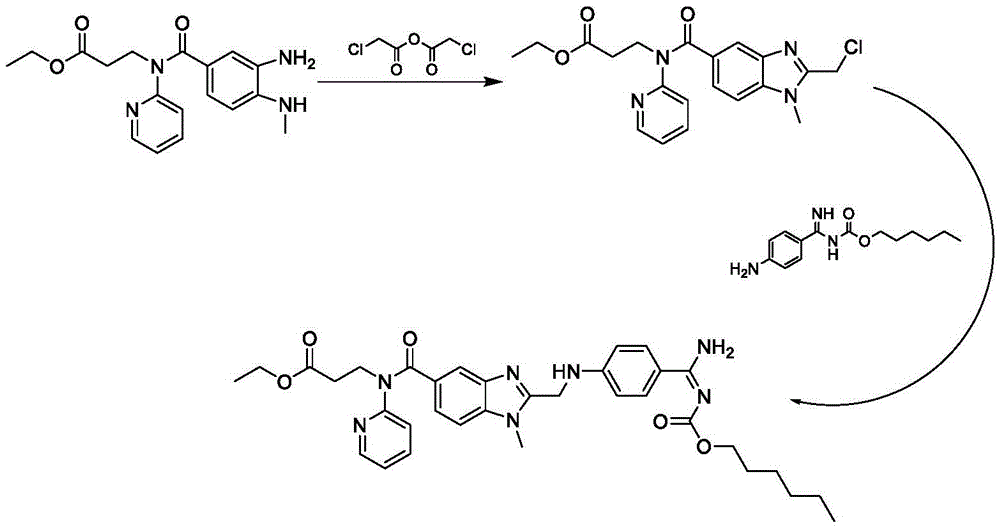
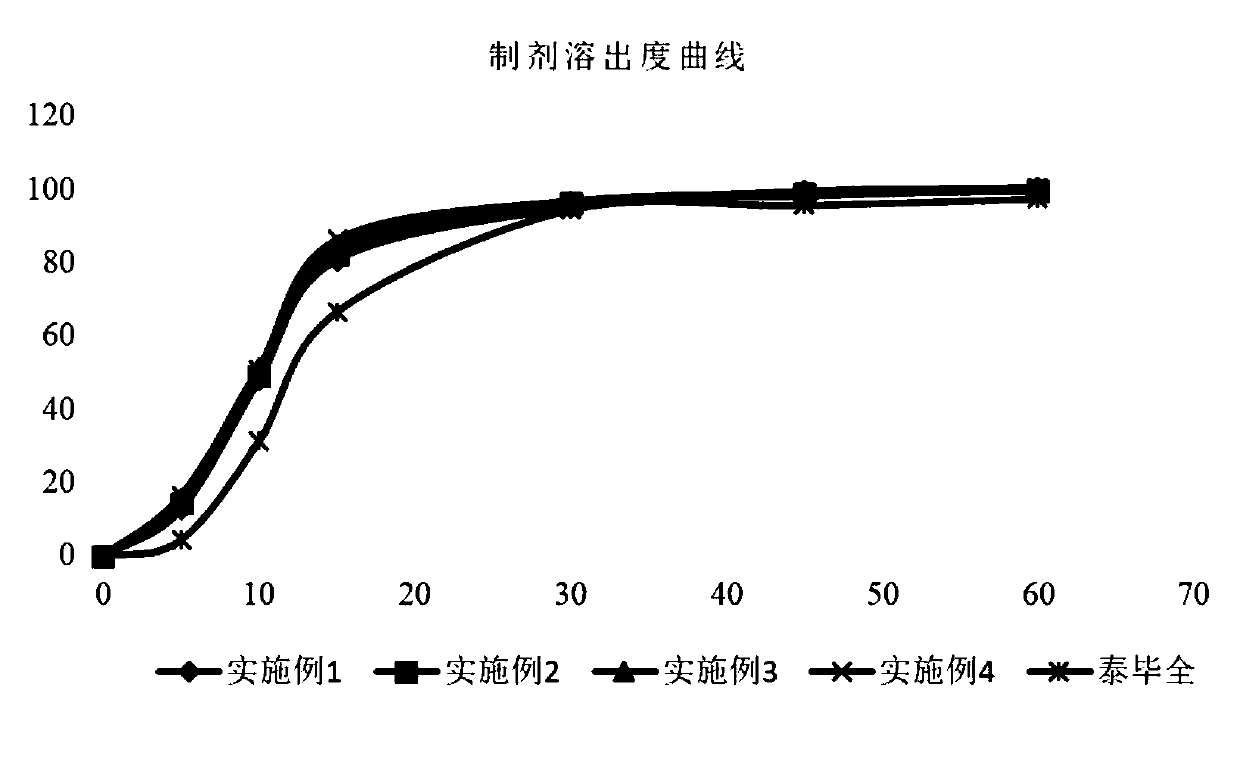
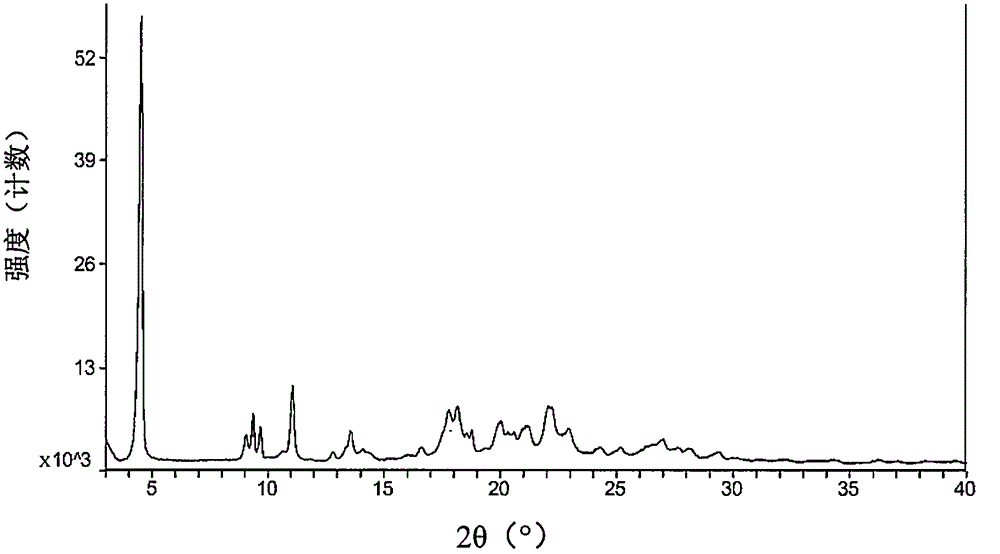
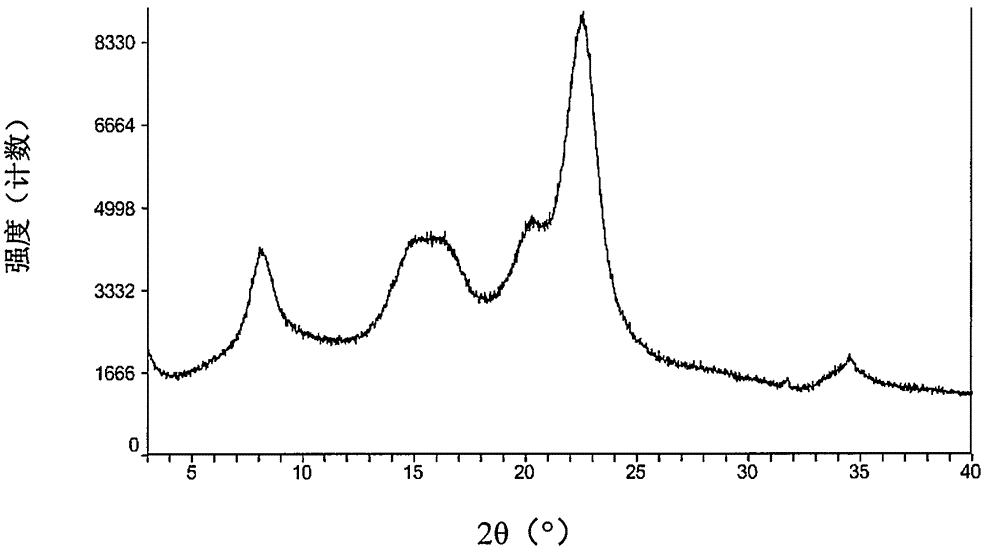
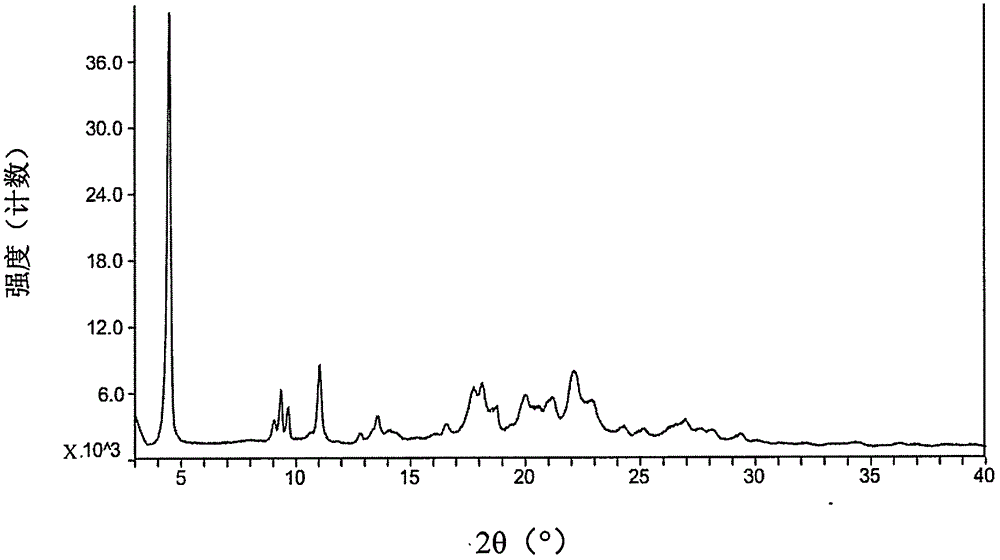
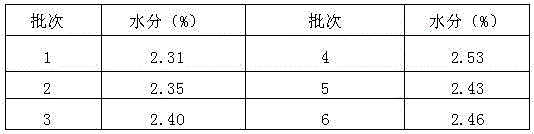
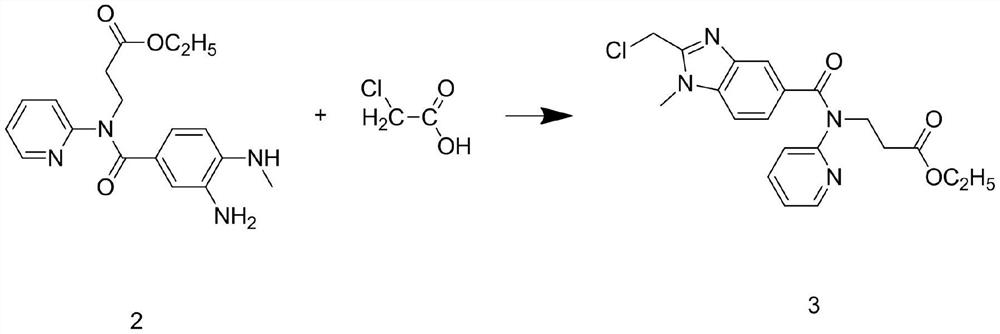
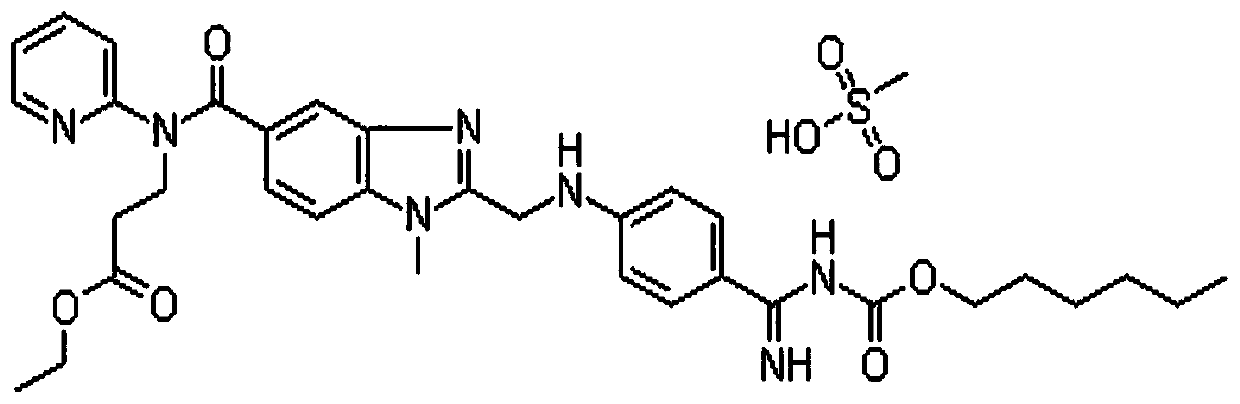
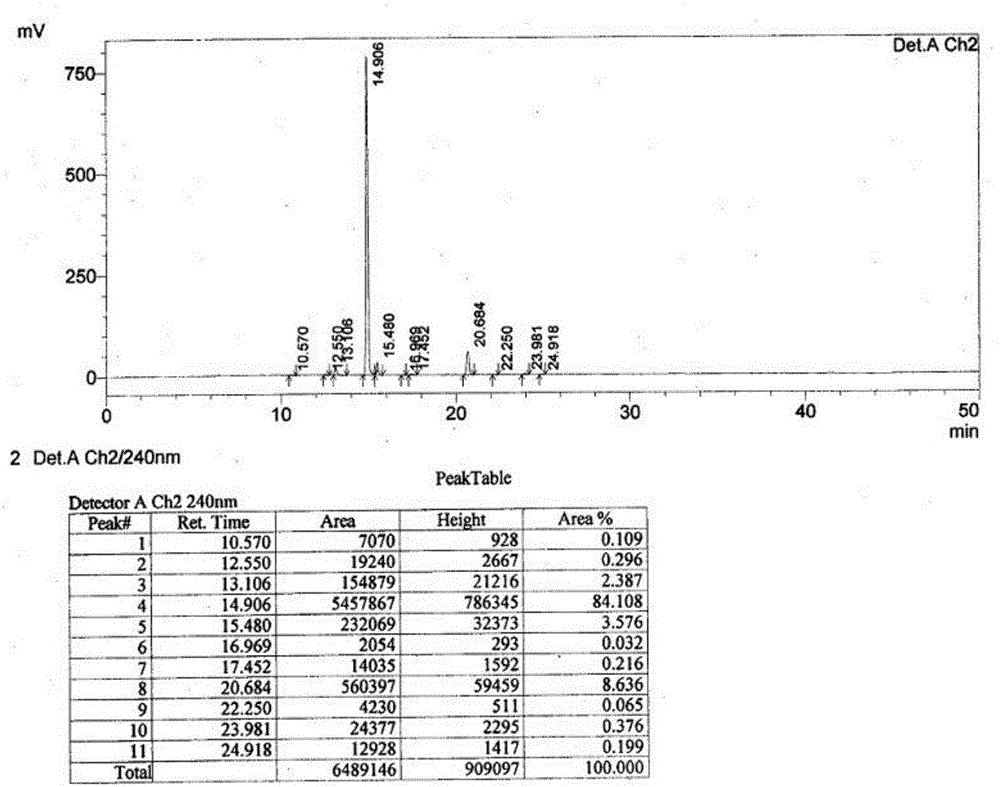
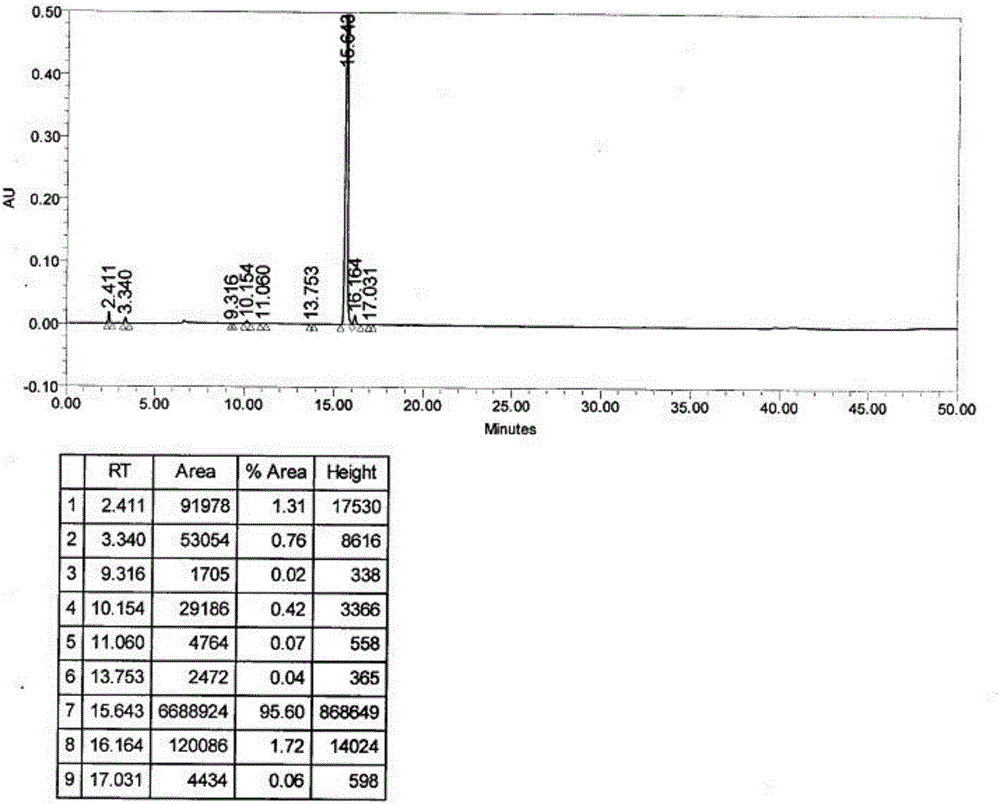
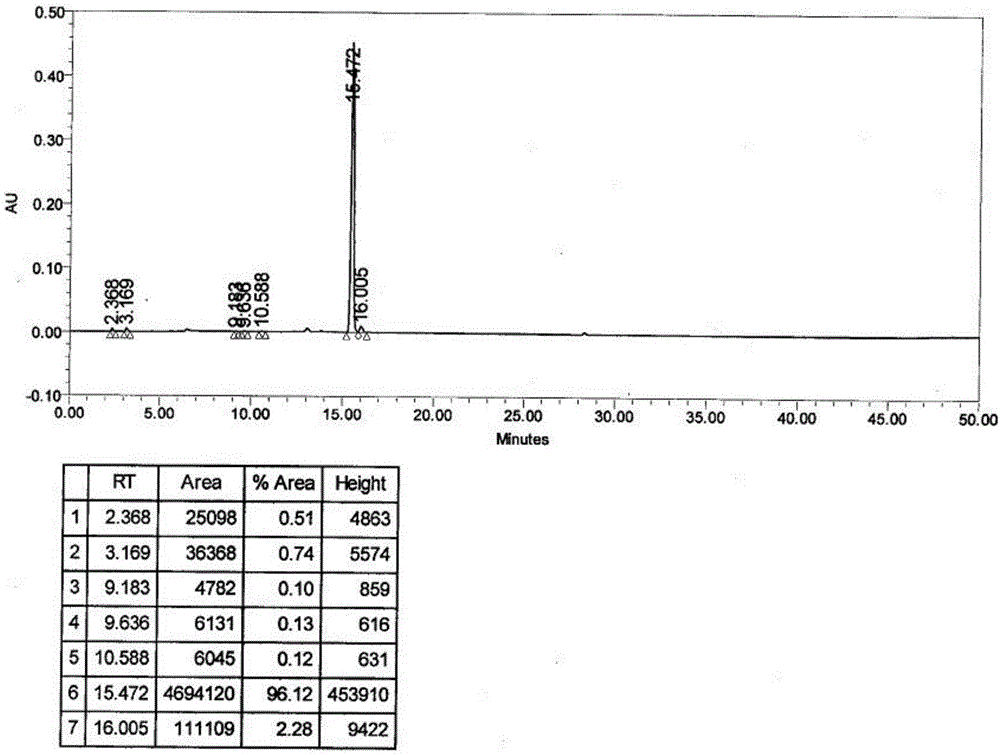
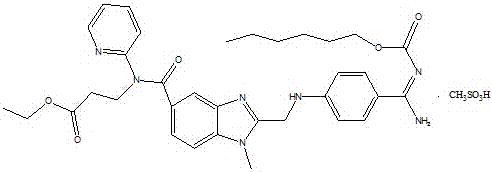
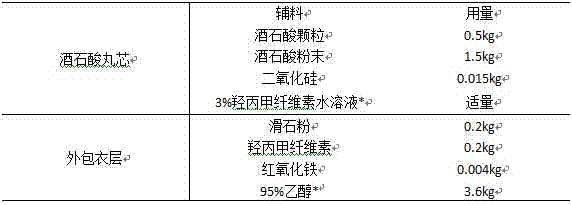
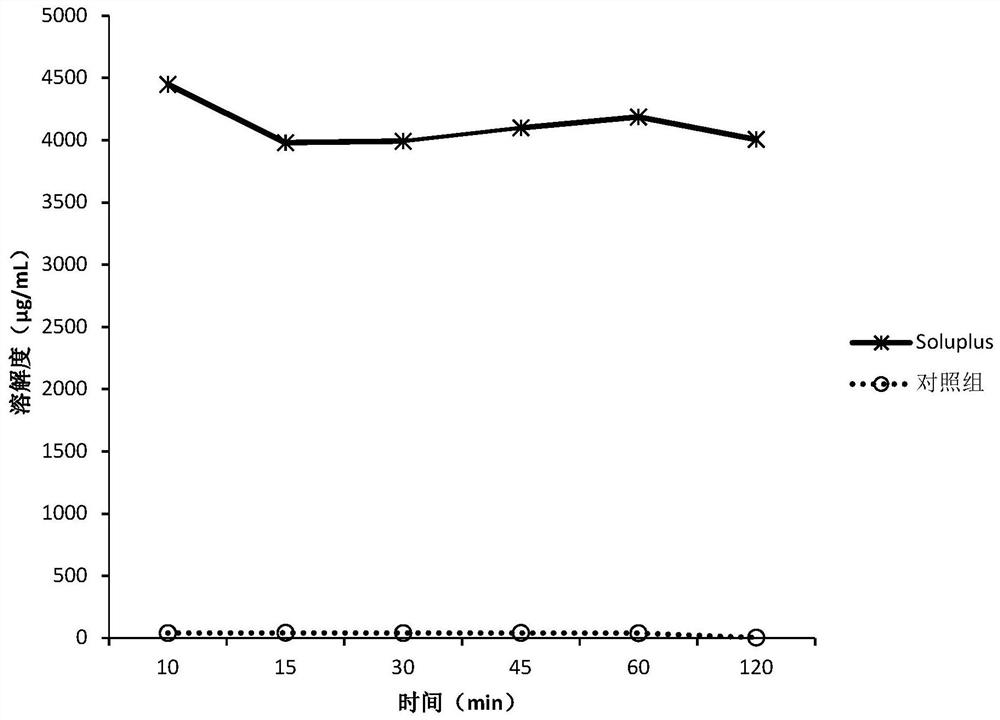
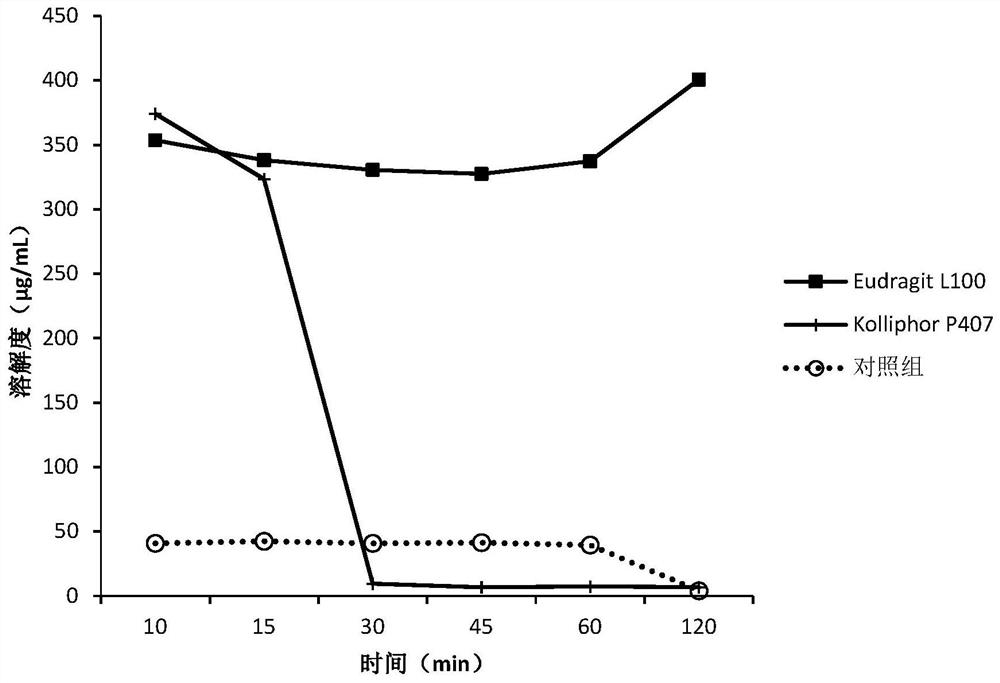
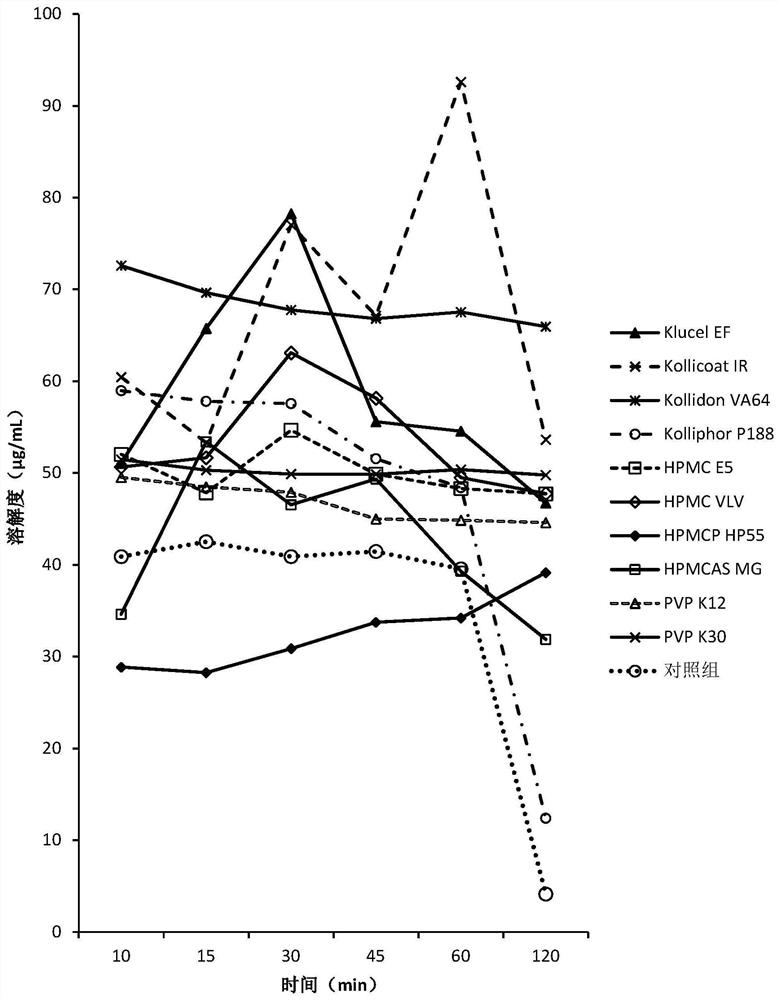
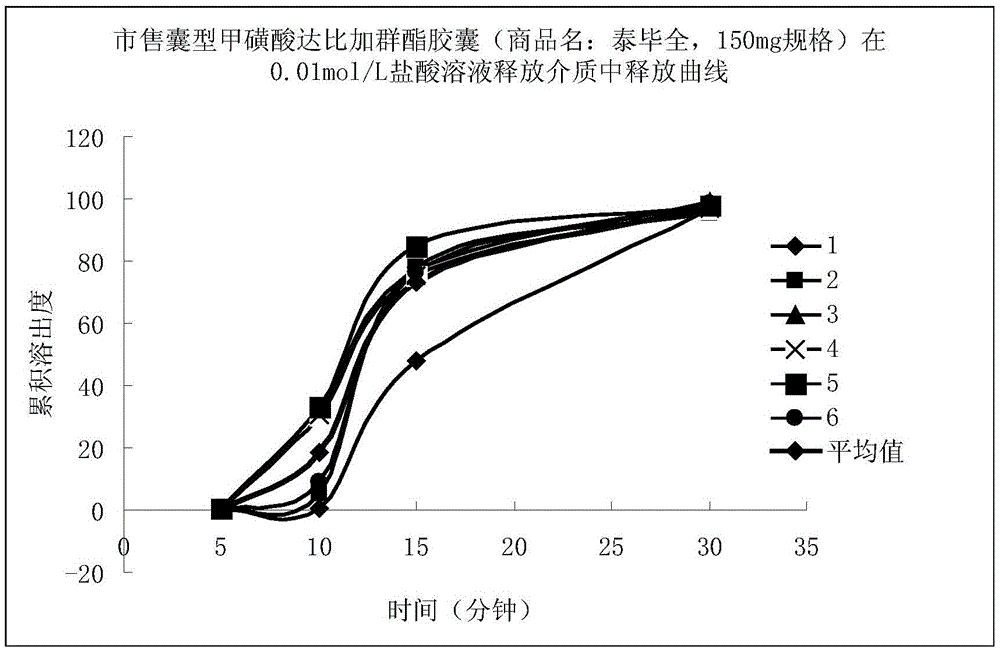
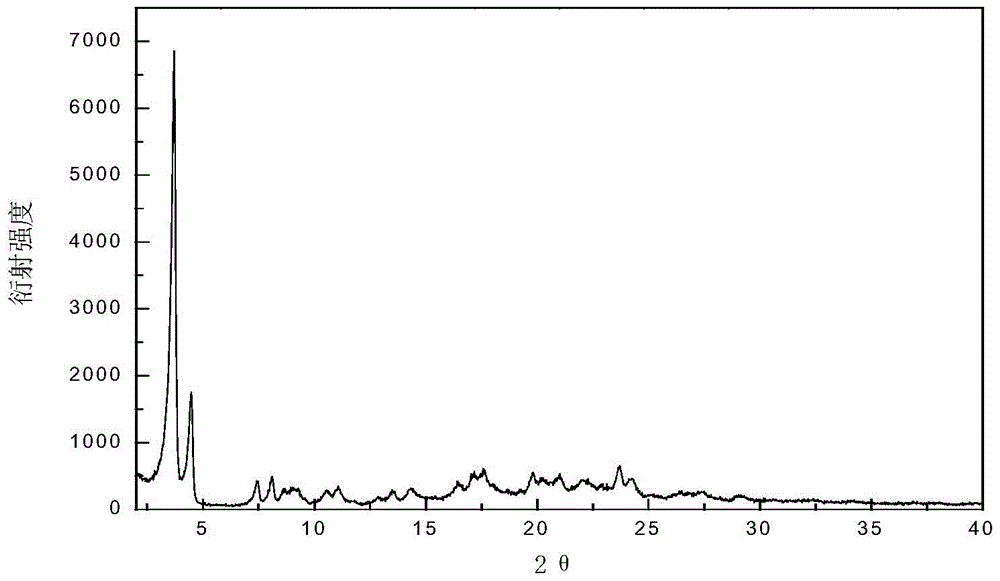
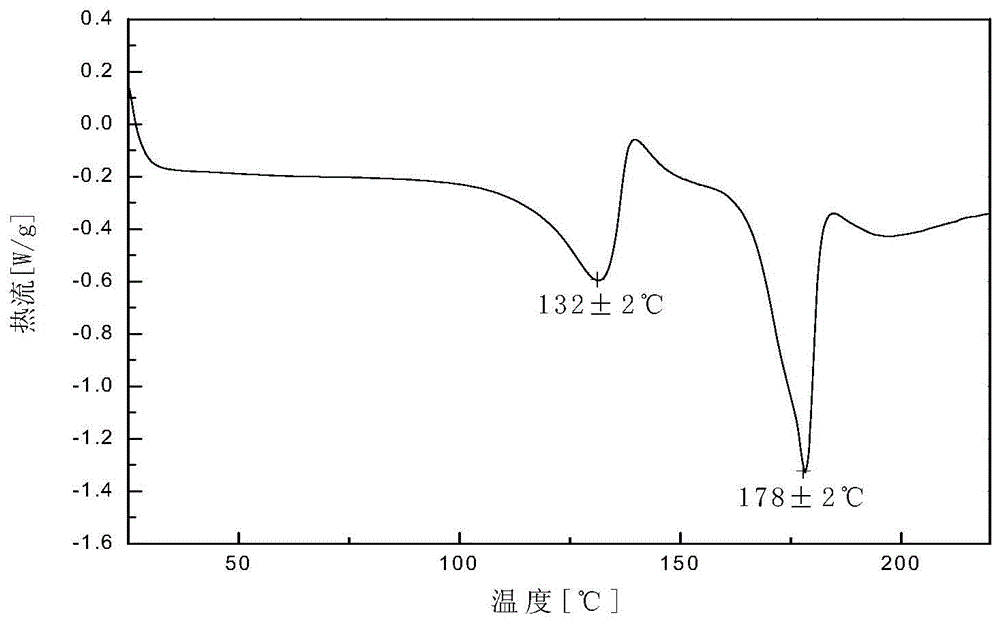
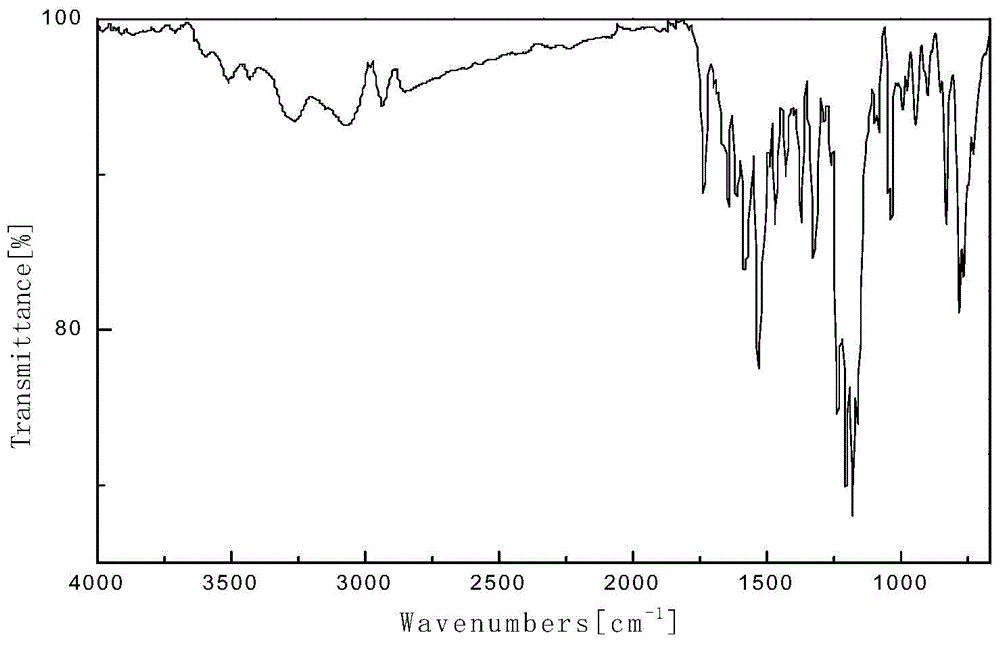
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Dabigatran Etexilate Mesylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
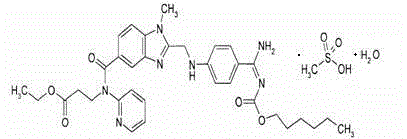
An orally available mesylate salt form of the etexilate prodrug of dabigatran, a benzimidazole and direct thrombin inhibitor with anticoagulant activity. Upon administration, dabigatran etexilate is hydrolyzed by esterases and is converted into dabigatran. Dabigatran reversibly binds to and inhibits the activity of thrombin, a serine protease that converts fibrinogen into fibrin. This disrupts the coagulation cascade and inhibits the formation of blood clots.
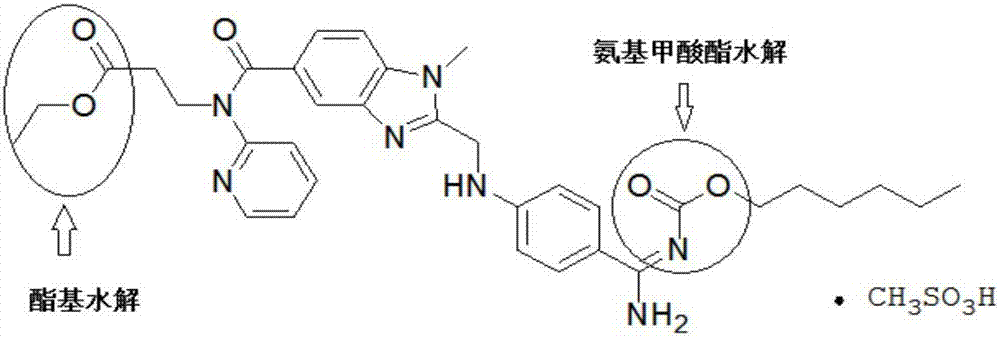
Method for preparing dabigatran etexilate hydrolysis impurities
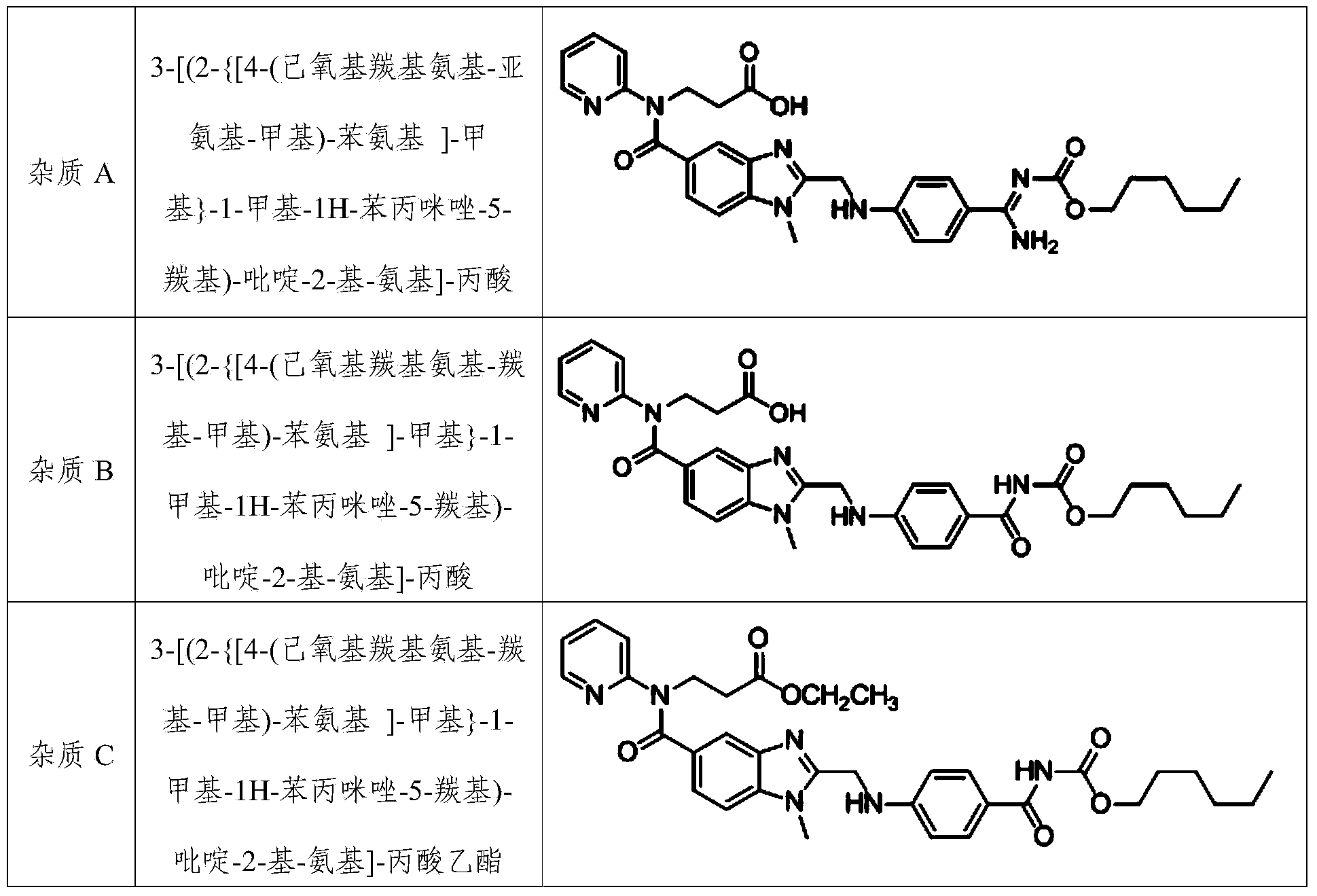
The invention provides a method for preparing a dabigatran etexilate hydrolysis impurity A. The method comprises steps as follows: (1) dabigatran etexilate or dabigatran etexilate mesylate is used as a raw material and dissolved in a mixed medium of an organic solvent and an alkaline aqueous solution for a hydrolysis reaction; (2) a mixed liquid obtained in Step (1) is adjusted to be acidic, and grease is separated out; (3) the organic solvent is added to the grease, crystals are separated out, and the impurity A is obtained. The invention further provides a method for preparing a dabigatran etexilate hydrolysis impurity B. The method comprises steps as follows: (1) the dabigatran etexilate hydrolysis impurity A is used as a raw material and dissolved in a mixed medium of the organic solvent and an acidic aqueous solution for a hydrolysis reaction; (2) a mixed liquid obtained in Step (1) is depressurized and distilled until grease is produced, and extraction is performed with dichloromethane; (3) an obtained extraction liquid is purified with medium-pressure preparation chromatography, the solvent is removed, and the grease is obtained; (4) ethyl acetate is added, crystals are separated out after dissolution, and the impurity B is obtained.
Owner:BENGBU BBCA MEDICINE SCI DEV
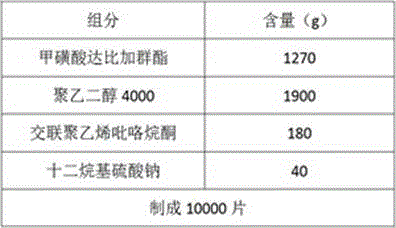
Multi-layer tablets containing dabigatran etexilate mesylate
ActiveCN104042588AIncrease absorption rateAddress disintegration risksOrganic active ingredientsPharmaceutical delivery mechanismSolubilityOrganic acid
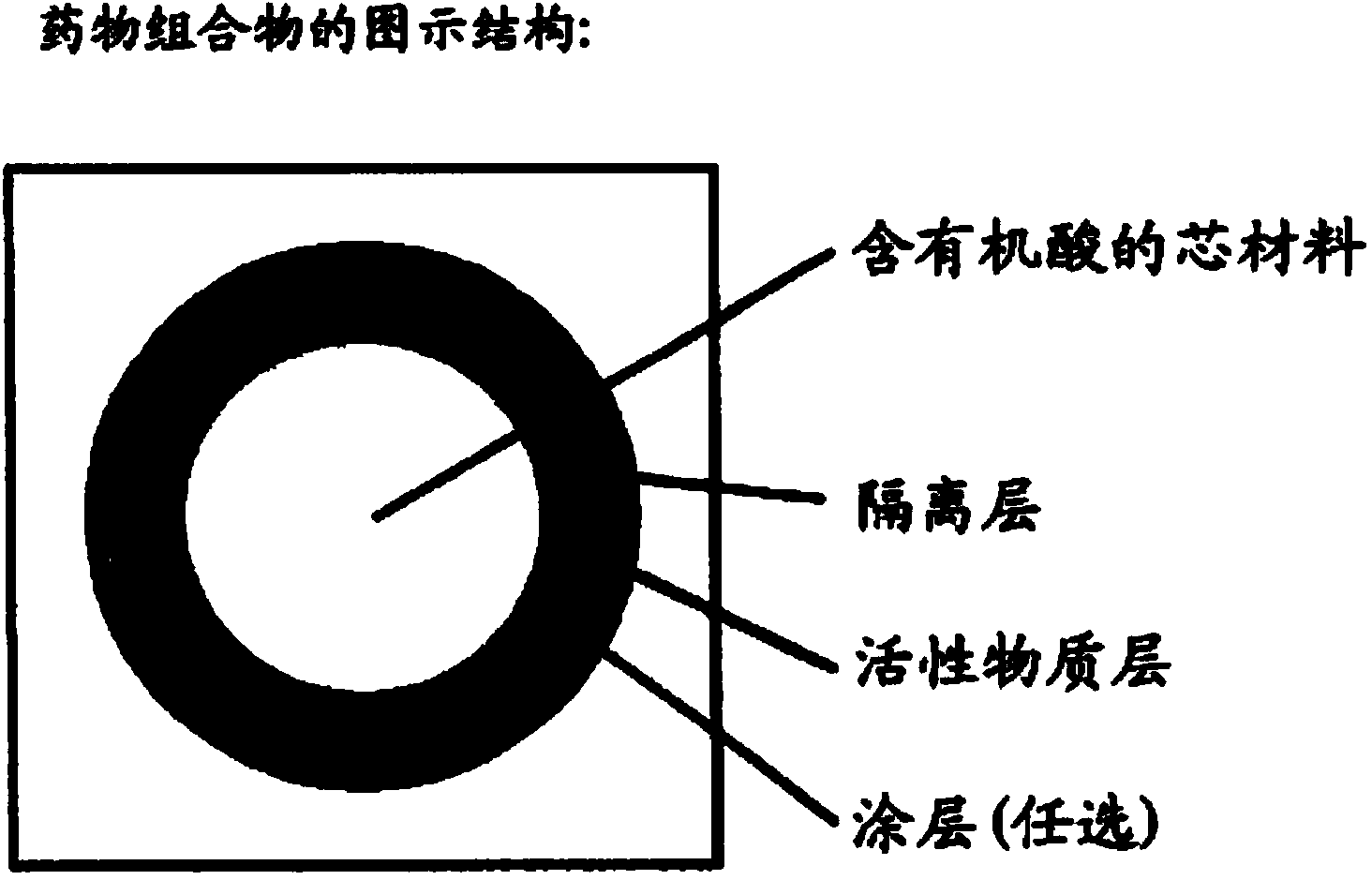
The invention discloses a multi-layer tablet containing dabigatran etexilate mesylate. The multi-layer tablet comprises a tablet core and a coating film; the tablet core comprises at least one drug-containing layer containing dabigatran etexilate mesylate and at least one organic acid layer. The multi-layer tablet is capable of increasing the solubility in vivo and the absorption rate in vivo, and also capable of effectively controlling the release time in vivo, and further is low in batch difference, and therefore, the safety of drug use is improved. Meanwhile, the multi-layer tablet is used for solving the problem that other substances are generated due to crystal transformation in the production and storage processes, and the production quality is improved and the storage time is prolonged. In addition, the multi-layer tablet further has the characteristics of convenience for swallowing, simple production process and high reproducibility.
Owner:ZHEJIANG JINGXIN PHARMA
Preparation method of dabigatran etexilate mesylate
InactiveCN105566297ASimple and fast operationHigh selectivityOrganic chemistryPropanoic acidChloroformate
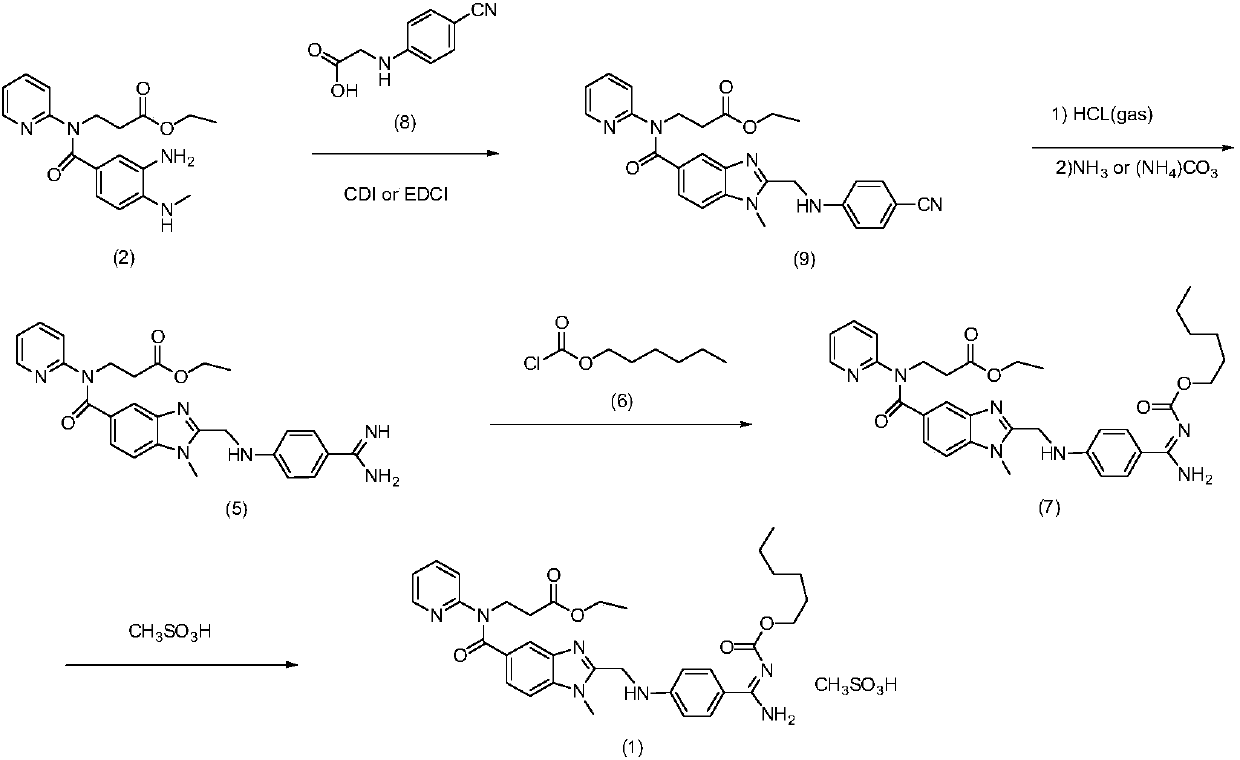
The invention discloses a preparation method of dabigatran etexilate mesylate, and belongs to the technical field of medicine. The preparation method comprises the following steps: taking 3-[(3-amino-4-methylaminobenzoyl)pyridine-2-ylamino]ethyl propanoate and N-(4-cyanphenyl)amino acetic acid as the raw materials to synthesize an intermediate (S3); making the intermediate (S3) carry out ring-closure reactions to generate an intermediate (S4); subjecting the intermediate (S4) to acid splitting in the presence of a hydrogen chloride-ethanol solution at first, then carrying out ammonification in the presence of ammonia water to generate an intermediate (S5); carrying out reactions between the intermediate (S5) and n-hexyl chloroformate under an alkaline condition to generate an intermediate (S6); dissolving the intermediate (S6), and finally carrying out reactions between the intermediate (S6) and methylsulfonic acid to obtain dabigatran etexilate mesylate. The preparation method has the advantages of simpleness, controllable and mild conditions, high yield, high product purity, stable product property, and suitability for industrial production.
Owner:HARBIN PHARMA GROUP TECH CENT
Dabigatran etexilate mesylate solid pharmaceutical preparation and preparation method thereof
InactiveCN111150714AImprove roundnessUniform quality and good stabilityOrganic active ingredientsBlood disorderCelluloseFluidized bed
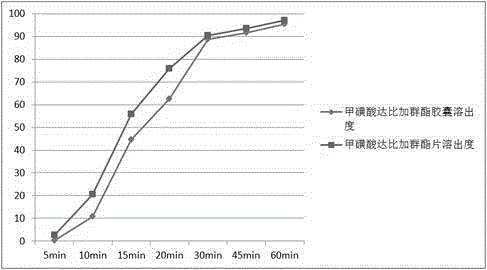
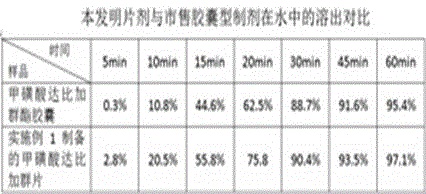
The invention provides a dabigatran etexilate mesylate solid pharmaceutical preparation and a preparation method thereof, and belongs to the field of pharmaceutical preparations. In the present invention, a fluidized bed bottom-spraying coating process is used to sequentially coat an isolation layer containing talcum powder, hydroxypropyl methylcellulose and dimethicone, and a drug-containing layer containing dabigatran etexilate mesylateactive ingredientson surface of tartaric acidpellets to obtain drug-containing pellets, and then the drug-containing pellets are encapsulated in capsules. Theresulting dabigatran etexilate mesylate solid pharmaceutical preparation has more than 95% of dissolution rate within 30 min and improved dissolution rate. The drug-containing pellets are uniform inweight and good in stability, higher in roundness and narrower in particle size distribution. Meanwhile, the preparation method is simple and controllable, saves tartaric acid auxiliary materials andimproves feasibility of industrial production.
Owner:南京嘉晨医药科技有限公司
Method for detecting related substance imidazole in starting material F of dabigatran etexilate mesylate
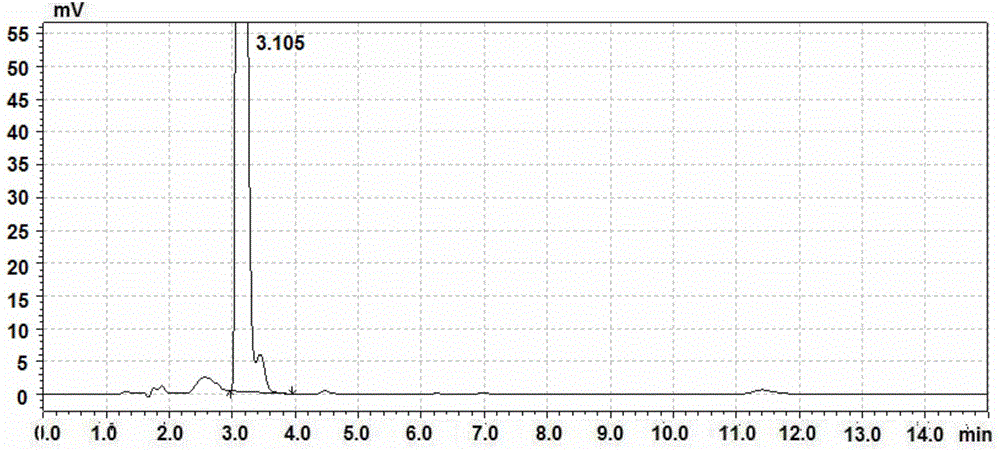
The invention belongs to field of pharmaceutical analysis, and more specifically relates to a method for detecting a related substance imidazole in a starting material F of dabigatran etexilate mesylate. The method which can be operated simply, rapidly and accurately is used for detecting the related substance imidazole in the starting material F; concretely, liquid chromatogram is employed for detection, and key HPLC detection conditions are controlled: the proportion between a mobile phase acetonitrile and water is 10-30:90-100, an amide-bonded silica gel chromatographic column is used, and the detection wavelength is 200-230nm. Key detection parameters are controlled in order to realize the purposes of simple, rapid and accurate detection, and the method can be simply operated; the detection result is accurate and reliable; the specificity is good; the retention time of the starting material F is 3-4 minutes, the retention time of imidazole is 6-7 minutes, the detection time is short, and a brand new selection is provided for detecting related substances in the product, controlling product quality, and guarantying safety of medicines.
Owner:CHENGDU BAIYU PHARMA CO LTD
Method for preparing dabigatran etexilate hydrolysis impurity
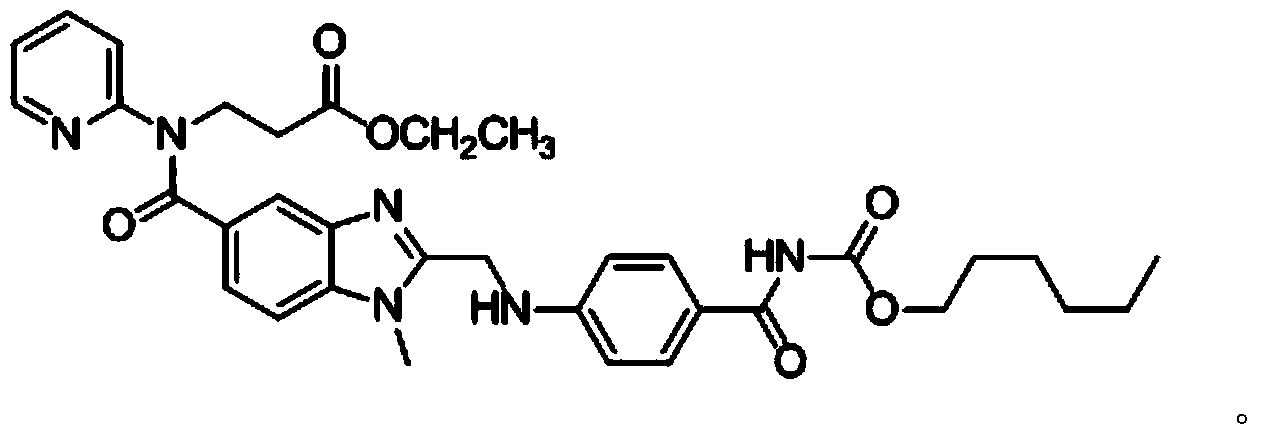
The invention relates to a method for preparing a dabigatran etexilate hydrolysis impurity C. The method comprises the following steps: (1) sufficiently dissolving dabigatran etexilate or dabigatran etexilate mesylate as a raw material in a mixed medium consisting of an organic solvent and an acid solution, and performing hydrolysis reaction at 10-50 DEG C, thereby obtaining a mixed liquid after the reaction is completed; (2) performing reduced pressure distillation on the obtained mixed liquid obtained in the step (1) till an oily matter is generated, removing the solvent, adding dichloromethane into the oily matter to extract, and collecting the extract; (3) purifying the extract of the step (2) by using a medium-pressure preparative chromatography method, performing pressure reduction distillation to remove the solvent so as to obtain an oily matter, and washing the oily matter with distilled water, wherein alkyl silica gel is adopted as the solid phase, and an organic solvent and an aqueous solution with the pH value of 4.0-5.0 are adopted as the flow phase; and (4) adding ethyl acetate into the oily matter obtained in the step (3), heating till the oily matter is completely dissolved, cooling, separating out crystals, performing suction filtration, and drying in vacuum, thereby obtaining a product, that is, the dabigatran etexilate hydrolysis impurity C.
Owner:BENGBU BBCA MEDICINE SCI DEV
Dabigatran etexilate mesylate content detection method
ActiveCN105572275ASimple processImprove product qualityComponent separationAcetic acidInjection volume
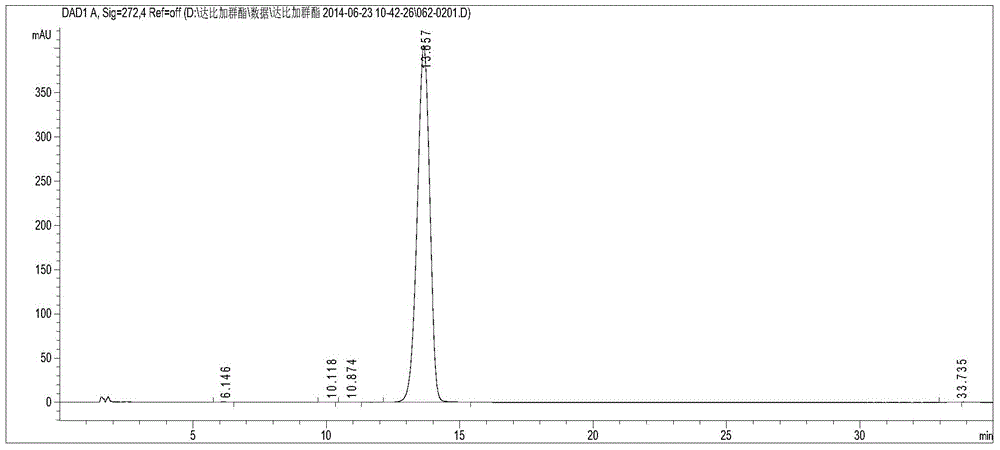
The present invention relates to a dabigatran etexilate mesylate content detection method, which concretely comprises that: 1) the chromatographic conditions of HPLC for determining the dabigatran etexilate mesylate comprise that the chromatographic column is phenomenex GeminiC18 (150*4.6 mm, 5 um), the mobile phase A is a 0.01 mol / L ammonium acetate aqueous solution, the pH value is adjusted to 6.5 with acetic acid, the mobile phase B is methanol, isocratic elution is performed according to a ratio of A to B of 35:65, the flow rate is 1 ml / min, the column temperature is 30 DEG C, the detection length of the DAD detector is 272 nm, and the injection volume is 10 [mu]l. According to the present invention, through the effective high performance liquid chromatography, the dabigatran etexilate mesylate content is detected, the dabigatran etexilate mesylate content is precisely detected, the process is easily optimized, and the product quality is easily improved.
Owner:HUAREN PHARMACEUTICAL CO LTD
Pharmaceutical composition containing dabigatran etexilate and preparation method thereof
ActiveCN110339193ARandom combinationStable concentrationOrganic active ingredientsPowder deliverySide effectAdditive ingredient
The invention discloses a pharmaceutical composition containing dabigatran etexilate and a preparation method thereof. The pharmaceutical composition comprises active pharmaceutical ingredients and anamphoteric polymer; wherein the pharmaceutical active ingredients are dabigatran etexilate and / or dabigatran etexilate mesylate (DEM), and the amphoteric polymer is a graft copolymer of polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol; and a mass ratio of the active pharmaceutical ingredients to the amphoteric polymer is 1: 0.23-1: 3. The pharmaceutical composition can not only improvethe bioavailability of the active pharmaceutical ingredients, but also reduce the absorption variability, and simultaneously provide a more stable concentration of dabigatran in blood plasma, therebyreducing adverse side effects and reducing the possibility of gastrointestinal tract (GIT) bleeding. In addition, due to the enhanced absorption, there is a significant reduction in non-absorbed DEMin gastrointestinal tracts, which can further alleviate the GIT massive bleeding.
Owner:SHANGHAI WD PHARM CO LTD
Preparing method of dabigatran etexilate mesylate crystal form I
InactiveCN104725360AHigh crystal purityQuality improvementOrganic chemistry methodsMedicinal chemistryDabigatran Etexilate Mesylate
An embodiment of the invention discloses a preparing method of dabigatran etexilate mesylate crystal form I. The method includes the steps of adding dabigatran etexilate into acetone solution before heating for solution, and adding methylsulfonic acid to generate dabigatran etexilate mesylate; stirring the dabigatran etexilate mesylate in ester solvent or ether solvent under heating so that the dabigatran etexilate mesylate with low crystal form I (containing little crystal form II or part of semihydrate) is converted into the dabigatran etexilate mesylate with high content of the crystal form I. The dabigatran etexilate mesylate crystal form I prepared by the method is high in purity, lower in water content and more stable in quality.
Owner:CHONGQING TOPTECH PHARMA TECH
Dabigatran etexilate mesylate anhydrous compound
InactiveCN104418840AHigh purityGood stability at room temperatureOrganic active ingredientsOrganic chemistryCombinatorial chemistryMedicinal chemistry
The invention belongs to the technical field of medicines, and in particular relates to a new medicinal crystal form of dabigatran etexilate mesylate. Prepared dabigatran etexilate mesylate disclosed by the invention has the advantages that the chemical purity is 99.9%, the maximum impurity content is less than 0.1%, and the optical purity reaches 99.96%ee; and dabigatran etexilate mesylat is good in stability.
Owner:TIANJIN HANRUI PHARMA
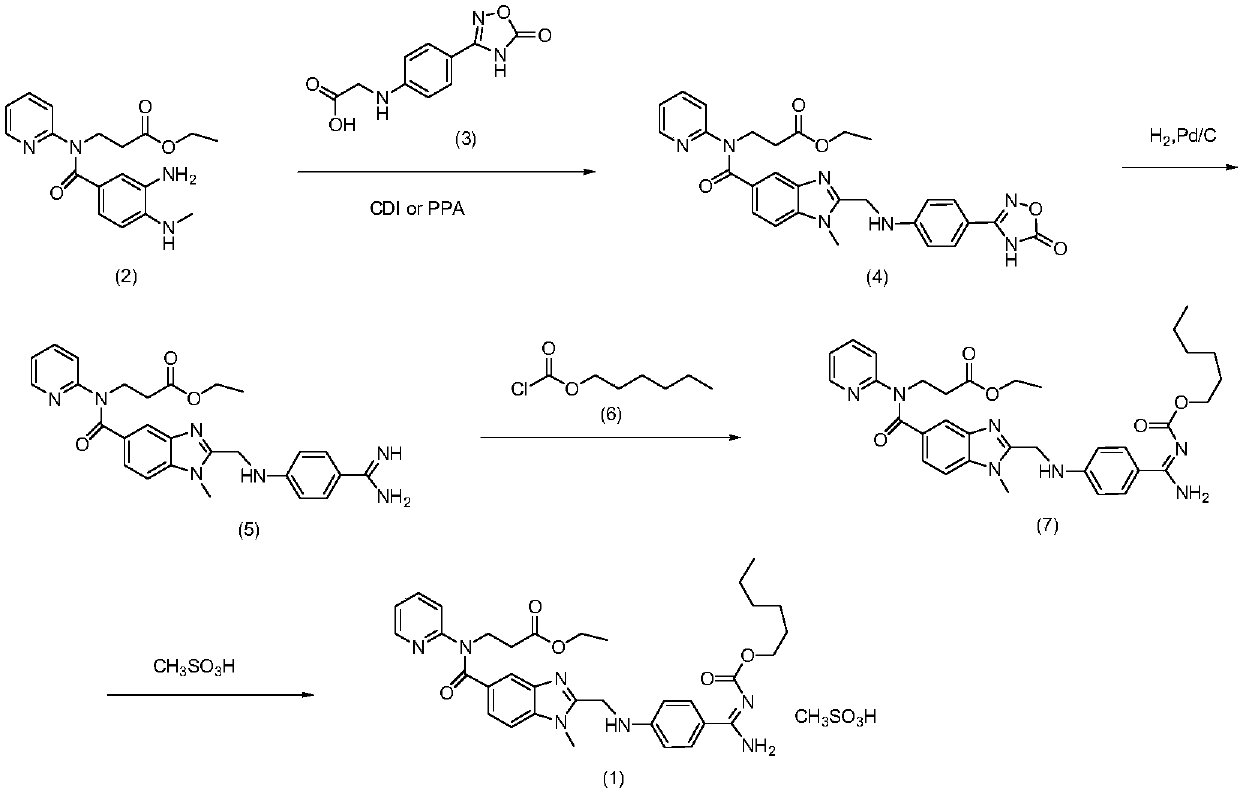
Dabigatran etexilate mesylate preparation method
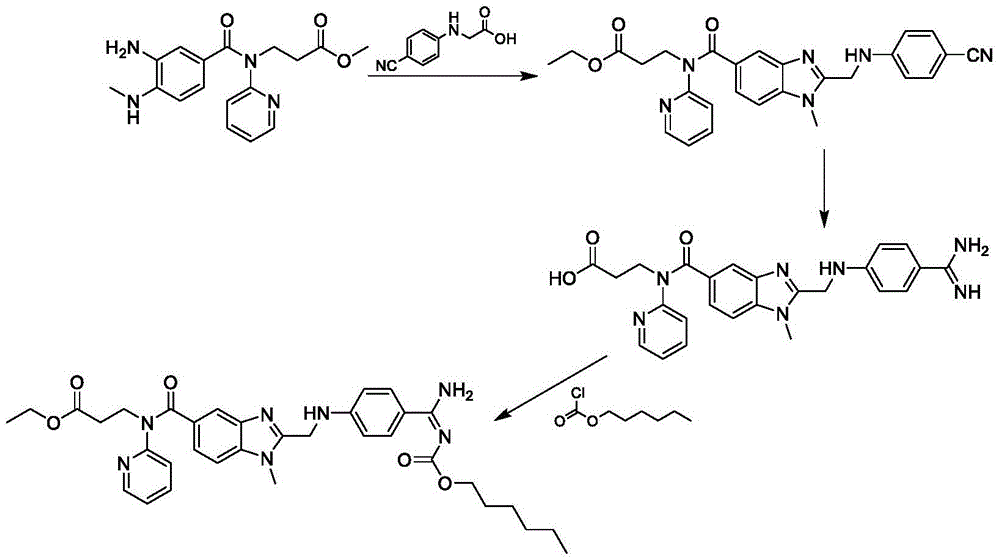
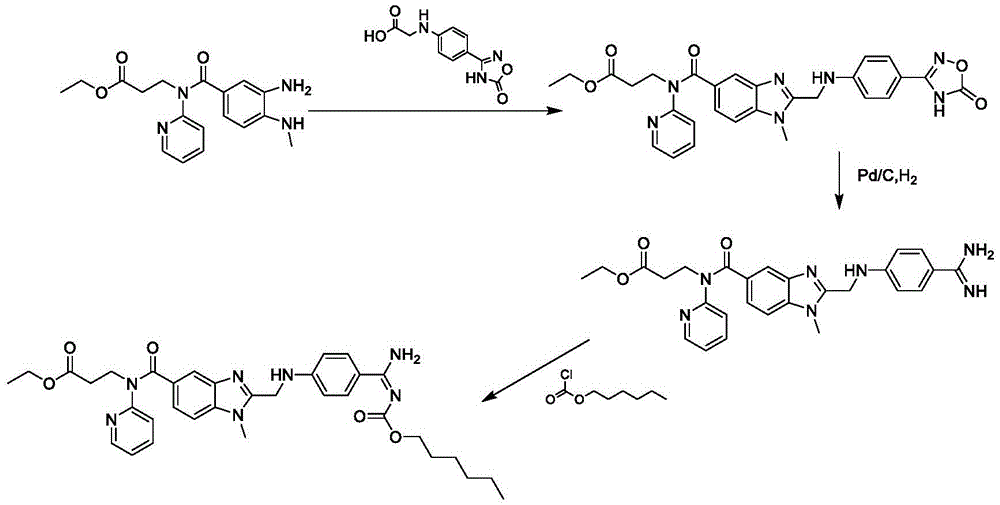
The invention belongs to the field of pharmaceutical synthesis, and provides a dabigatran etexilate mesylate preparation method, which comprises: carrying out a ring closure reaction on 3-[(3-amino-4-methylaminobenzoyl)(pyridine-2-yl)amino] ethyl propionate and chloroacetic anhydride to generate N-[[2-(chloromethyl)-1-methyl-1H-benzimidazole-5-yl]carbonyl]-N-2-pyridyl-beta-alanine ethyl ester, carrying out a condensation reaction on the N-[[2-(chloromethyl)-1-methyl-1H-benzimidazole-5-yl]carbonyl]-N-2-pyridyl-beta-alanine ethyl ester and 4-aminobenzamidine dihydrochloride to obtain 3-({2-[(4-amidino-phenylimino)-methylene]-1-methylene-1H-benzimidazole-5-carbonyl}-pyridine-2-imine)-ethyl propionate, carrying out ester forming on the 3-({2-[(4-amidino-phenylimino)-methylene]-1-methylene-1H-benzimidazole-5-carbonyl}-pyridine-2-imine)-ethyl propionate and hexyl chloroformate to obtain dabigatran etexilate, and carrying out salt forming on the dabigatran etexilate and methanesulfonic acid to obtain dabigatran etexilate mesylate. According to the present invention, the route of the method has characteristics of high yield, mild condition and convenient intermediate purification, and meets the requirements of industrial production.
Owner:辽宁博美医药科技有限公司
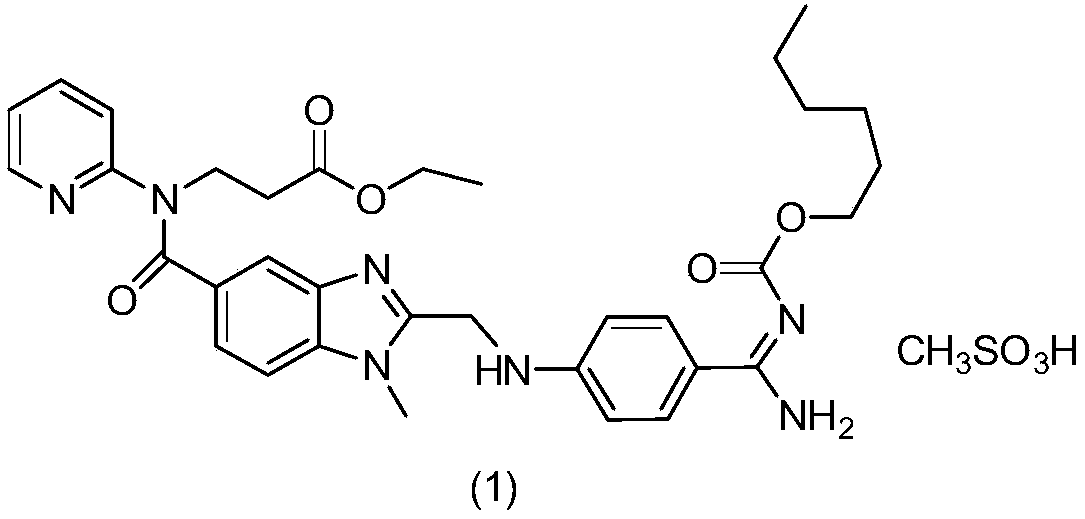
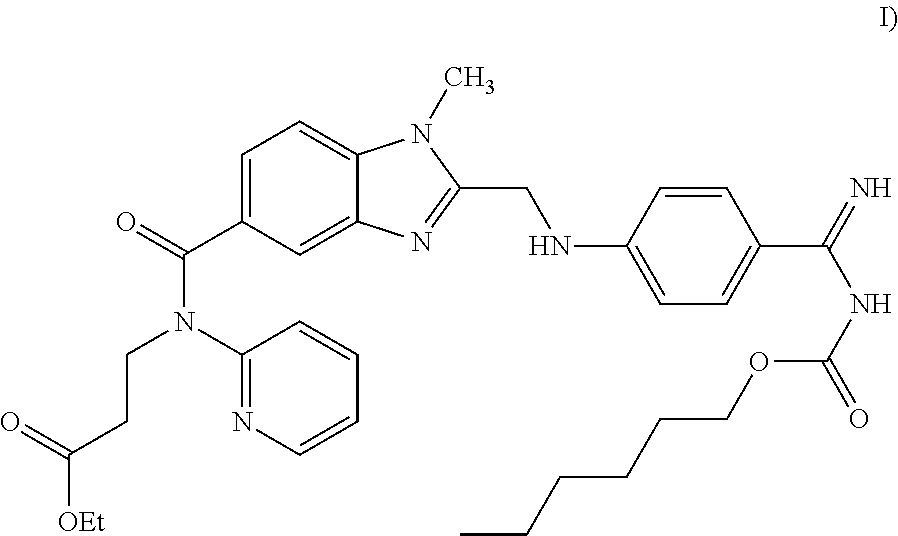
Pharmaceutical oral dosage forms comprising dabigatran etexilate and its pharmaceutically acceptable salts
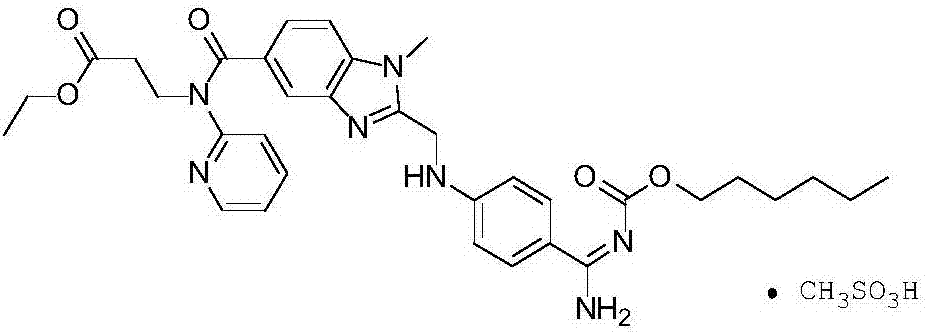
The invention relates to pharmaceutical oral dosage forms of the active substance ethyl 3-[(2-{[4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1H-benzimidazole-5-carbonyl)-pyridin-2-yl-amino]-propionate (dabigatran etexilate) and the pharmacologically acceptable salts thereof, in particular dabigatran etexilate methanesulfonate.
Owner:KRKA TOVARNA ZDRAVIL D D
Process for the preparation of dabigatran etexilate mesylate and polymorphs of intermediates thereof
The present invention provides crystalline form of intermediates of Formula 2A,The present invention also provides process for the preparation of dabigatran etexilate mesylate; polymorph of intermediates thereof; particularly processes for the preparation of crystalline form of intermediates. The present invention also relates to the use of crystalline intermediates for the preparation of dabigatran etexilate mesylate.
Owner:CADILA HEALTHCARE LTD
Pharmaceutical composition containing pradaxa mesylate and preparation method thereof
ActiveCN104825422AEase of industrial scale productionSimple processOrganic active ingredientsPharmaceutical delivery mechanismMedicinal chemistryDabigatran Etexilate Mesylate
The invention relates to a pharmaceutical composition containing pradaxa mesylate and a preparation method thereof. Compared with prior art, the preparation method has the advantages of simple process, low production cost and environmental protection. The obtained pharmaceutical composition has the advantages of stable crystal form of active substance, good storage stability and high dissolvability.
Owner:SEASONS BIOTECHNOLOGY (TAIZHOU) CO LTD
Stable dabigatran etexilate mesylate compound
InactiveCN104447691AHigh purityImprove stabilityOrganic active ingredientsSulfonic acids salts preparationSulfonateHigh humidity
Belongs to the technical field of medicines, the invention in particular relates to a hydrate of dabigatran etexilate mesylate and a preparation method thereof. The hydrate of dabigatran etexilate mesylate obtained by the invention contains a crystal water, and has the advantages of high purity, good stability, and non-obvious moisture absorption weight gain even under a high humidity condition.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Dabigatran etexilate mesylate oral solid preparation and preparation method thereof
InactiveCN105534939AAvoid acid decompositionImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsDrug contentMedicine
The invention provides a dabigatran etexilate mesylate oral solid preparation. With a specification of 110mg (based on free dabigatran etexilate), each tablet of the dabigatran etexilate mesylate oral solid preparation comprises the following components: 127mg of dabigatran etexilate mesylate (110mg of dabigatran etexilate), 180-190mg of water-soluble polymer, 15-20mg of disintegrant and 3.0-5.0mg of lubricant. According to the solid medicinal preparation provided by the invention, main drugs are dispersed in a water-soluble carrier, thus the dissolution rate of the preparation is increased, and the drug content uniformity is guaranteed. The dabigatran etexilate mesylate oral solid preparation provided by the invention is simple in preparation technology and suitable for industrial production.
Owner:郑州大明药物科技有限公司 +1
Preparation method of dabigatran etexilate mesylate
The invention discloses a preparation method of dabigatran etexilate mesylate. The method comprises a, adding a dabigatran etexilate basic group into an organic solvent and heating to dissolve the solution, b, filtering the solution to obtain a dabigatran etexilate basic group solution, c, adding methylsulfonic acid into the filtrate drop by drop so that the dabigatran etexilate mesylate is formed through acidification, and d, carrying fluid exhaustion, drying and crushing to obtain a dabigatran etexilate mesylate product. The organic solvent used in the step a is ethyl acetate, and in the filtration process of the step b, diatomaceous earth as a filter aid is used. The method has simple processes and is suitable for industrial production. The product has low related substance content and high purity.
Owner:NANJING LIFENERGY R & D
Method for purifying kilogram-grade dabigatran etexilate free alkali
InactiveCN105601615AReasonably control the contentHigh purityOrganic chemistryPurification methodsEthyl propanoate
The invention relates to a method for purifying a kilogram-grade dabigatran etexilate free alkali crude product, wherein a compound specifically refers to 3-(2-(4-amidino(hexyloxy carbonyl)-phenylamino)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzo[d]imidazole-5-amido)ethyl propionate, and has the main use for preparing an anti-coagulant drug dabigatran etexilate mesylate. With adopting of a relatively simple purification method, the content of new impurities appearing in a process of kilogram-grade preparation of the dabigatran etexilate free alkali is effectively reduced, so that design and development of new pharmacological and toxicological tests are avoided. The purity of the product is also improved, and the preparation cost is reduced.
Owner:YANTAI DONGCHENG PHARMA GRP
Method for refining dabigatran etexilate and method for controlling specific degradation impurities of dabigatran etexilate
InactiveCN113307792ALow content of specific degradation impuritiesLess categoriesOrganic chemistryPropanoic acidMeth-
The invention provides a method for refining dabigatran etexilate and a method for controlling specific degradation impurities of dabigatran etexilate. The dabigatran etexilate compound is 3-[(2-{[4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1H-benzimidazole-5-carbonyl)-pyridine-2-yl-amino]-ethyl propionate mesylate, the invention also relates to a control method of specific degradation impurities in the refining process of the compound. The method comprises the following steps: stirring and mixing dabigatran etexilate and acetonitrile, then heating to a reflux state, carrying out heat preservation for 1-2 hours in the reflux state, then carrying out slow cooling, filter pressing, vacuum drying and other technological processes to obtain dabigatran etexilate, and salifying the dabigatran etexilate and methanesulfonic acid to obtain dabigatran etexilate mesylate. The dabigatran etexilate obtained by the refining method provided by the invention has the advantages of high purity, no solvent residue, mild reaction conditions and easiness in industrial production.
Owner:杭州国瑞生物科技有限公司
Dabigatran etexilate mesylate and preparation method thereof
InactiveCN111606885AHigh yieldReduce manufacturing costSulfonic acids salts preparationPropanoic acidChloroacetic acids
The invention discloses a dabigatran etexilate mesylate and a preparation method thereof. The preparation method comprises the following steps: carrying out amide reaction on 4-aminobenzamidine dihydrochloride and n-hexyl chloroformate to prepare an intermediate 1; reducing 3-(4-(methylamino)-3-nitro-N-(pyridine-2-yl) benzoylamino) ethyl propionate by using tin powder and concentrated hydrochloricacid; reducing nitro groups to amino groups to prepare an intermediate 2, reacting the intermediate 2 with chloroacetic acid for cyclization to obtain an intermediate 3; condensing the intermediate 3with the intermediate 1 under the combined action of potassium iodide and potassium bicarbonate to prepare an intermediate 4, reacting the intermediate 4 with a methanesulfonic acid to form a salt, so that the dabigatran etexilate mesylate is prepared. The dabigatran etexilate mesylate prepared by the preparation method disclosed by the inventionis high in yield, and compared with the existing preparation method, most of the used raw materials are low-price raw materials, so that the production cost of the dabigatran etexilate mesylate is greatly reduced.
Owner:安徽鼎旺医药有限公司
Method for detecting dabigatran etexilate mesylate
InactiveCN110441426AGood tailing factorImprove applicabilityComponent separationSilica gelLength wave
The invention relates to a method for detecting dabigatran etexilate mesylate. The method comprises the following steps: preparing a test solution and a reference solution, and then injecting the testsolution and the reference solution into a high performance liquid chromatograph for detection, wherein the test solution is prepared by dissolving a sample by using acetonitrile and water accordingto a ratio of (1 : 1), and carrying out filtering by using a microporous filter membrane; and chromatographic conditions are that octadecylsilane bonded silica is taken as a filler, a mobile phase is0.02 mol / L of ammonium acetate (the pH value is adjusted to 8.0 by triethylamine)-acetonitrile (35 : 65), a chromatographic column is the Phenomenex Gemin C18 chromatographic column with the parameters of 4.6mmX250mm and 5 microns, and the detection wavelength is 220 nm. The method disclosed by the invention has the advantages that each component obtained by analysis is good in peak shape and highin separation degree; and the method is simple to operate, high in precision, high in recovery rate and good in stability and repeatability.
Owner:JIANGXI GUOYAO PHARMA LLC
Method for purifying dabigatran etexilate mesylate intermediate
InactiveCN106397403AMeet quality requirementsOrganic compound preparationOrganic chemistry methodsPurification methodsSolvent
The invention relates to a method for purifying a dabigatran etexilate mesylate intermediate. The method comprises the following step: purifying the dabigatran etexilate mesylate intermediate in benign solvent through control of gradient crystallization. The purification method can make the purity of the dabigatran etexilate mesylate intermediate be more than 95%, and make other unknown single impurity be less than 0.15%; the intermediate can be used for preparing a dabigatran etexilate mesylate product which meets the quality requirement of a first-researching drug factory.
Owner:苏州正济药业有限公司
Stable capsule preparation containing dabigatran etexilate mesylate and preparation method of stable capsule preparation
InactiveCN107569467AQuality is easy to controlGuaranteed stabilityOrganic active ingredientsPharmaceutical non-active ingredientsAqueous solutionPharmaceutical formulation
The invention relates to the field of medicinal preparations, and in particular relates to a stable capsule preparation containing dabigatran etexilate mesylate and a preparation method of the stablecapsule preparation. Under the state of water solution, especially in acidic and alkaline media, dabigatran etexilate mesylate is prone to oxidation and hydrolysis. Due to the adoption of a specific process and selection for specific auxiliary materials, the preparation is good in stability, the loading differences conform to the limitation requirement, in addition, a preparation process has repeatability, and large-scale production can be realized.
Owner:TIANJIN HANRUI PHARMA
Preparation method of dabigatran etexilate mesylate
InactiveCN108864048AReduce difficulty of reactionReduce energy consumptionOrganic compound preparationSulfonic acids salts preparationHazardous substanceChloroformate
The invention belongs to the technical field of medicine preparation, and in particular relates to a preparation method of a novel oral anticoagulant drug dabigatran etexilate mesylate; the method comprises the following steps: (1) adding and mixing 3-[[[2-[[(4-amidino-ylphenyl)amino]methyl]-1-methyl-1H-benzimidazole-5-carbonyl](pyridine-2-yl)amino]-ethyl propionate tosilate and potassium carbonate in a solvent; (2) adding hexyl chloroformate for reaction; (3) adding a refining solvent to dissolve to obtain dabigatran etexilate; 4) adding a reactive solvent for dissolving, and dropwise addingmethanesulfonic acid for reaction; in general, the preparation method of the dabigatran etexilate mesylate provided by the application has mild reaction conditions, short reaction period, and no toxicand harmful substance in purification process of a finished product, and the yield of the final product dabigatran etexilate mesylate is high, so the preparation method has better practical value andpopularization and application significance.
Owner:TOPFOND PHARMA CO LTD
Pharmaceutical composition containing dabigatran etexilate and preparation method thereof
ActiveCN110339193BRandom combinationStable concentrationPowder deliveryOrganic active ingredientsSide effectPolythylene glycol
The invention discloses a pharmaceutical composition containing dabigatran etexilate and a preparation method thereof. The pharmaceutical composition includes a pharmaceutical active ingredient and an amphoteric polymer; wherein, the pharmaceutical active ingredient is dabigatran etexilate and / or dabigatran etexilate mesylate, and the amphoteric polymer is polyvinyl caprolactam-polymer Vinyl acetate-polyethylene glycol graft copolymer; and the mass ratio of the active pharmaceutical ingredient and the amphoteric polymer is 1:0.23-1:3. The pharmaceutical composition can not only improve the bioavailability of the active ingredients of the drug, but also reduce the variability of absorption while providing a more stable concentration of dabigatran in plasma, thereby reducing adverse side effects and reducing the possibility of GIT bleeding. In addition, due to enhanced absorption, there was a significant reduction in unabsorbed DEM in the gastrointestinal tract, which further alleviated GIT hemorrhage.
Owner:SHANGHAI WD PHARM CO LTD
Multilayer tablet containing dabigatran etexilate mesylate
ActiveCN104042588BIncrease absorption rateAddress disintegration risksOrganic active ingredientsPharmaceutical delivery mechanismSolubilityOrganic acid
The invention discloses a multi-layer tablet containing dabigatran etexilate mesylate. The multi-layer tablet comprises a tablet core and a coating film; the tablet core comprises at least one drug-containing layer containing dabigatran etexilate mesylate and at least one organic acid layer. The multi-layer tablet is capable of increasing the solubility in vivo and the absorption rate in vivo, and also capable of effectively controlling the release time in vivo, and further is low in batch difference, and therefore, the safety of drug use is improved. Meanwhile, the multi-layer tablet is used for solving the problem that other substances are generated due to crystal transformation in the production and storage processes, and the production quality is improved and the storage time is prolonged. In addition, the multi-layer tablet further has the characteristics of convenience for swallowing, simple production process and high reproducibility.
Owner:ZHEJIANG JINGXIN PHARMA
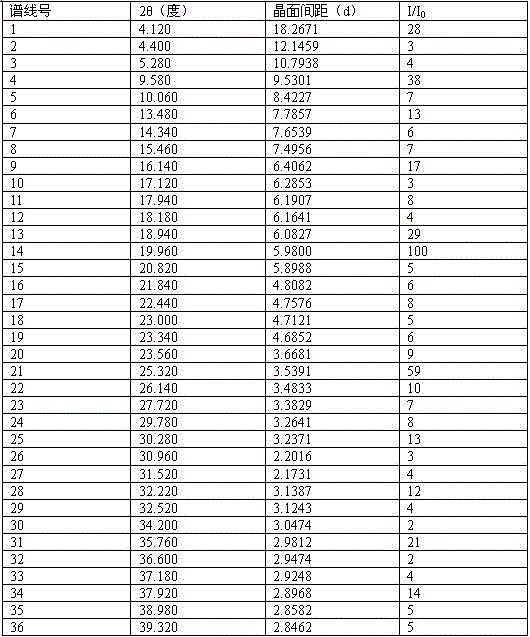
Crystal V of dabigatran etexilate mesylate and preparation method thereof
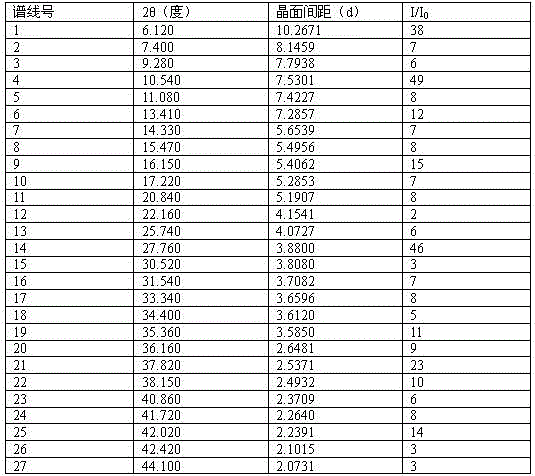
ActiveCN103951654BHigh purityImprove stabilityOrganic active ingredientsOrganic chemistryAlcoholFiltration
The invention relates to a dabigatran etexilate mesylate crystal V and a preparation method thereof. The crystal V is defined by characteristic peaks of an X-ray powder diffraction spectrum at a diffraction angle 2 theta and DSC. The preparation method of the dabigatran etexilate mesylate crystal V comprises the following steps: adding an amorphous dabigatran etexilate mesylate crystal solid or a known dabigatran etexilate mesylate crystal solid with any crystal form to an alcohol solvent so as to prepare 400-600g / L of suspension liquid, completely dissolving the solid at the temperature of 5-15 DEG C, then cooling a solution to -5 DEG C to 5 DEG C, and then dropwise adding a solventing-out agent to the mixed solution, wherein the using amount of the solventing-out agent is 6-10 times of the volume of the solvent; and carrying out suction filtration on the formed suspension liquid, and drying a dabigatran etexilate mesylate crystal product to constant weight, thus obtaining the dabigatran etexilate mesylate crystal V. The preparation method of the dabigatran etexilate mesylate crystal V has the advantages that a technology is simple, the consumed time is short, and the prepared product has high purity, good thermodynamic stability and the like.
Owner:NANJING LIFENERGY R & D
Novel crystal form of dabigatran etexilate mesylate and preparation method of novel crystal form
InactiveCN108864049AImprove solubilityReduce dosageOrganic active ingredientsOrganic compound preparationMedicinal chemistryDabigatran Etexilate Mesylate
The invention provides a novel crystal form of dabigatran etexilate mesylate and a preparation method of the novel crystal form. The method comprises the following steps: dissolving dabigatran etexilate mesylate into dichloromethane; and adding tetrahydrofuran to perform recrystallization.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Process for the preparation of dabigatran etexilate mesylate and polymorphs of intermediates thereof
InactiveUS9212166B2Organic chemistryThin material handlingDabigatran Etexilate MesylateCrystallization
The present invention provides crystalline form of intermediates of Formula 2A,The present invention also provides process for the preparation of dabigatran etexilate mesylate; polymorph of intermediates thereof; particularly processes for the preparation of crystalline form of intermediates. The present invention also relates to the use of crystalline intermediates for the preparation of dabigatran etexilate mesylate.
Owner:CADILA HEALTHCARE LTD
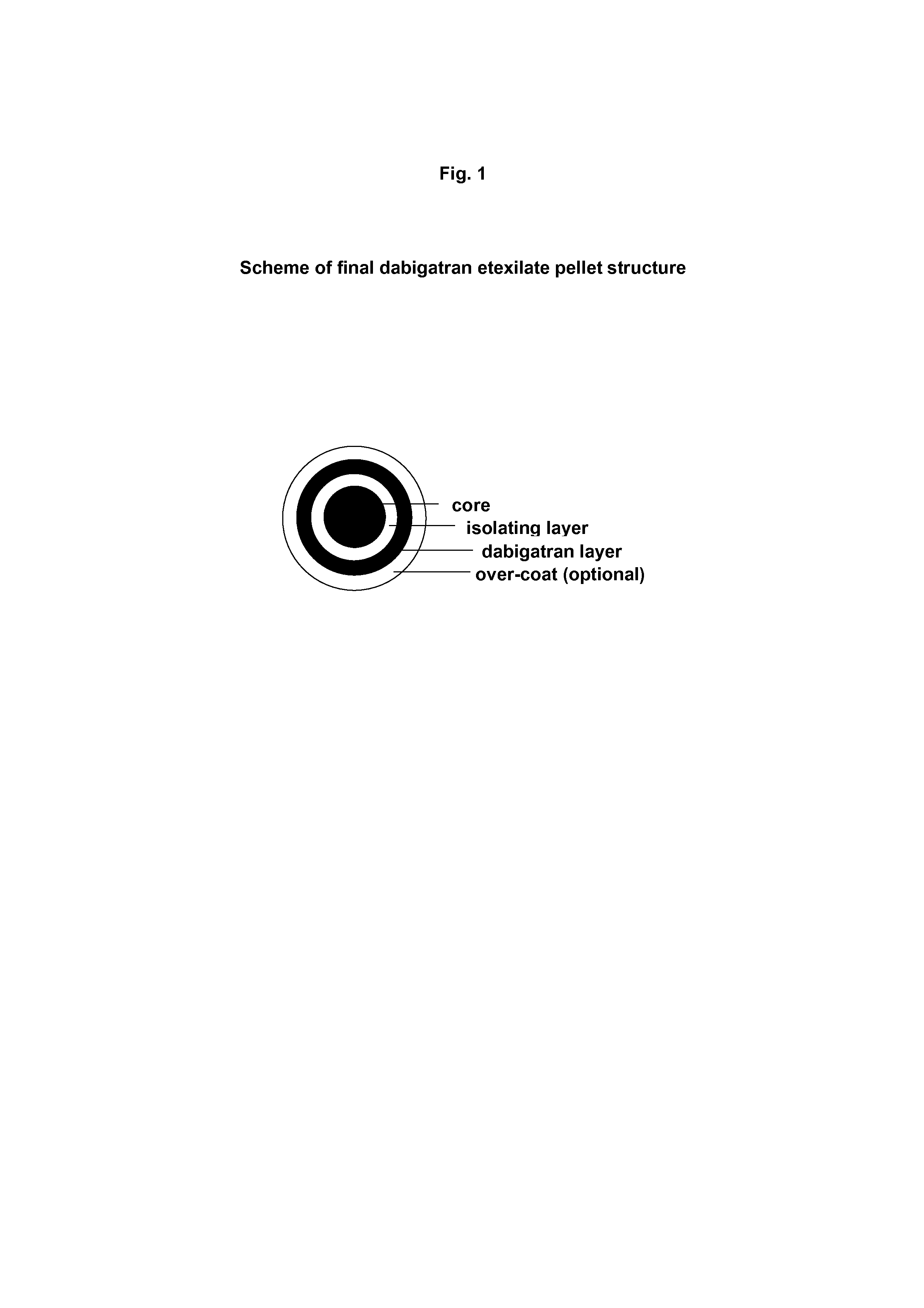
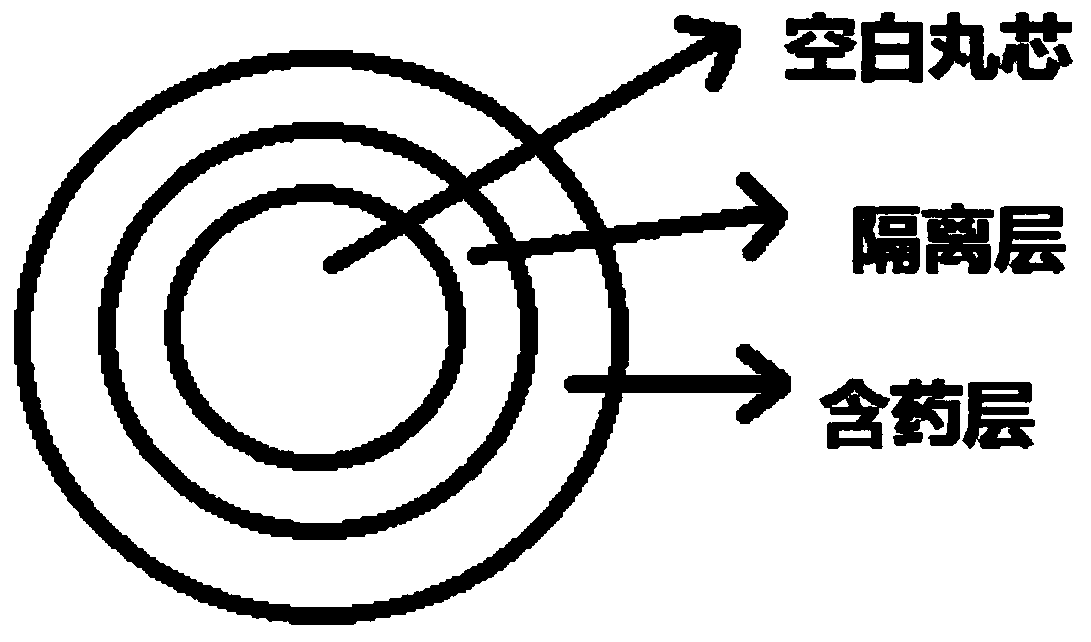
A kind of dabigatran etexilate mesylate pellet and preparation method thereof
ActiveCN106727414BHelp releaseControllable release patternOrganic active ingredientsInorganic non-active ingredientsDiseaseCellulose
The invention discloses a methane sulfonic acid pradaxa pellet and a preparation method and belongs to the technical field of medicals. The structure of the pellet comprises a pellet core, an isolating layer and a drug-containing layer, wherein the isolating layer is wrapped on the outer layer of the pellet core; the drug-containing layer is wrapped on the outer layer of the isolating layer; glycine and hydroxypropyl methylcellulose are contained in the pellet core; hydroxypropyl methylcellulose phthalic ester and talcum powder are contained in the isolating layer; methane sulfonic acid pradaxa, methyl methacrylate and talcum powder are contained in the drug-containing layer. The surface of the isolating layer of the pellet prepared according to the invention is round and smooth; no so-called satellite particle exists; the completeness of the isolating layer is not influenced, so that the active ingredients and the glycine can be effectively isolated in space and the stability of the active ingredients of the preparation in a storage process can be guaranteed. The methane sulfonic acid pradaxa pellet prepared according to the invention has the effects of preventing and treating cerebral ischemia and systemic embolism diseases.
Owner:哈药集团股份有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com