Tripterine nano structure lipid carrier and preparation method and application thereof
A technology of nanostructured lipids and tripterine, applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of tripterine nanostructured lipid carrier
[0032] Prescription: take tripterine 60mg, monoglyceride 450mg, caprylic / capric glyceride 150mg, phospholipid 60mg, vitamin E polyethylene glycol succinate 60mg, poloxamer (1%, w / v) 150mL.
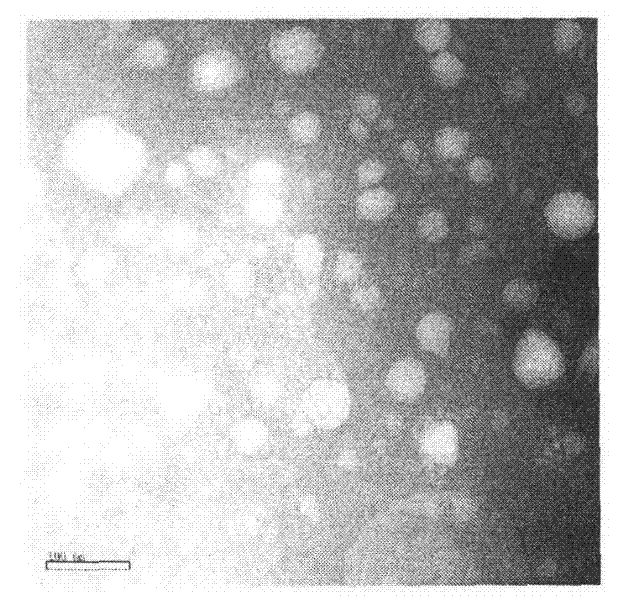
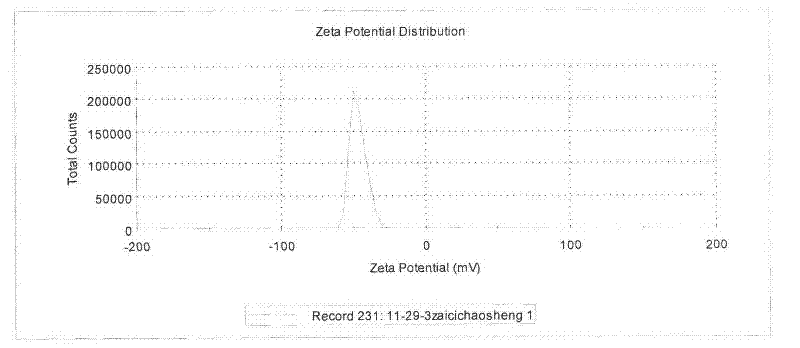
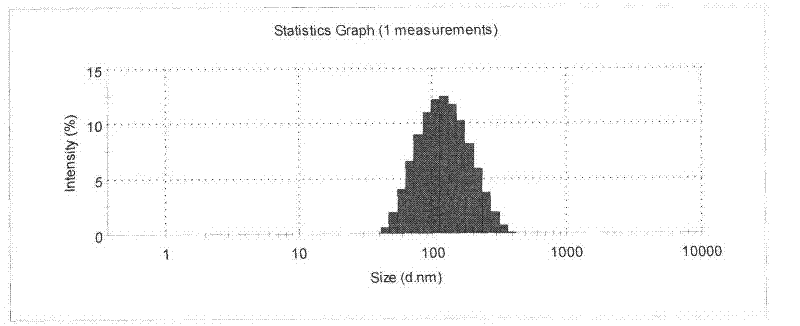
[0033] Preparation process: heat and melt monoglyceride at 80°C, then add caprylic / capric glycerides, phospholipids, vitamin E polyethylene glycol succinate and tripterine, fully dissolve and stir vigorously (4000r / min) Disperse the molten liquid into 150mL aqueous phase containing poloxamer (1%, w / v) at the same temperature to obtain colostrum, and emulsify the colostrum through a high-pressure homogenizer (500bar), and circulate for 3- 6 times; then cooling and solidifying in a low-temperature water phase (0-2° C.) to obtain tripterine nanostructured lipid carrier. The average particle size of the obtained nanoparticles is: 102.3nm, the electric potential is -38.7mv, the encapsulation efficiency is 97.4%, and the drug load...
Embodiment 2
[0035] Preparation of tripterine nanostructured lipid carrier lyophilized powder
[0036]Add 5% mannitol to the obtained tripterine nanostructured lipid carrier suspension prepared in Example 1 and freeze-dry. The resulting freeze-dried powder has good redispersibility. After redispersing, the particle diameter of nanoparticles, Potential is almost constant.
Embodiment 3
[0038] Preparation of tripterine nanostructured lipid carrier ointment
[0039] The tripterygine nanostructured lipid carrier lyophilized powder prepared in Example 2 was pulverized and passed through a 60-mesh sieve for later use. Take 335040g of polyethylene glycol and 40060g of polyethylene glycol, mix and heat to 65°C on a water bath, add 5g of vitamin E and 5g of sorbic acid, stir until condensed, and obtain a water-soluble ointment base. Add the sieved tripterine nanostructured lipid carrier into the above-mentioned matrix, and stir evenly to obtain.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com