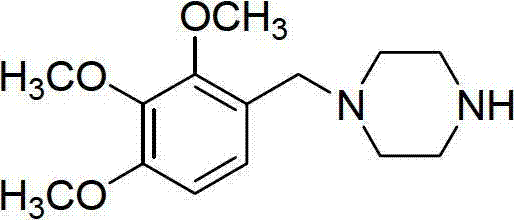
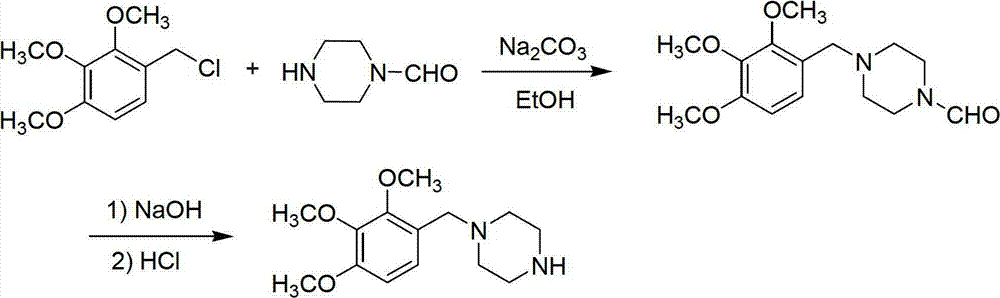
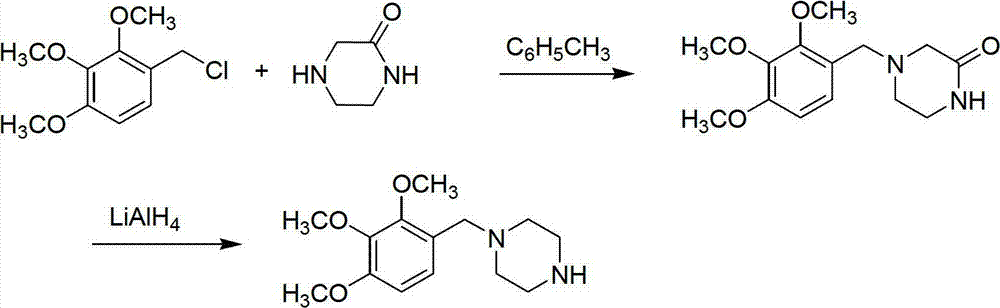
Preparation method of trimetazidine
A technology of trimetazidine and a production method, which is applied in the field of preparation of pharmaceutical compounds, can solve problems such as difficult industrial production, unfavorable production, and unfavorable amplification, and achieve the effects of high yield, enhanced safety, and reduced synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Put 180g of 2,3,4-trimethoxybenzaldehyde, 553g of piperazine, and 800mL of methanol into the reactor, quickly raise the temperature of the reaction system to 63±3°C, then add 733g of formic acid into the reaction system, raise the temperature to 85°C and keep At this temperature for 3 hours, methanol was distilled off under reduced pressure, then 800mL of 40% sodium hydroxide solution was added to the reaction system, the temperature was raised to 100°C and kept at this temperature for 1 hour, the pH was adjusted to 11, the temperature was lowered, and used at 12±2°C 8N hydrochloric acid to adjust the pH value of the material liquid to 1, wash 3 times with 200mL of dichloromethane each, and then neutralize the aqueous phase with 520g40% sodium hydroxide to a pH value of 12, extract 3 times with 100mL of toluene each, anhydrous sulfuric acid Magnesium drying, vacuum rotary evaporation to obtain trimetazidine 217.3g, yield: 89%.
Embodiment 2
[0038] Put 180g of 2,3,4-trimethoxybenzaldehyde, 620g of piperazine, and 800mL of toluene into the reactor, quickly raise the temperature of the reaction system to 103±3°C, then add 648g of formic acid into the reaction system, raise the temperature to 105°C and keep At this temperature for 3.5 hours, toluene was evaporated under reduced pressure, then 800mL of 40% sodium hydroxide solution was added to the reaction system, the temperature was raised to 105°C and kept at this temperature for 1.5 hours, the pH was adjusted to 12, the temperature was lowered, and used at 8±3°C Adjust the pH value of the feed solution to 1.5 with concentrated hydrochloric acid, wash with 200mL of chloroform three times, neutralize the water phase with 490g of 40% sodium hydroxide to pH 10, extract with 100mL of toluene three times, and dry over anhydrous magnesium sulfate , 202.7 g of trimetazidine was obtained by rotary evaporation under reduced pressure, yield: 83%.
Embodiment 3
[0040] Put 180g of 2,3,4-trimethoxybenzaldehyde, 720g of piperazine, and 800mL of toluene into the reactor, quickly raise the temperature of the reaction system to 103±3°C, then add 648g of formic acid into the reaction system, raise the temperature to 110°C and keep At this temperature for 5 hours, toluene was evaporated under reduced pressure, then 800mL of 40% sodium hydroxide solution was added to the reaction system, the temperature was raised to 105°C and maintained at this temperature for 1.5 hours, the pH was adjusted to 13, the temperature was lowered, and used at 5-10°C Concentrated hydrochloric acid to adjust the pH value of the feed solution to 2, wash with 200mL of chloroform for 3 times, then neutralize the water phase with 490g of 40% sodium hydroxide until the pH value is 10, extract with 100mL of toluene for 3 times, and dry over anhydrous magnesium sulfate , 202.7 g of trimetazidine was obtained by rotary evaporation under reduced pressure, yield: 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com