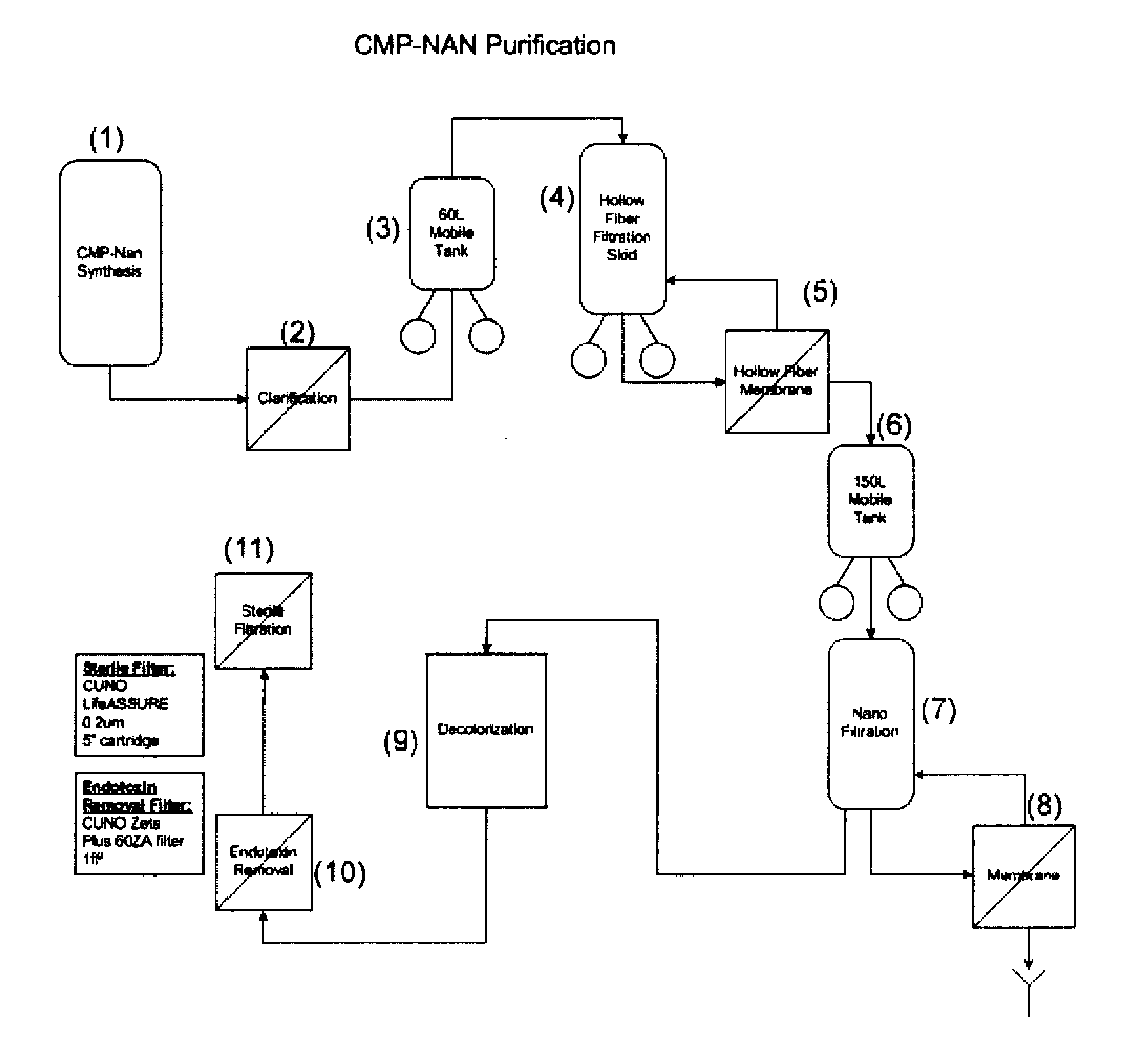
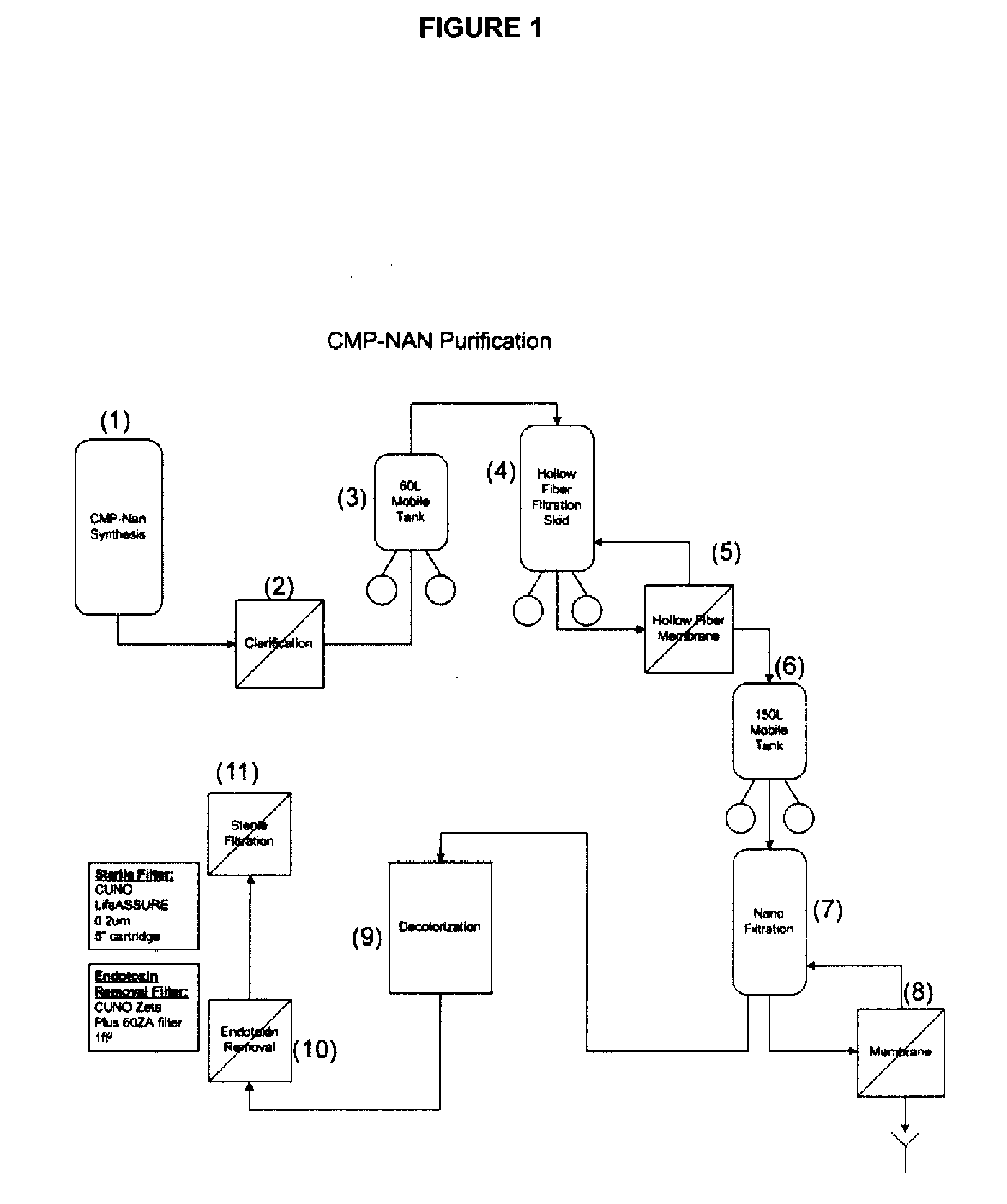
Nucleotide Sugar Purification Using Membranes
a technology of nucleotide sugar and membrane, which is applied in the direction of sugar derivates, organic chemistry, chemistry apparatus and processes, etc., can solve the problems of unfavorable presence of contaminants, high cost of chromatographic purification methods, and inability to use them for commercial production of saccharides, etc., to achieve a higher carbohydrate ratio and carbohydrate ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fmoc-Glycyl-Mannosamine Synthesis and Purification
[0146]The synthesis of Fmoc-glycyl-mannosamine (FGM) occurred in a non-aqueous solution involving two main compounds: D-Mannosamine HCl and Fmoc-Glycyl-OSu. Both materials were dry powders that were introduced into a system comprised of anhydrous methanol and sodium methoxide. The reaction was agitated at 25° C. for 1 hThe reaction was complete when the FGM concentration was greater than 15 mg / mL, determined by HPLC. The FGM synthesis was then rotovapped (20° C.) to about 8% of the initial volume. The chromatographic purification was performed using a Biotage pre-packed silica column. The FGM solution was loaded onto the column in a 50:50 CHCl3:CH3OH solution. The silica column was then washed with 18 column volumes (CV) of 3% CHCl3 / 97% CH3OH. Following the wash, FGM was eluted from the column using 14 CV of 15% CHCl3 / 85% CH3OH. Fractions containing material were pooled and then rotovapped (20° C.) to dryness and stored at 4° C. The ...
example 2
I. Description of CMP-Glycyl-Sialic Acid and CMP-Sialic Acid-PEG Synthesis and Purification
[0149]The production of CMP-Sialic Acid-PEG (PSC) was performed in two segments. First, a key intermediate, CMP-Glycyl-Sialic Acid (GSC), was synthesized, purified, and dried, and second, this intermediate was PEGylated, purified, and dried. A synthetic pathway for CMP-SA-PEG is shown below.
Synthetic Pathway for CMP-SA-PEG
[0150]The first step in the synthesis of GSC was the reaction of mannosamine with Fmoc-Gly-OSu in methanol under basic conditions. The resulting Fmoc-glycyl-mannosamine was purified on a silica flash chromatography column. The purified Fmoc-glycyl-mannosamine then entered a two step enzymatic reaction. Fmoc-glycyl-mannosamine (FGM) was reacted with pyruvate to convert to Fmoc-glycyl-sialic acid. This reaction was catalyzed by a commercially available sialic acid aldolase. Fmoc-glycyl-sialic acid was then coupled to cytidine-5′-monophosphate through a CMP-NAN synthetase cataly...
example 3
I. Summary of Consistency Batches of 10k and 20k Cmp-SA-PEG After Process Development
[0164]Consistency batches were performed for 10K and 20K CMP-Sialic Acid-PEG after development of the synthesis and purification operations. These batches demonstrated that a reproducible process had been developed to produce high-purity CMP-SA-PEG with very low contaminant levels, suitable for the glycopegylation projects.
[0165]10K CMP-SA-PEG was produced at greater than 80% purity at overall process yields of approximately 60%, and 20K CMP-SA-PEG was produced at greater than 70% purity at overall process yields of approximately 50%. The products were low in endotoxin, bioburden, and protein, and NMR has shown that the balance of the material was nearly all mPEG-OH, a by-product of the synthesis process.
Materials and Methods
[0166]CMP-SA-PEG (PSC) was produced in a reaction of CMP-Glycyl-Sialic Acid (GSC) with paranitrophenyl-carbomate-polyethylene glycol (pNP-PEG). The reaction conditions for the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
| Atomic weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com