Apparatus and method for hydrogen generation from gaseous hydride
a technology of gaseous hydride and apparatus, which is applied in the direction of electrochemical generators, other chemical processes, separation processes, etc., can solve the problems of global pollution, carbon dioxide release, and carbon dioxide release in the atmosphere, and achieve the effects of reducing the number of carbon dioxide emissions, and reducing the amount of carbon dioxide released into the atmospher
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
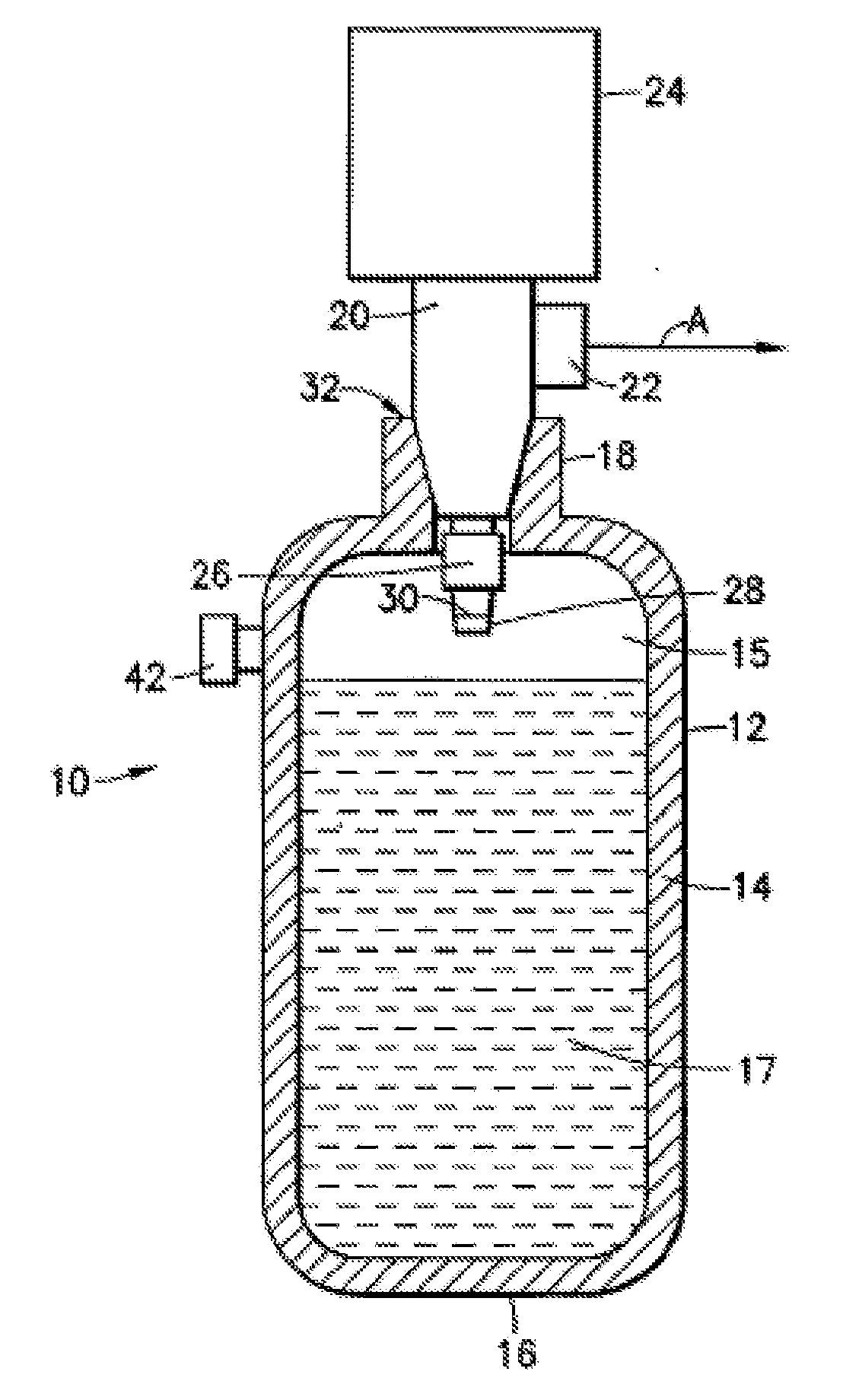
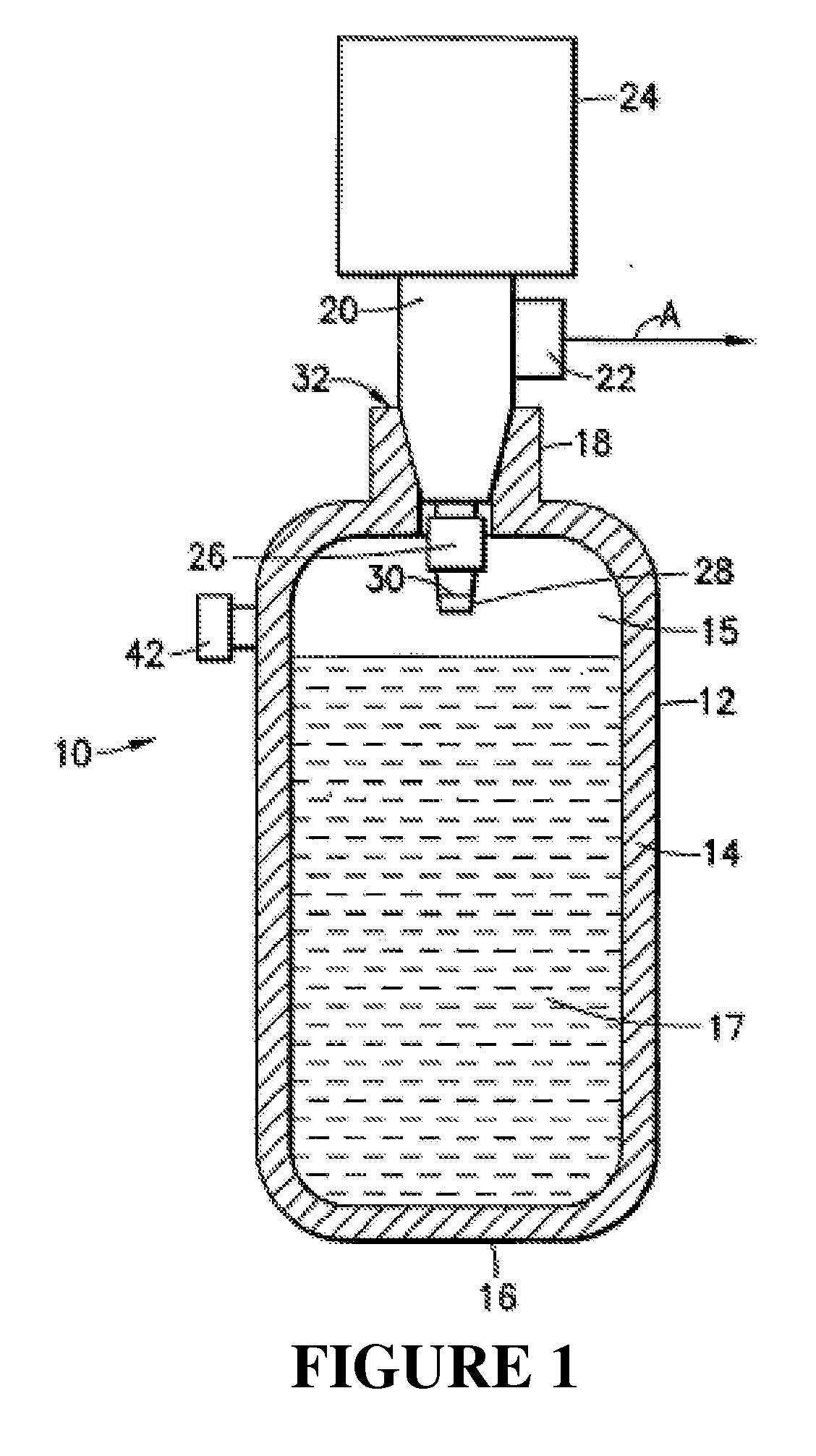
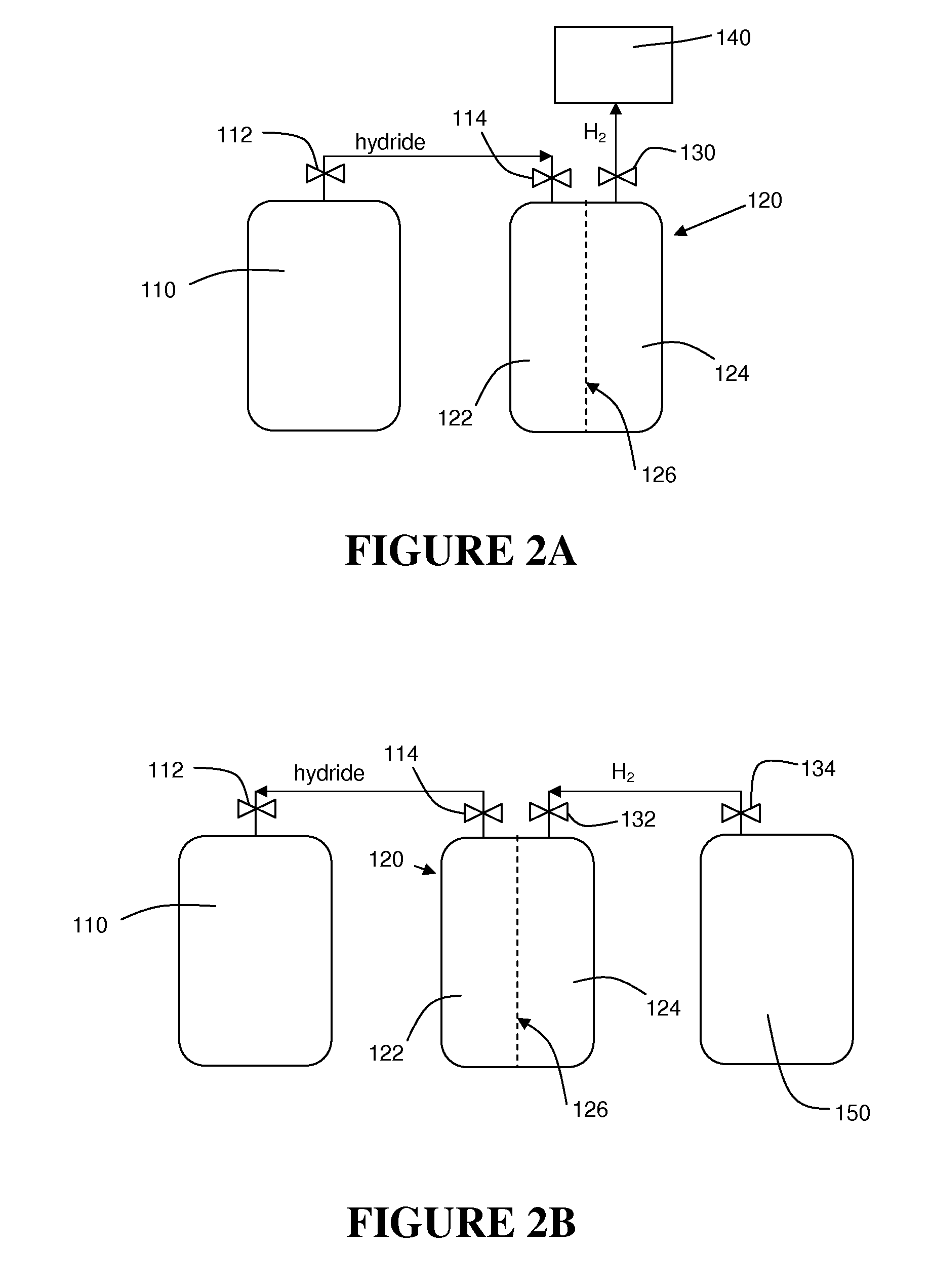
[0044]The instant invention resolves the problem of insufficient hydrogen storage capacity in a vessel or tank, while simultaneously permitting the safe and efficient generation of hydrogen fuel. Specifically, this invention relates to an apparatus and method using storage and dispensing vessels that safely store and dispense gaseous hydrides, and in which the gaseous hydride is decomposed to generate hydrogen gas.
[0045]U.S. Pat. No. 5,528,518, issued May 21, 1996 in the names of Glenn M. Tom and James V. McManus, and U.S. Pat. No. 6,089,027, issued Jul. 18, 2000 in the names of Luping Wang and Glenn M. Tom, are hereby incorporated by reference herein in their entireties.
[0046]The invention provides an alternative to conventional hydrogen storage methods. The invention embodies the following operational aspects:[0047]A. Safe storage of a gaseous hydride material in a reduced pressure vessel; and[0048]B. Decomposition of the gaseous hydride in order to generate H2 according to the fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Division | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| vapor pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com