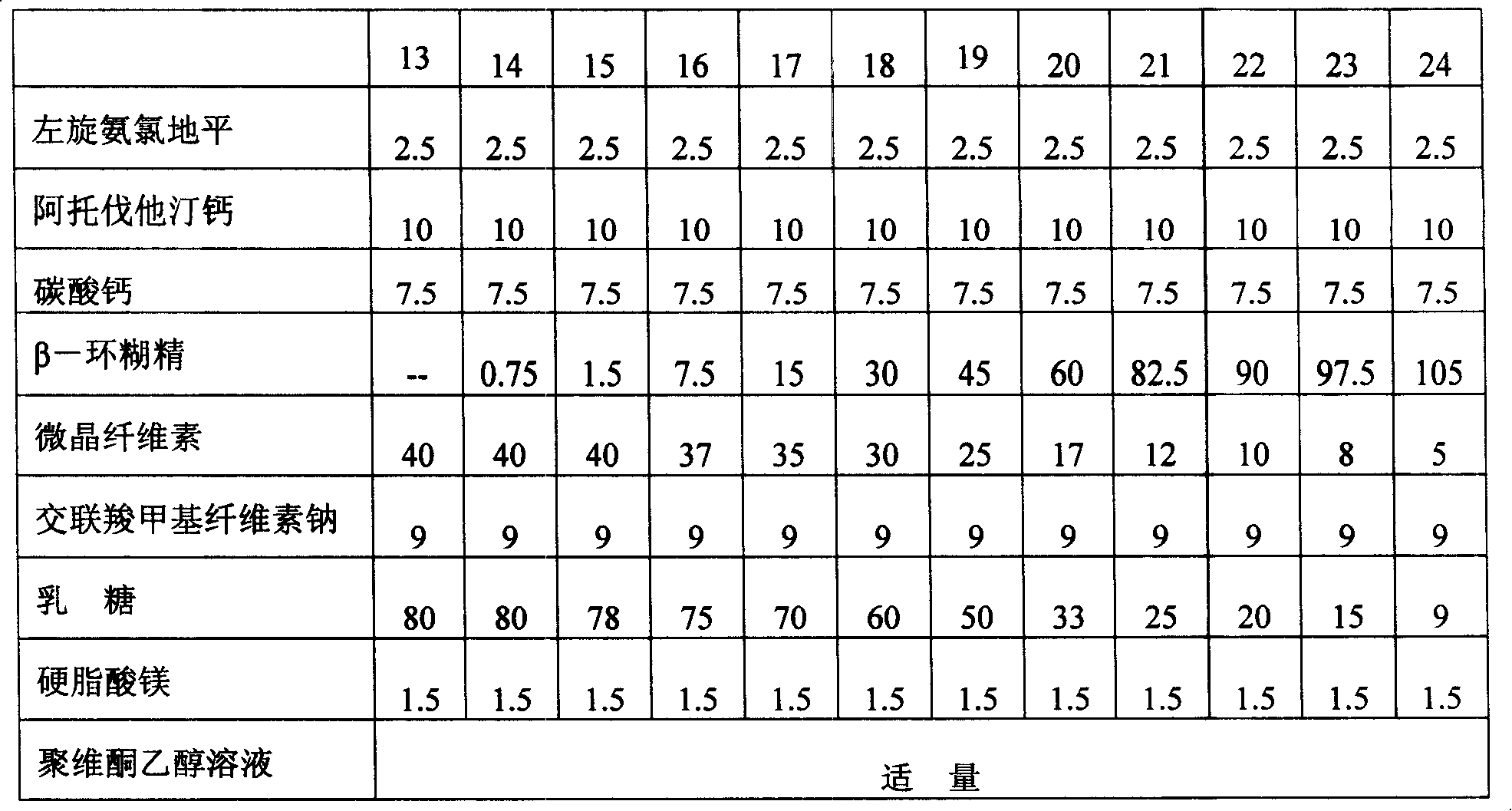
[0067] Embodiment 1: Atorvastatin calcium and levamlodipine maleate tablets
[0068] prescription:
[0069] Name Amount (g) Weight percent (%)
[0070] Levoamlodipine maleate 2.5 1.7
[0071] (equivalent to levamlodipine)
[0072] Atorvastatin Calcium 10 6.7
[0073] Calcium carbonate 1.5 1
[0074] Low-substituted hydroxypropyl cellulose 42 28
[0075] Croscarmellose sodium 9 6
[0076] β-cyclodextrin 15 10
[0077] Compressible starch 65 43.3
[0078] Magnesium stearate 1.5 1
[0079] Proper amount of povidone ethanol solution
[0080] Total weight 150g
[0081] Opadry Coating Powder Appropriate amount
[0082] The specific preparation method is as follows: the raw and auxiliary materials are pulverized respectively, and passed through a 100-mesh sieve for subsequent use; After mixing sodium carboxymethylcellulose, β-cyclodextrin, and compressible starch evenly, add an appropriate amount of the prepared adhesive povidone ethanol solution to make soft materials, g...
Embodiment 2
[0083] Embodiment 2: Atorvastatin calcium and levamlodipine maleate tablets
[0084] prescription:
[0085] Name Amount (g) Weight percent (%)
[0086] Levoamlodipine maleate 2.5 1.3
[0087] (equivalent to levamlodipine)
[0088] Atorvastatin Calcium 20 11.1
[0089] Calcium carbonate 5.4 3
[0090] Microcrystalline cellulose 102.4 56.9
[0091] Crospovidone 8 5
[0092] β-cyclodextrin 36 20
[0093] Hydroxypropyl methylcellulose ethanol solution qs 2.3
[0094] Magnesium stearate 1.5 1
[0095] Total weight 180
[0096] Opadry Coating Powder Appropriate amount
[0097] The specific preparation method is as follows: the raw and auxiliary materials are pulverized respectively, and passed through a 100-mesh sieve for subsequent use; atorvastatin calcium, levamlodipine maleate, calcium carbonate, microcrystalline cellulose, and crospovidone are weighed. , β-cyclodextrin mixed evenly, add the prepared adhesive hydroxypropyl methylcellulose ethanol solution to make soft...
Embodiment 3
[0098] Embodiment 3: Atorvastatin and levamlodipine besylate tablets
[0099] prescription:
[0100] Name Amount (g) Weight percent (%)
[0101] Levoamlodipine Besylate
[0102] (equivalent to levamlodipine) 2.5 1.0
[0103] Atorvastatin Calcium 40 16.7
[0104] Calcium carbonate 12 5
[0105] Microcrystalline cellulose 50 20.8
[0106] Crospovidone 12 5
[0107] Methyl-β-cyclodextrin 24 10
[0108] Lactose 92 38.3
[0109] Proper amount of hydroxypropyl methylcellulose ethanol solution
[0110] Magnesium stearate 1.5 0.6
[0111] Total weight 240
[0112] Opadry Coating Powder Appropriate amount
[0113] The specific preparation method is as follows: the raw and auxiliary materials are pulverized respectively, and passed through a 100-mesh sieve for subsequent use; the prescribed amount of atorvastatin calcium, levamlodipine besylate, calcium carbonate, microcrystalline cellulose, and crospovidone are weighed. , methyl-β-cyclodextrin, and lactose are mixed evenly,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com