A kind of dabigatran etexilate solid lipid nanoparticles and preparation method thereof
A technology of solid lipid nanometer and dabigatran etexilate, which is applied in the field of medicine, can solve the problems of easy degradation, instability and low solubility of active ingredients, achieves good process feasibility and repeatability, avoids degradation and leakage, and achieves biological stability. High utilization effect
Active Publication Date: 2022-06-03
ZEIN BIOTECHNOLOGY CO LTD
View PDF6 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The dosage form on the market needs to be taken twice a day, and the stability is poor. It needs to be packaged in a hard double aluminum blister pack with extremely high moisture resistance. After the capsule is taken out, it is very unstable when exposed to ambient humidity, and the active ingredients are easy to degrade; Environmental humidity and product moisture control are extremely strict, which increases production costs and risks
[0007] Most of the research on dabigatran etexilate preparations in the prior art is to continue the idea that the commercially available preparations only solve the problem of low solubility, but have not studied to solve the technical problem of P-gp efflux
Chinese patent (publication number CN105997868A) discloses a kind of dabigatran etexilate nano-mixed micelles, and adopts the film dispersion method to prepare the soluplus / VitaminE-TPGS nano-mixed micelles of dabigatran etexilate, although the improvement of solubility and solution The problem of P-gp efflux, but the preparation process of this dosage form is currently difficult to achieve industrial production, and requires the use of a large amount of organic solvents, which seriously pollutes the environment and does not meet the increasingly stringent environmental protection requirements
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
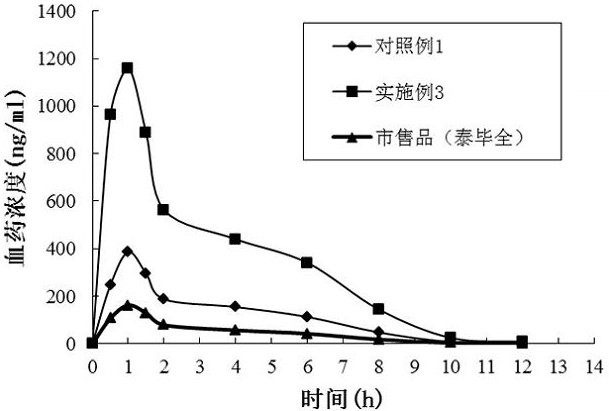
The invention provides a dabigatran etexilate solid lipid nanoparticle, which is composed of dabigatran etexilate or a pharmaceutically acceptable salt thereof and a solid lipid nanoparticle carrier material. The solid lipid nanoparticles of the present invention are prepared by a melting-homogenization method. The solid lipid nanoparticles of the present invention can significantly improve the solubility of dabigatran etexilate in aqueous solution, effectively avoid the degradation and leakage of the drug in the gastrointestinal tract, and can control the release behavior of the drug, reducing the number of single-day administration, Improve the dissolution and absorption of dabigatran etexilate in the gastrointestinal tract, and effectively inhibit the efflux of intestinal P-gp receptors to promote absorption, thereby significantly improving the bioavailability of the drug. The solid lipid nanoparticle of the invention has good stability, high bioavailability, easy access to commonly used auxiliary materials, good process feasibility and repeatability, and basically does not generate three wastes.
Description
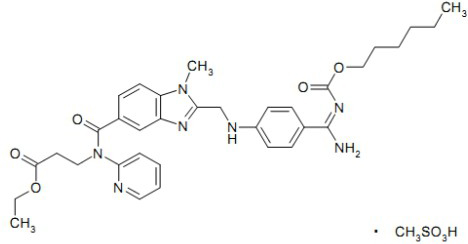
A kind of dabigatran etexilate solid lipid nanoparticles and preparation method thereof technical field The invention belongs to the technical field of medicine, be specifically related to a kind of solid lipid nanoparticles of dabigatran etexilate and preparation thereof methods and applications. Background technique Dabigatran etexilate mesylate Pradaxa (dabigatran etexilate mesylate, structure such as formula one) It was developed by Boehringer Ingelheim, Germany, and was listed in Germany and the United Kingdom in April 2008, and in the United States in 2010. Launched in Japan in 2011 for the prevention of stroke and blood clotting in patients with abnormal heart rhythms (atrial fibrillation). It is a new type of anticoagulant drug with various characteristics that has been successfully developed recently, which is the first launch in 50 years after warfarin. A new class of oral anticoagulants that are comparable in efficacy to warfarin without increasing the r...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K9/51A61K47/14A61K47/24A61K47/10A61K31/4439A61P7/02
CPCA61K9/5123A61K9/5146A61K31/4439
Inventor 陈海军肖玉梅覃东张先华牟祥
Owner ZEIN BIOTECHNOLOGY CO LTD
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com