Therapeutic applications of laminin and laminin-derived protein fragments
a technology of laminin and laminin-derived protein, which is applied in the field of discovery, identification and use of laminin, laminin-derived protein fragments, and laminin-derived polypeptides, can solve the problems of neuronal cell death, toxicity and neuronal cell death, and the fact that this potential fibrillar protein remains soluble in circulating biological fluids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Binding of Laminin to the Beta-Amyloid Protein (Aβ) of Alzheimer's Disease
[0073]2 μg of Aβ (1-40)(Bachem Inc., Torrance, Calif. USA; Lot #WM365) in 40 μl of Tris-buffered saline (TBS)(pH 7.0) was allowed to bind overnight at 4° C. to microtiter wells (Nunc plates, Maxisorb). The next day all of the microtiter wells were blocked by incubating with 300 μl of Tris-buffered saline containing 100 mM Tris-HCl, 50 MM NaCl, 0.05% Tween-20, and 3 mM NaN3 (pH 7.4)(TTBS) plus 2% bovine serum albumin (BSA). Various dilutions (ie. 1:10, 1:30, 1:90, 1:270, 1:810, 1:2430 and 1:7290) of Engelbreth-Holm-Swarm (EHS) mouse tumor laminin (1 mg / ml)(Sigma Chemical Co., St. Louis, Mo., USA) in 250 μl of TBS (pH 7.4) were placed in wells (in triplicate) either containing substrate bound Aβ (1-40) or blank, and allowed to bind overnight at 4° C. overnight. The next day, the wells were rinsed 3 times with TTBS, and then probed for 2 hours with 100 μl of rabbit anti-laminin antibody (Sigma Chemical Company, S...
example 2
Inhibition of Alzheimer's Disease Aβ Fibril Formation by Laminin
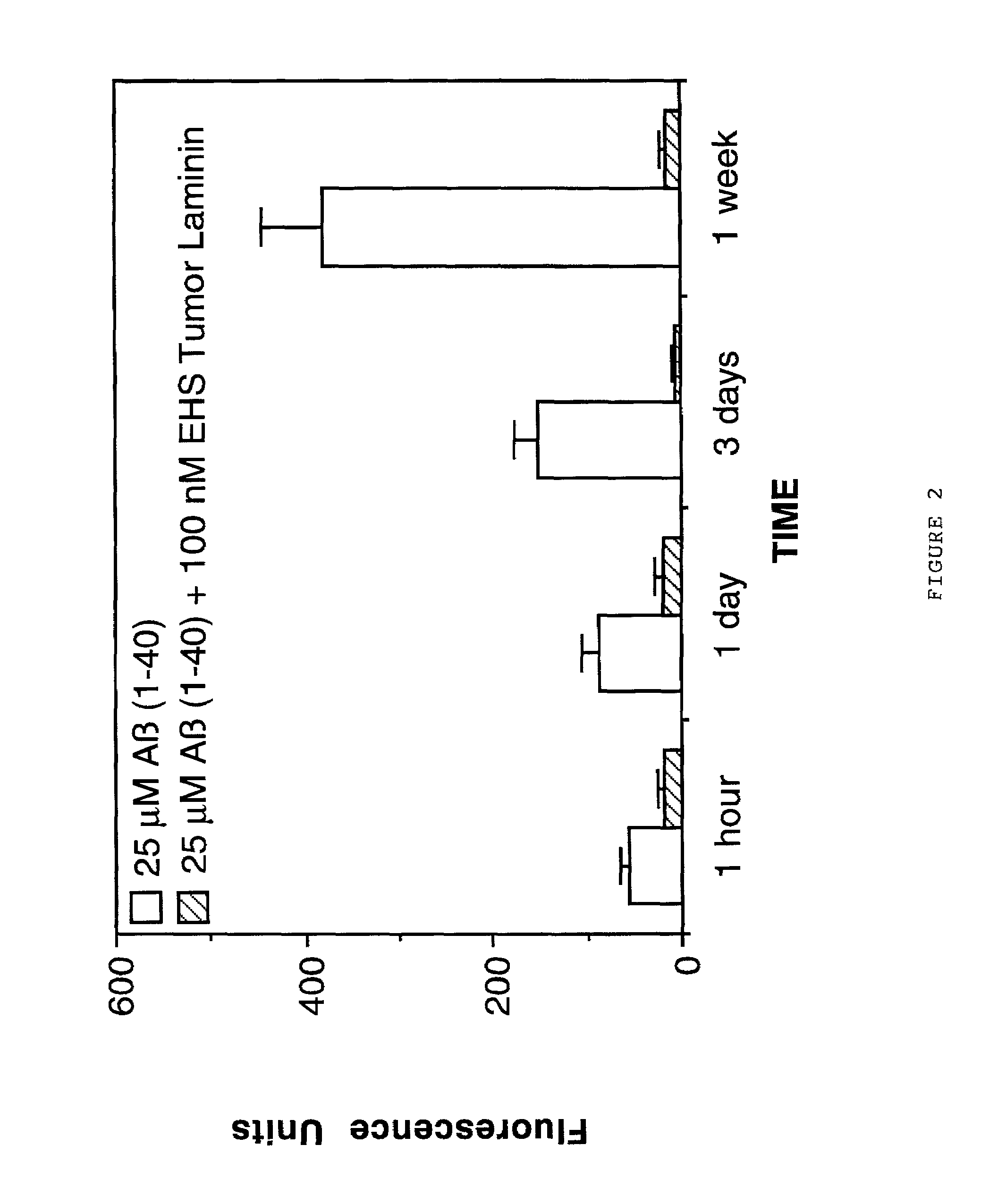
[0077]The effects of laminin on Aβ fibrillogenesis was also determined using the previously described method of Thioflavin T fluorometry (Naiki et al, Lab. Invest. 65:104-110, 1991; Levine III, Protein Sci. 2:404-410, 1993; Levine III, Int. J. Exp. Clin. Invest. 2:1-6, 1995; Naiki and Nakakuki, Lab. Invest. 74:374-383, 1996). In this assay, Thioflavin T binds specifically to fibrillar amyloid and this binding produces a fluorescence enhancement at 480 nm that is directly proportional to the amount of amyloid fibrils formed (Naiki et al, Lab. Invest. 65:104-110, 1991; Levine III, Protein Sci. 2:404-410, 1993; Levine III, Int. J. Exp. Clin. Invest. 2:1-6, 1995; Naiki and Nakakuki, Lab. Invest. 74:374-383, 1996). In a first study, the effects of EHS laminin on Aβ (1-40) fibrillogenesis was assessed. For this study, 25 μM of freshly solubilized Aβ (1-40)(Bachem Inc., Torrance, Calif., USA; Lot #WM365) was incubated in micro...
example 3
Laminin Causes Dose-Dependent Dissolution of Pre-Formed Alzheimer's Disease Amyloid Fibrils
[0082]The next study was implemented to determine whether laminin was capable of causing a dose-dependent dissolution of pre-formed Alzheimer's disease Aβ (1-40) amyloid fibrils. This type of activity would be important for any potential anti-Alzheimer's amyloid drug which can be used in patients who already have substantial amyloid deposition in brain. For example, Alzheimer's disease patients in mid-to late stage disease have abundant amyloid deposits in their brains as part of both neuritic plaques and cerebrovascular amyloid deposits. A therapeutic agent capable of causing dissolution of pre-existing amyloid would be advantageous for use in these patients who are at latter stages of the disease process.
[0083]For this study, 1 mg of Aβ (1-40)(Bachem Inc., Torrance, Calif., USA; Lot #WM365) was dissolved in 1.0 ml of double distilled water (1 mg / ml solution) and then incubated at 37° C. for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com