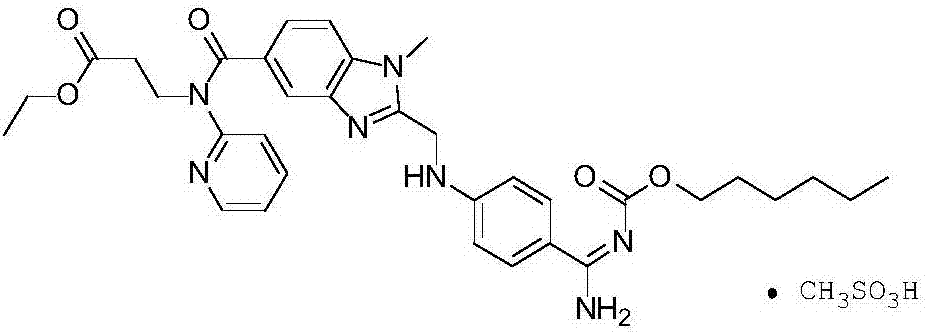
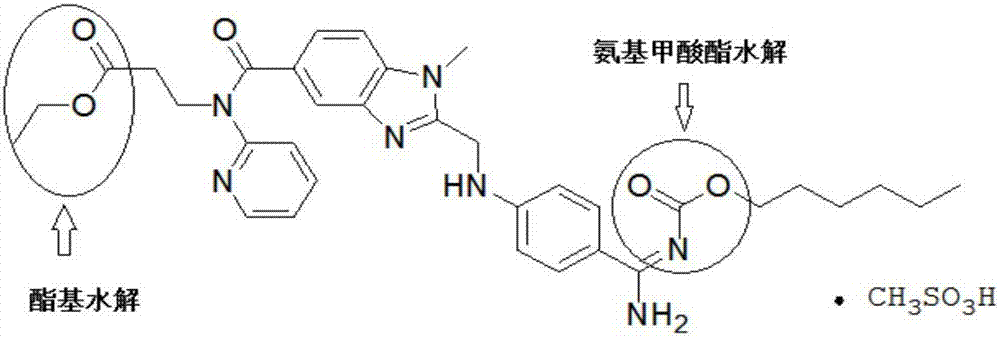
Preparation method of dabigatran etexilate mesylate
A technology of dabigatran etexilate mesylate and dabigatran etexilate, applied in the field of preparation of dabigatran etexilate mesylate, to achieve the effects of reducing related substances, low price, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
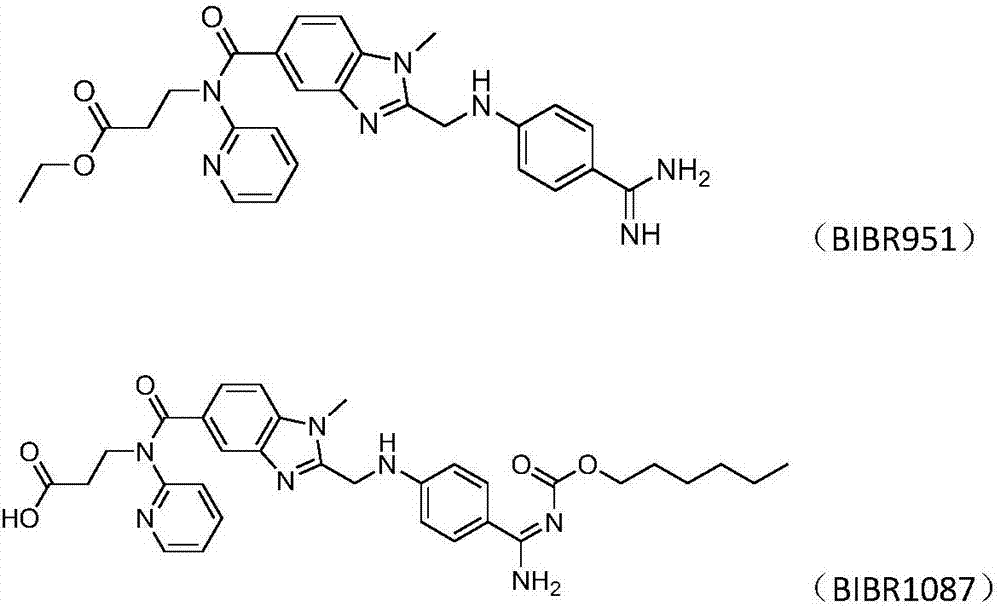
[0027] 5.0g (8.0mmol) dabigatran etexilate base (purity 95.73%, BIBR951: 0.12%, BIBR1087: 0.09%, the same below) and 100.0g ethyl acetate are joined in the 250ml there-necked flask, heated to 60 ℃ to Dissolved completely, filtered through 2.0g of diatomaceous earth (Celite STD: D50: 22μm, D90: 38μm), added dropwise 0.77g (8.0mmol) methanesulfonic acid to the obtained filtrate at 50°C, cooled to room temperature after the dropwise addition, and passed Filter, dry, pulverize to obtain dabigatran etexilate mesylate 5.3g, yield 91.9% (purity 99.50%, BIBR951: 0.02%, BIBR1087: 0.04%)
Embodiment 2
[0029] 5.0g (8.0mmol) dabigatran etexilate base and 100.0g ethyl acetate were added in a 250ml three-necked flask, heated to 60°C to dissolve completely, and passed through 2.0g diatomaceous earth (Celite 503: D50: 29.6 μm, D90: 64.2μm) hot filtration, the resulting filtrate was added dropwise with 0.77g (8.0mmol) methanesulfonic acid at 50°C, cooled to room temperature after the dropwise addition, filtered, dried and pulverized to obtain 5.2g of dabigatran etexilate mesylate , yield 90.2% (purity 99.30%, BIBR951: 0.07%, BIBR1087: 0.08%)
Embodiment 3
[0031] 5.0g (8.0mmol) dabigatran etexilate base and 100.0g ethyl acetate were added in a 250ml three-necked flask, heated to 60°C to dissolve completely, and passed through 2.0g diatomaceous earth (Celite 512: D50: 22.5 μm, D90: 38 μm) hot filtration, the resulting filtrate was added dropwise with 0.77g (8.0mmol) methanesulfonic acid at 50°C, cooled to room temperature after the dropwise addition, filtered, dried, and pulverized to obtain 5.1g of dabigatran etexilate mesylate, Yield 88.5% (purity 99.56%, BIBR951: 0.03%, BIBR1087: 0.03%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com