Orally taken control released trimetazidine medicine composition
A technology for trimetazidine and controlled-release drugs, applied in the field of oral trimetazidine pharmaceutical compositions, can solve the problems of incomplete drug release, prone to time lag, and incomplete utilization of dosage, and achieve plasma drug concentration Smooth, effective and safe effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
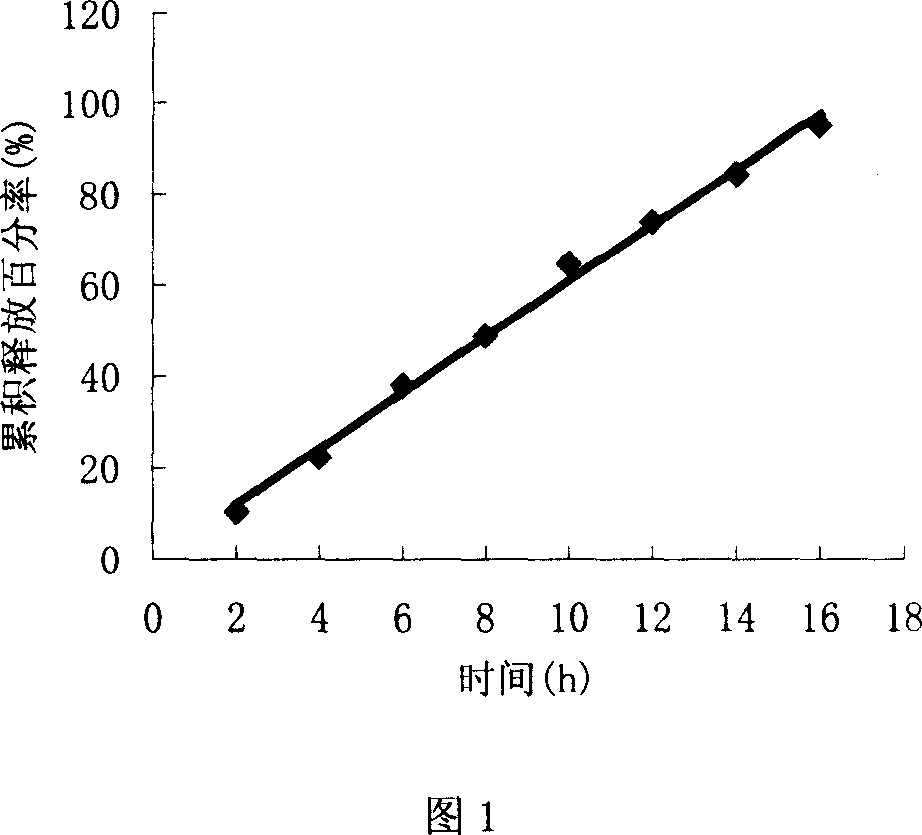
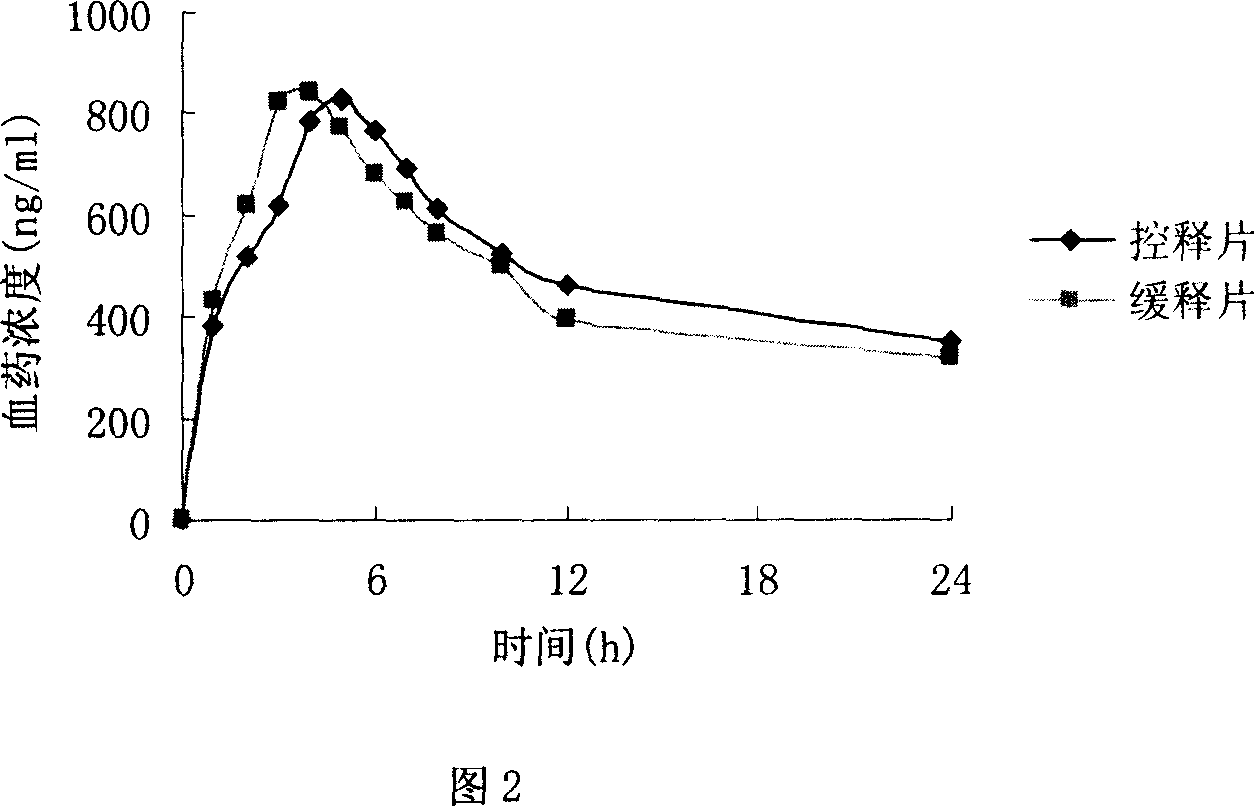
Image
Examples
Embodiment 1
[0096] (1) Drug-containing layer
[0097] Trimetazidine Hydrochloride 35g
[0098] Polyoxyethylene 50g
[0099] Hypromellose 4g
[0100] Magnesium stearate 0.6g
[0101] (2) booster layer
[0102] Polyoxyethylene 30g
[0104] Hypromellose 2g
[0105] Iron oxide red 0.5g
[0106] Magnesium stearate 0.5g
[0107] Makes 1000 pieces
[0108] (3) Prescription of coating solution (1000 tablets dosage)
[0109] Cellulose acetate 10g
[0110] Polyethylene glycol 0.5g
[0111] Proper amount of acetone
[0112] Anhydrous ethanol amount
[0113] Preparation:
[0114] 1. Sieve, mix and granulate the raw and auxiliary materials of the drug-containing layer and the booster layer according to the above-mentioned prescription amount, and press the drug-containing layer and the booster layer into a double-layer tablet core by two-time tablet compression technology;
[0115] 2. Preparation of coating solution: mix acetone and absolute ethanol, then ad...
Embodiment 2
[0119] (1) Drug-containing layer
[0120] Trimetazidine Hydrochloride 60g
[0122] Polyoxyethylene 80g
[0123] Hypromellose 6g
[0125] 75% ethanol appropriate amount
[0126] (2) booster layer
[0127] Polyoxyethylene 45g
[0129] Hypromellose 4g
[0130] Iron oxide 1g
[0131] Magnesium Stearate 1g
[0132] 75% ethanol appropriate amount
[0133] Makes 1000 pieces
[0134] (3) Prescription of coating solution (1000 tablets dosage)
[0135] Cellulose acetate 15g
[0136] Polyethylene glycol 1g
[0137] Proper amount of acetone
[0138]Anhydrous ethanol amount
[0139] Preparation:
[0140] 1. Sieve and mix the raw and auxiliary materials of the drug-containing layer and the booster layer according to the above-mentioned prescription quantities, and then mix wet granulation, and use the two-time compression technology to compress the drug-containing layer and the booster l...
Embodiment 3
[0143] (1) Drug-containing layer
[0144] Trimetazidine Hydrochloride 70g
[0145] Sodium chloride 5g
[0146] Polyoxyethylene 104g
[0147] Hypromellose 8g
[0148] Magnesium Stearate 2g
[0149] Silica 2g
[0150] (2) booster layer
[0151] Polyoxyethylene 58g
[0152] Sodium chloride 44g
[0153] Hypromellose 6g
[0154] Iron oxide red 1g
[0155] Magnesium Stearate 1g
[0156] Makes 1000 pieces
[0157] (3) Prescription of coating solution (1000 tablets dosage)
[0158] Cellulose acetate 30g
[0159] Polyethylene glycol 1.5g
[0160] Proper amount of acetone
[0161] Preparation method: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com