Embolic Device Delivery Systems
a technology of embolic devices and delivery systems, applied in the field of embolic device delivery systems, can solve the problems of potentially pathological thrombosis within the parent vessel, the procedure may become even more complicated, and the physician's skill may be even greater, so as to improve the device trackability, delivery and the ability to recapture, and the effect of minimizing in-catheter/sheath forces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0071]Various exemplary embodiments of the invention are described below. Reference is made to these examples in a non-limiting sense. They are provided to illustrate more broadly applicable aspects of the present invention. Various changes may be made to the invention described and equivalents may be substituted without departing from the true spirit and scope of the invention. In addition, many modifications may be made to adapt a particular situation, material, composition of matter, process, process act(s) or step(s) to the objective(s), spirit or scope of the present invention. All such modifications are intended to be within the scope of the claims made herein.
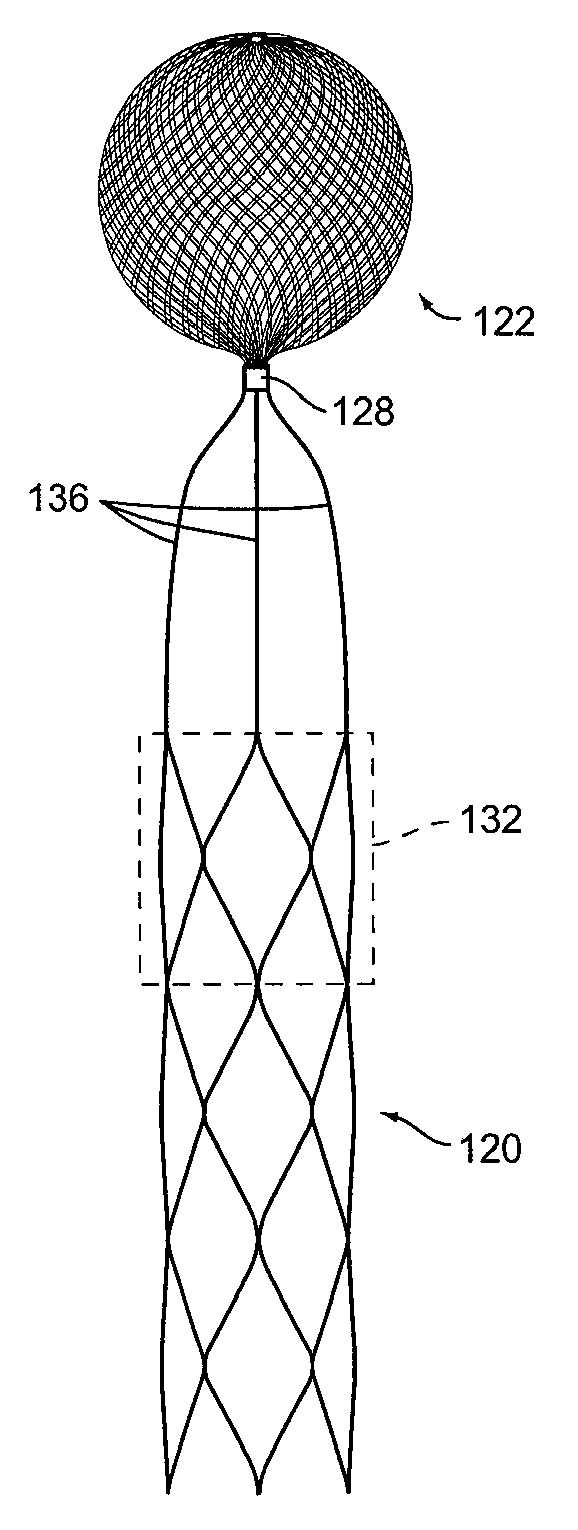
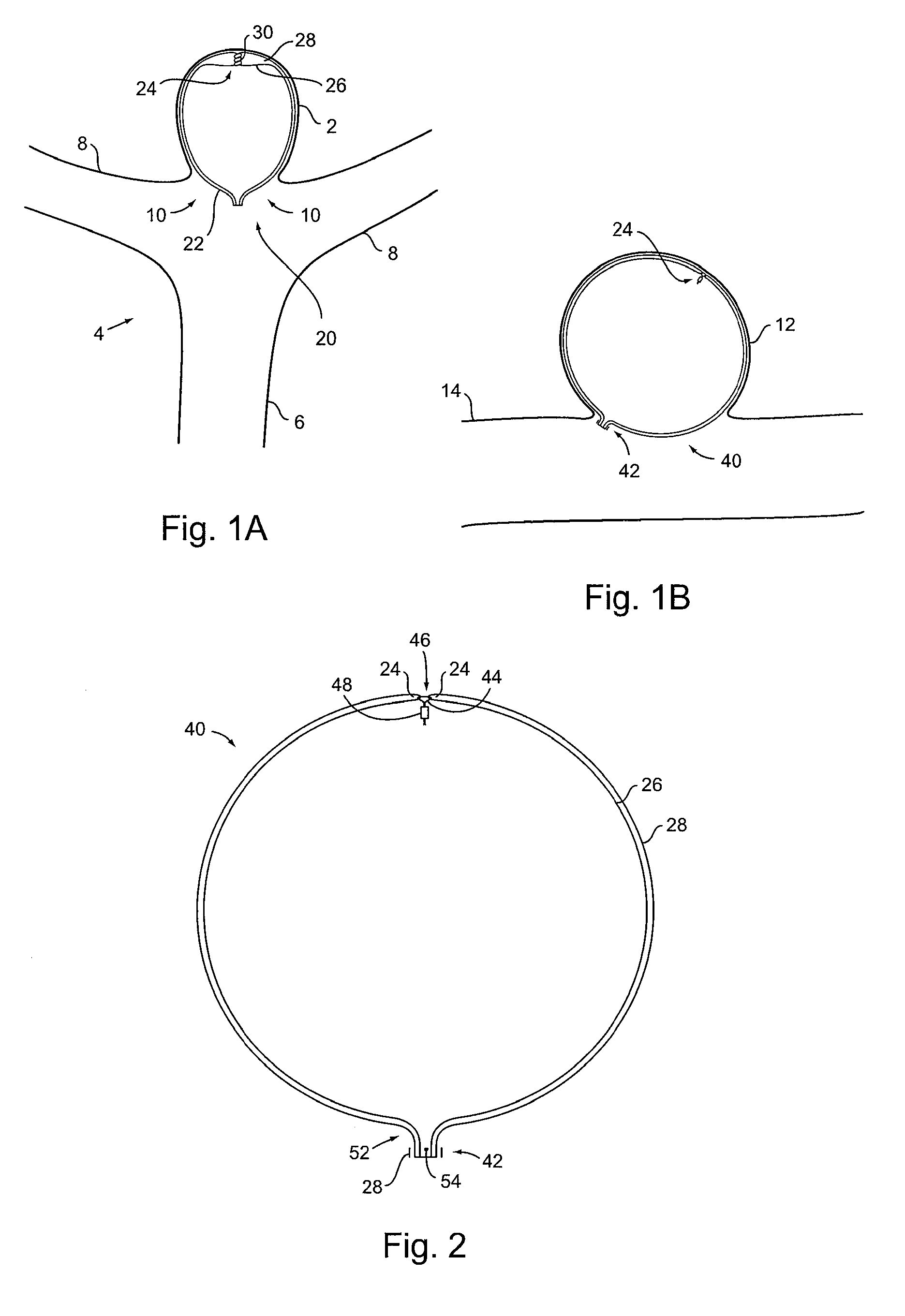
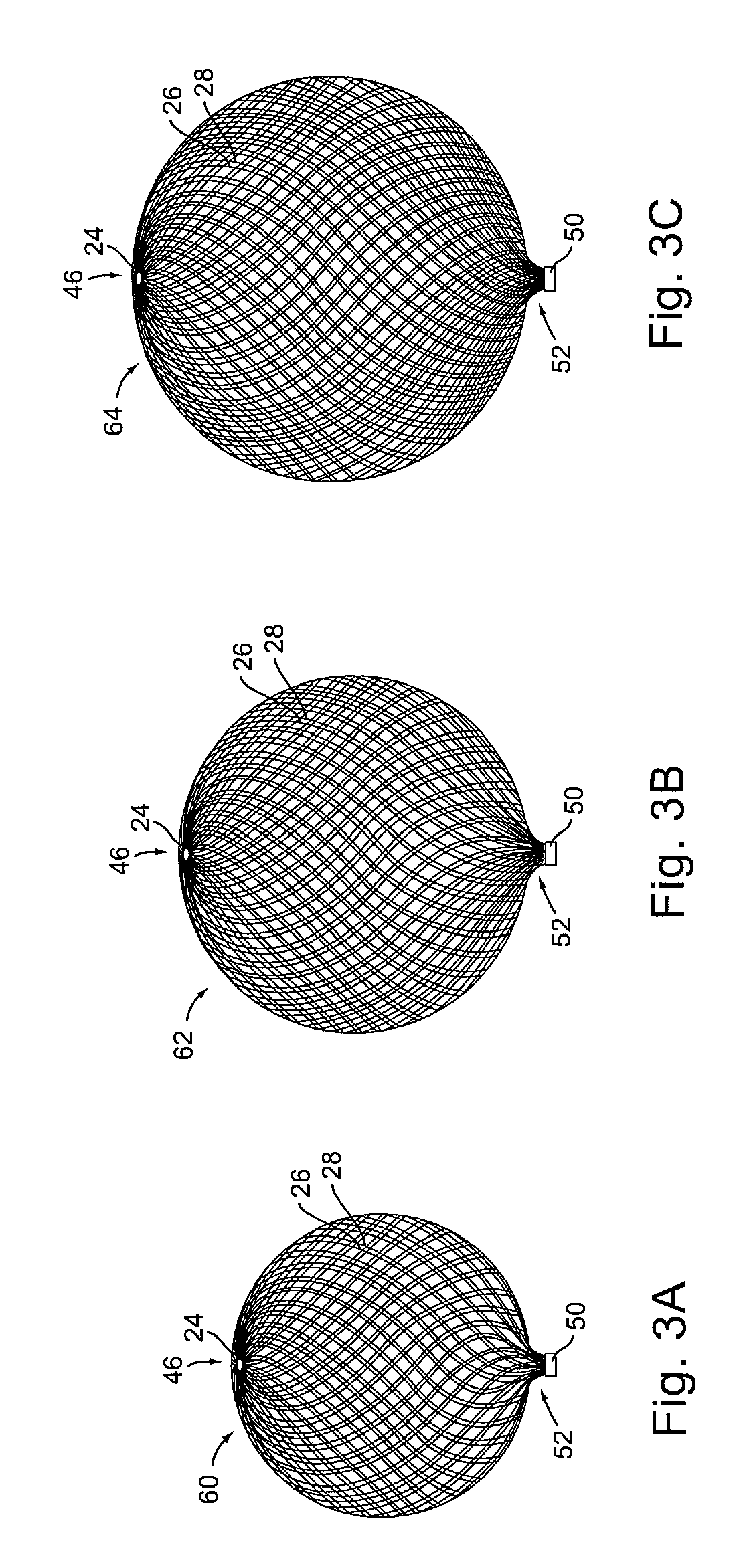
[0072]Turning to FIG. 1A, it shows a first implant 20 according to the present invention. It is formed from tubular braid stock comprising a resilient material such as Nitinol, that defines an open volume (generally round, spherical, ovular, heart-shaped, etc.) in an uncompressed / constrained state.
[0073]Implant 20 is set...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com