A kind of solid pharmaceutical composition containing trimetazidine or its salt and its preparation method
A composition and drug technology, applied in the field of trimetazidine solid pharmaceutical compositions, can solve the problems of poor particle fluidity, unfavorable patients, increase in impurities and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] With trimetazidine hydrochloride, calcium hydrogen phosphate, povidone K30, according to the ratio in table 1, adopt the high-speed shearing wet granulation process, carry out granulation with purified water, dry processing then, dry granule (moisture is less than 2 %) for dry sizing, adding the prescribed amount of sustained-release matrix material and lubricant, mixing with a rotary blender, and compressing the obtained blended granules into tablets.
[0043] Table 1
[0044]
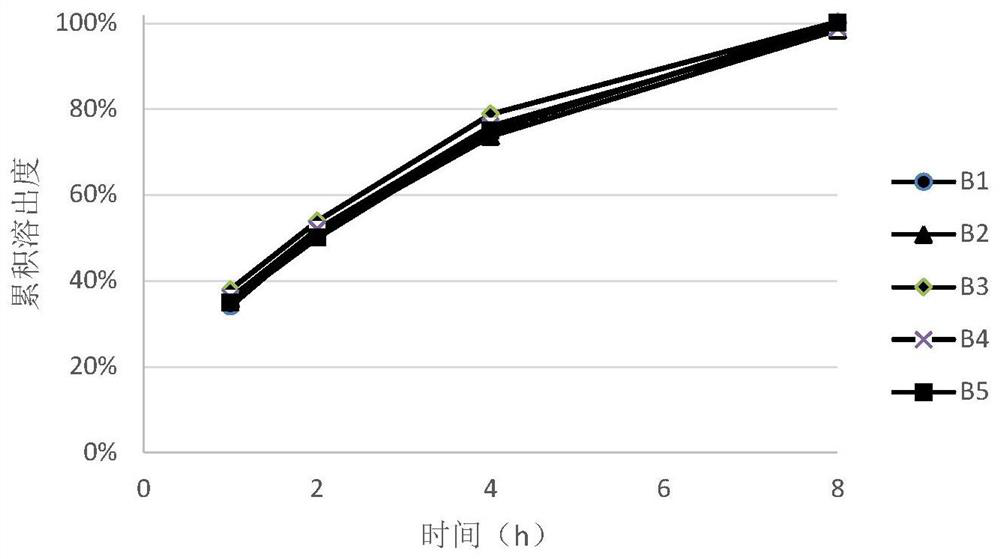
[0045] After the end of the blending, the blended granules with different prescriptions were taken to investigate the fluidity of the granules. During the tableting process, adjust the tableting parameters to ensure consistent tableting pressure, and investigate the hardness of the tablets made of granules prepared with different prescriptions under the same pressure conditions. After the tablet compression was completed, 10 tablets were randomly selected from the tablets of each prescripti...
Embodiment 2
[0051] Trimetazidine hydrochloride, calcium hydrogen phosphate, povidone K30, according to the ratio in table 3, adopt the high-speed shearing wet granulation process, carry out granulation with purified water, dry processing then, dry granule (moisture is less than 2%) ) for dry sizing, adding the prescribed amount of slow-release matrix material and lubricant, and mixing with a rotary blender. The resulting blended granules were compressed into tablets.
[0052] table 3
[0053]
[0054]
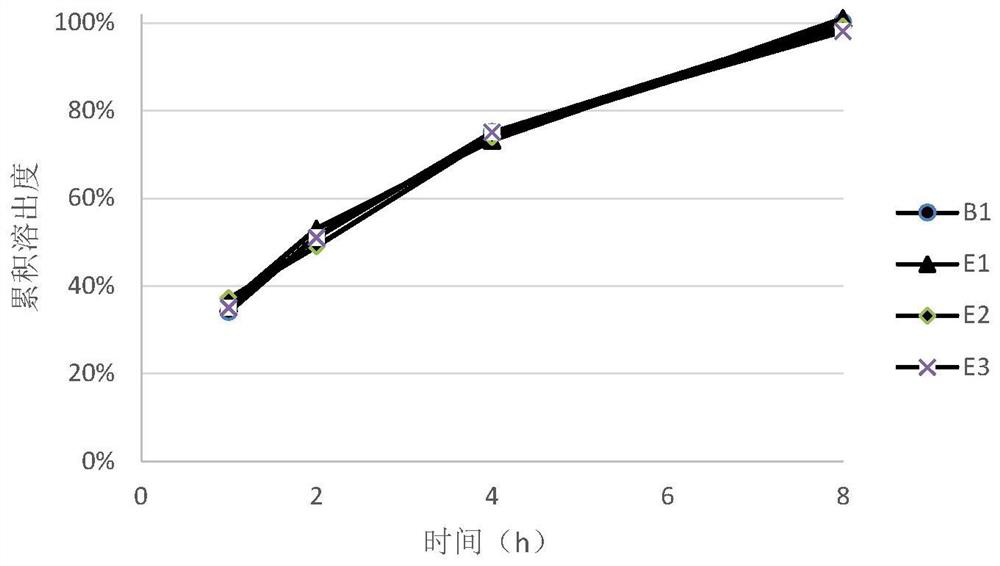
[0055] It can be seen from the measurement results of the angle of repose of the blended granules of each prescription in Table 3 that the preparation of the present invention is used, and the ratio of hydroxypropyl methylcellulose to polyoxyethylene in the prescription is within the range of 1:3 and 1:0.3 At the same time, the mixed granules of the prescription all showed excellent fluidity, and the content uniformity of the final tablet was all small, all of which could reach the ...
Embodiment 3
[0066] Trimetazidine hydrochloride, calcium hydrogen phosphate, povidone K30, according to the ratio in table 6, adopt the high-speed shearing wet granulation process, carry out granulation with purified water, dry processing then, dry granule (moisture is less than 2%) ) for dry sizing, adding the prescribed amount of slow-release matrix material and lubricant, and mixing with a rotary blender. The resulting blended granules were compressed into tablets. Wherein prescription C adopts the preparation method and prescription in the patent CN1166408C.
[0067] Table 6
[0068]
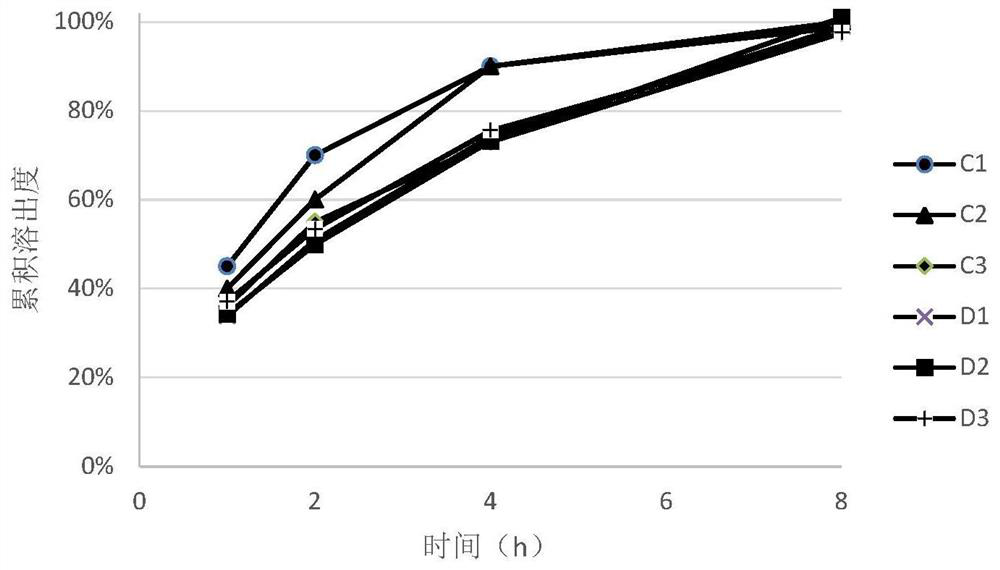
[0069] During the tablet compression process, prescription C and prescription D were respectively compressed into tablets with different hardness (see Table 7 for details), and the dissolution rates of tablets with different hardness were measured according to the aforementioned dissolution test method.
[0070] Table 7
[0071]
[0072] The trimetazidine hydrochloride sustained-release tablet o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com