Oral liquid sustained-release preparation containing codeine and chlorphenamine and preparation method thereof
A technology of chlorpheniramine and sustained-release preparations, which is applied in the preparation field of compound codeine oral sustained-release suspension and its industrial application, can solve the problem of increasing industrial production difficulty and production cost, being expensive and difficult to break through. Slow-release microcapsule coating and other problems, to achieve the effect of low production cost, good taste and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
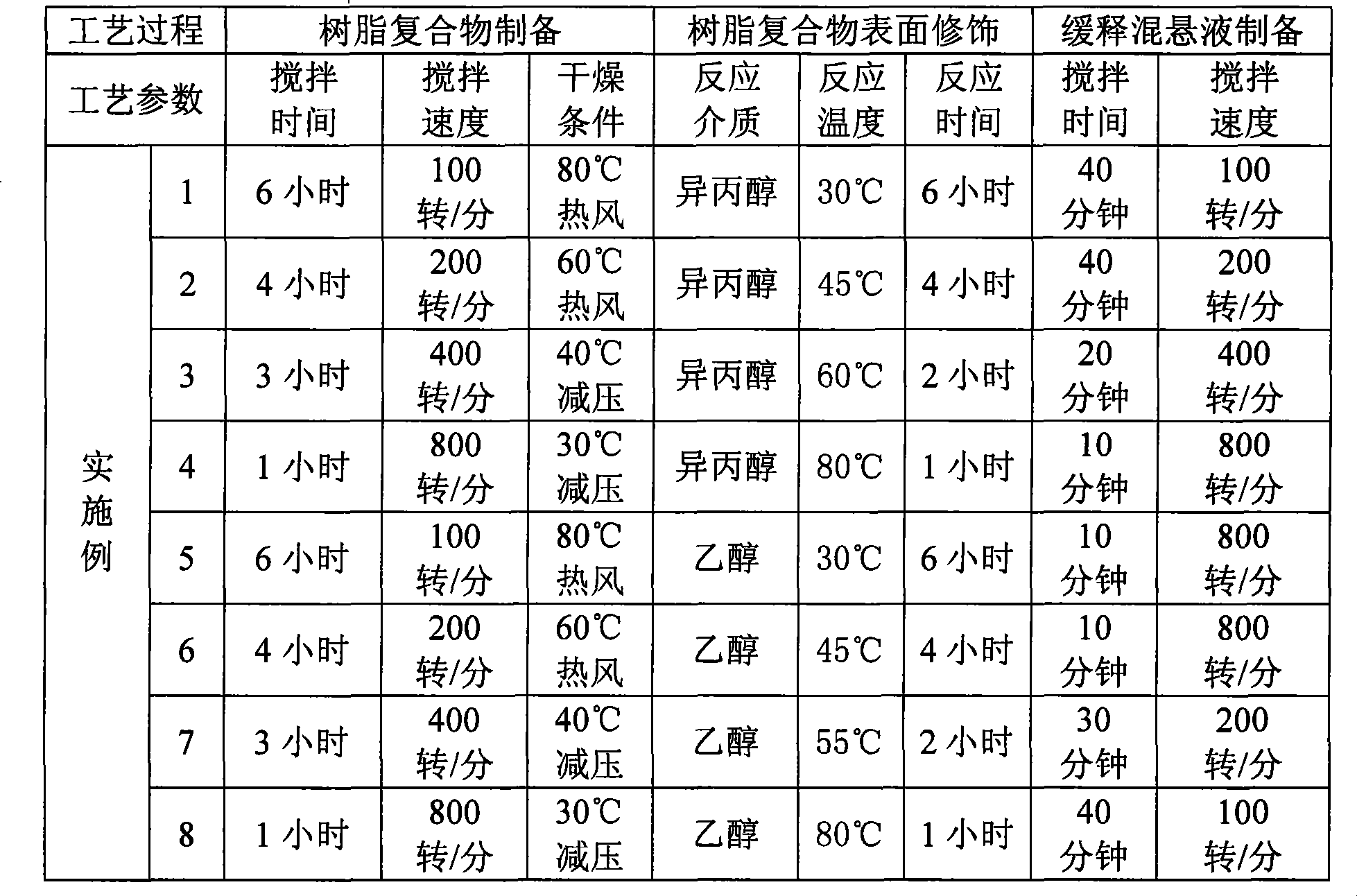
Examples
preparation example Construction
[0019] In the preparation method of the present invention, the active ingredients codeine and chlorpheniramine are respectively placed on the ion exchange resin to form microparticles with an average particle diameter of 50 microns; after the two kinds of microparticles are mixed according to the prescription amount, the compound formula is prepared by modifying the surface of the slow-release material Codeine-chlorpheniramine slow-release microparticles; slow-release microparticles are mixed with suspending agents, acidity regulators, heavy metal ion complexing agents, preservatives, flavoring agents, coloring agents and purified water commonly used in preparations to prepare Form a compound codeine-chlorpheniramine sustained-release suspension liquid preparation. The principle of the surface modification process is to use the slow-release material with ion-exchange ability groups to directly combine with the free groups after the ion-exchange resin adsorbs the drug, and form ...
Embodiment 1
[0029] Embodiment 1, 1000ml of oral liquid sustained-release preparation containing codeine and chlorpheniramine, formula:
[0030] Codeine Phosphate 1.48g
[0031] Chlorpheniramine Maleate 0.1g
[0032] Styrene strongly acidic cation exchange resin 1.0g
[0033] Xanthan Gum 3.0g
[0034] Disodium edetate 0.1g
[0035] Sodium dihydrogen phosphate 1.0g
[0036] Acrylic resin 0.01g
[0037] Hydroxypropyl Methyl Cellulose 1.2g
[0038] Microcrystalline Cellulose 1.0g
[0039] Paraben ethyl ester 2.7g
[0040] Sodium Benzoate 2.0g
[0041] Pigment 0.02g
[0042] Flavor 0.07g
[0043] Purified water up to 1000ml
[0044] Such as figure 1 with figure 2 As shown, the statistical mean diameter of the particle size is 50.39 microns.
Embodiment 2
[0045] Embodiment 2, 1000ml of oral liquid sustained-release preparation containing codeine and chlorpheniramine, formula:
[0046] Codeine Phosphate 14.8g
[0047] Chlorpheniramine Maleate 1.0g
[0048] Styrene strongly acidic cation exchange resin 105.0g
[0049] Sucrose 212.5g
[0050] EDTA 22.0g
[0051] Citric acid 50.0g
[0052] Sodium citrate 35.0g
[0053] Microcrystalline Cellulose 4.8g
[0054] Xanthan Gum 3.6g
[0055] Sodium Carboxymethyl Cellulose 1.2g
[0056] RL30D 52g
[0057] Paraben methyl ester 2.7g
[0058] Propylparaben 0.6g
[0059] Pigment 0.05g
[0060] Flavor 0.07g
[0061] Purified water up to 1000ml
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com