Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
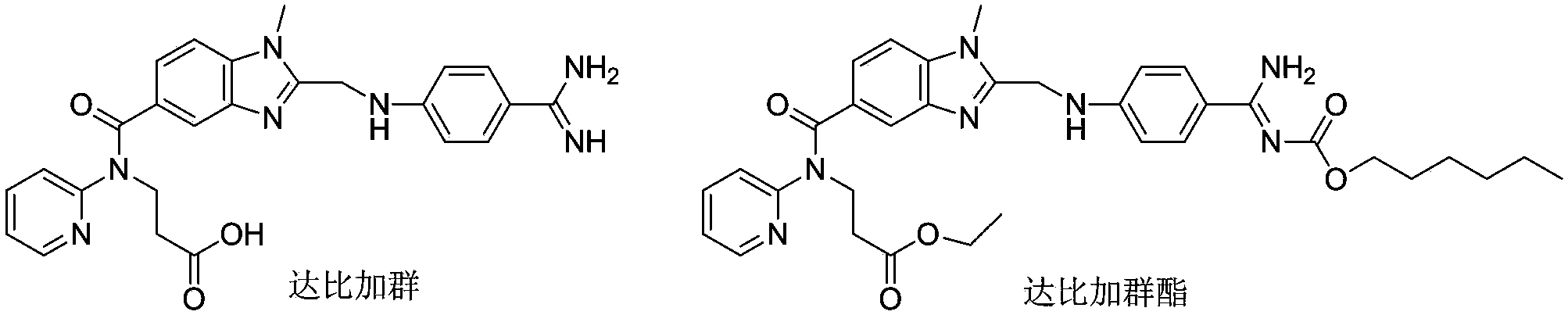
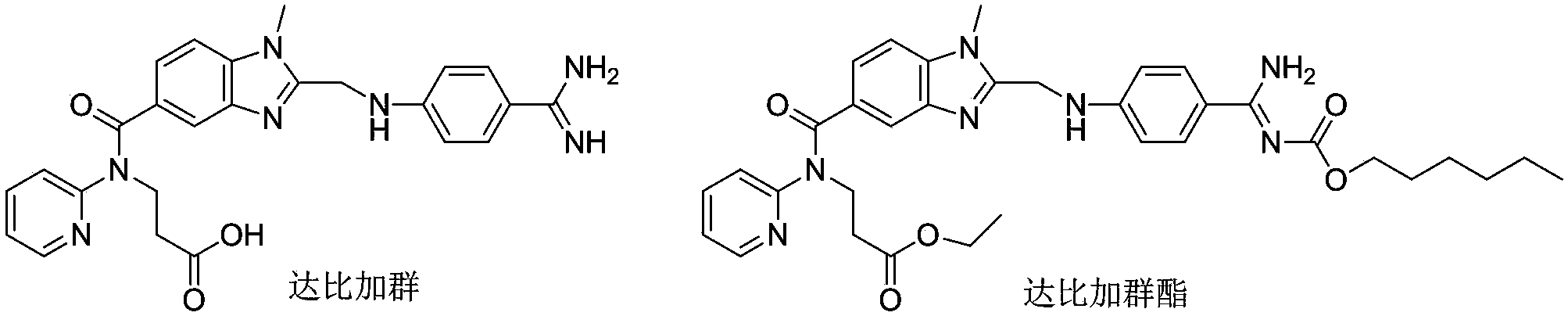
67 results about "Dabigatran ethyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dabigatran-containing solid dispersion and preparation method as well as application thereof
InactiveCN104644543AImprove bioavailabilitySimple processOrganic active ingredientsPharmaceutical delivery mechanismPolymer scienceDabigatran ethyl ester
The invention belongs to the technical field of medicines and specifically discloses a dabigatran-containing solid dispersion and a preparation method as well as application thereof. The solid dispersion comprises a dabigatran-containing active substance and a carrier material, wherein the hydrophilic high-molecular polymer accounts for 20%-98% of mass of the solid dispersion and the carrier material is a hydrophilic high-molecular polymer. The preparation of the solid dispersion is low in cost and excellent in technical reproducibility.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Dabigatran etexilate self-emulsifying dispersible tablets and preparation method thereof
InactiveCN104706609ASolve the technical problem of low oral bioavailabilitySimple production processOrganic active ingredientsPill deliveryEmulsionIn vivo
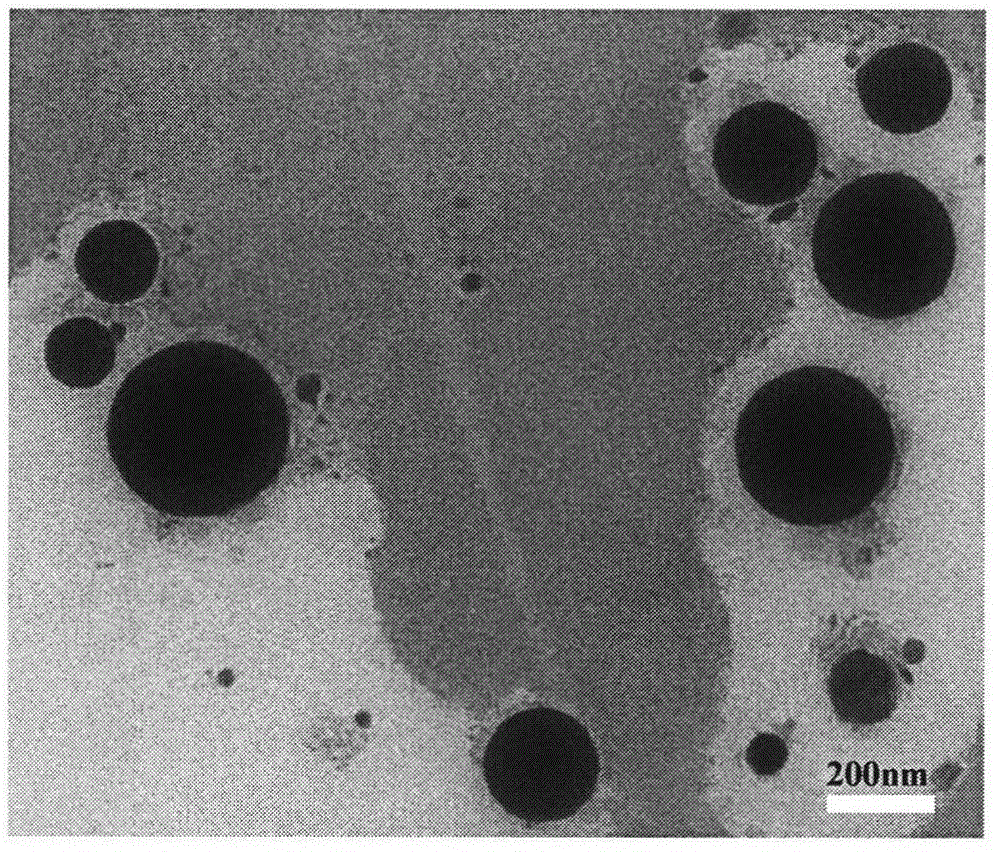
The invention relates to the technical field of medicament preparations, and discloses dabigatran etexilate self-emulsifying dispersible tablets and a preparation method thereof. The preparation method comprises the following steps: combining a certain proportion of dabigatran etexilate, an emulsifier and an assistant emulsifier into a dabigatran etexilate self-emulsifying solution; adsorbing by using a solid adsorbent to further obtain a solid self-emulsifying composition; and preparing the dabigatran etexilate self-emulsifying dispersible tablets by adopting a powder direct tabletting method. The dabigatran etexilate self-emulsifying solution and the self-emulsifying dispersible tablets prepared by the method disclosed by the invention can be self-emulsified in vivo after being orally taken to form a micro emulsion of which the particle size is about 200nm, so that the in vitro dissolution rate and in vivo intestinal absorption of dabigatran etexilate are significantly improved, and the in vivo bioavailability is improved.
Owner:CHINA PHARM UNIV
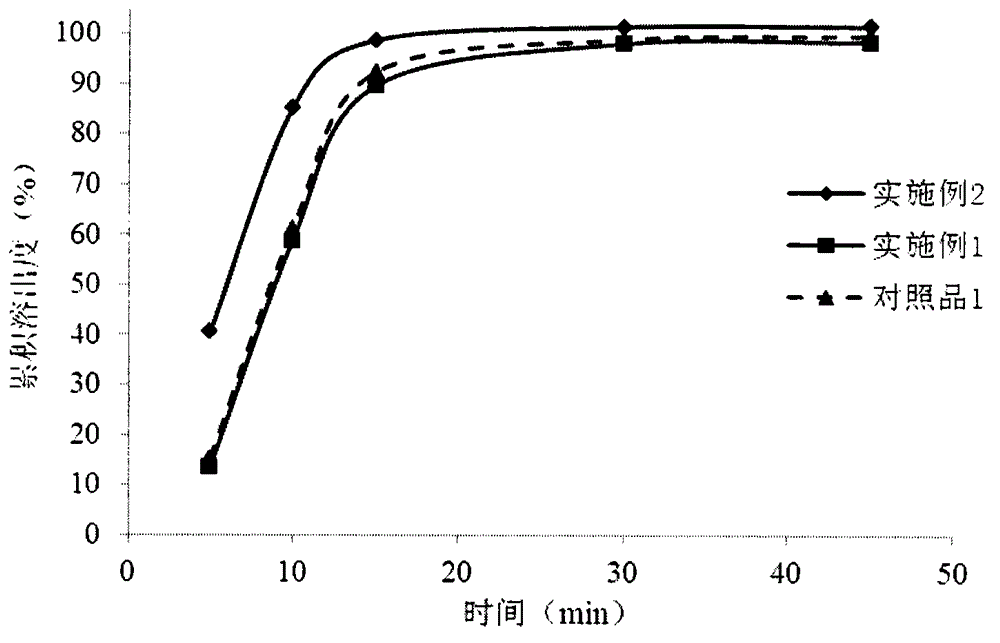
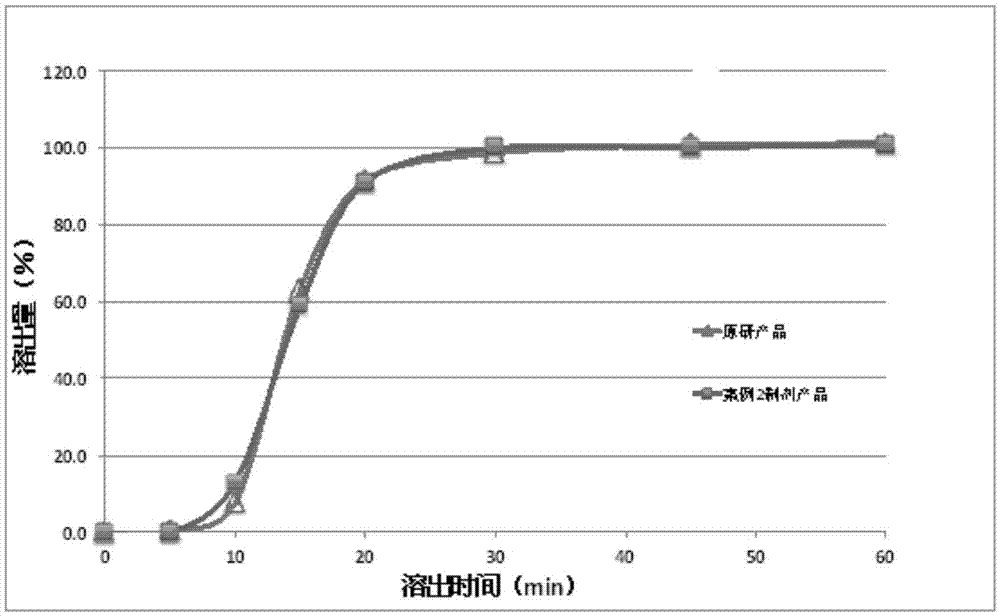
Dabigatran tablet, and preparation method and application thereof
ActiveCN105919962AReduce stimulationAvoid damageOrganic active ingredientsPharmaceutical non-active ingredientsMethyl celluloseDabigatran ethyl ester
The present invention provides a dabigatran tablet, including a dabigatran layer, an organic acid layer compounded on the surface of the dabigatran layer. The organic acid layer includes organic acid and a sustained-release material; the organic acid is citric acid or tartaric acid; the sustained-release material is one or more of hydroxypropyl cellulose, hydroxypropyl methyl cellulose, methyl cellulose, ethyl cellulose and hydroxyethyl cellulose; the dabigatran layer includes dabigatran and a pharmaceutically acceptable salt thereof; the content of the organic acid is 20wt%-50wt%; and the content of the slow release material is 5wt%-20wt%. The organic acid in the dabigatran tablet can release at a constant speed or slowly, thereby reducing the stimulation and injury of gastrointestinal drugs, but does not affect the bioavailability of the drug. At the same time, the preparation method is more simple and easy to operate, reduces the production cost, and is easy to realize industrialization.
Owner:LIANGJIANG MEDICINE CO LTD
Dabigatran etexilate medicine composition and preparation method thereof
InactiveCN104224754AImprove stabilityNarrow particle size distributionOrganic active ingredientsPharmaceutical delivery mechanismOrganic acidIsolation effect
The invention relates to a dabigatran etexilate medicine composition and a preparation method thereof. The dabigatran etexilate medicine composition consists of a pill I and a pill II, wherein the pill I contains an active ingredient, a blank pill core material, an adhesive, an anti-sticking agent and / or an isolation material, wherein the active ingredient is dabigatran etexilate or a pharmaceutically acceptable salt, hydrate or solvate thereof; and the pill II contains a medicinal organic acid, a pill core material, an adhesive, an anti-sticking agent and / or an isolation material. The discovery shows that the active ingredient and the organic acid are respectively contained in the two different pills, so that the requirement of dissolving out the active ingredient is satisfied, and the active ingredient is more uniformly dissolved out; and in addition, an isolation effect between the active ingredient and the organic acid is enhanced, so that the stability of the dabigatran etexilate medicine composition is improved.
Owner:SICHUAN HAISCO PHARMA CO LTD
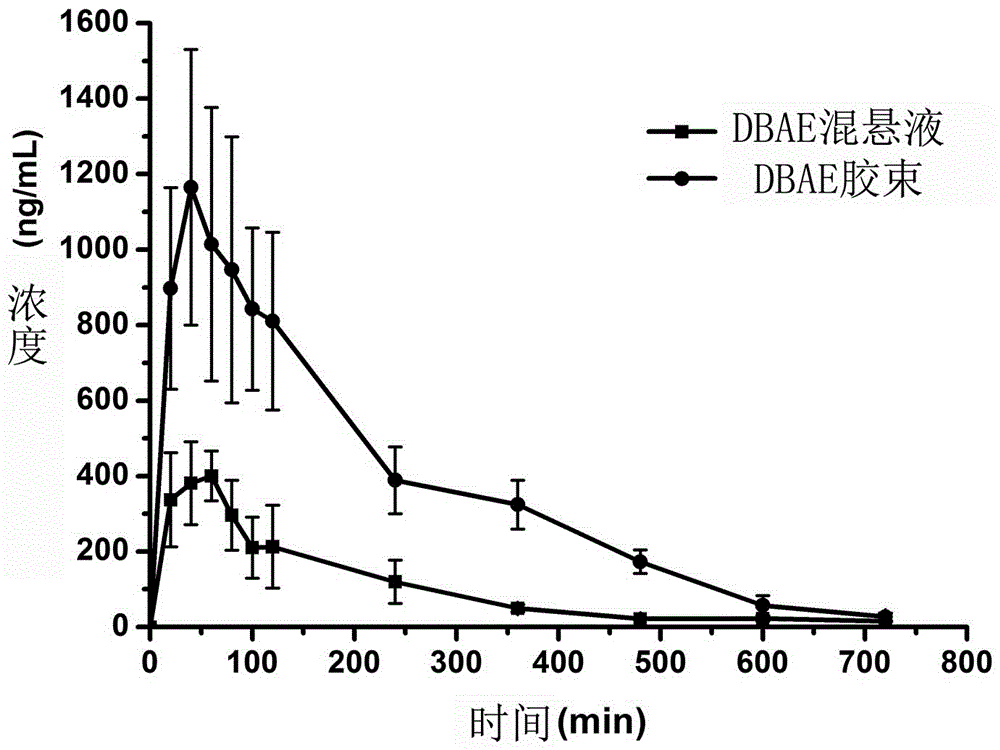
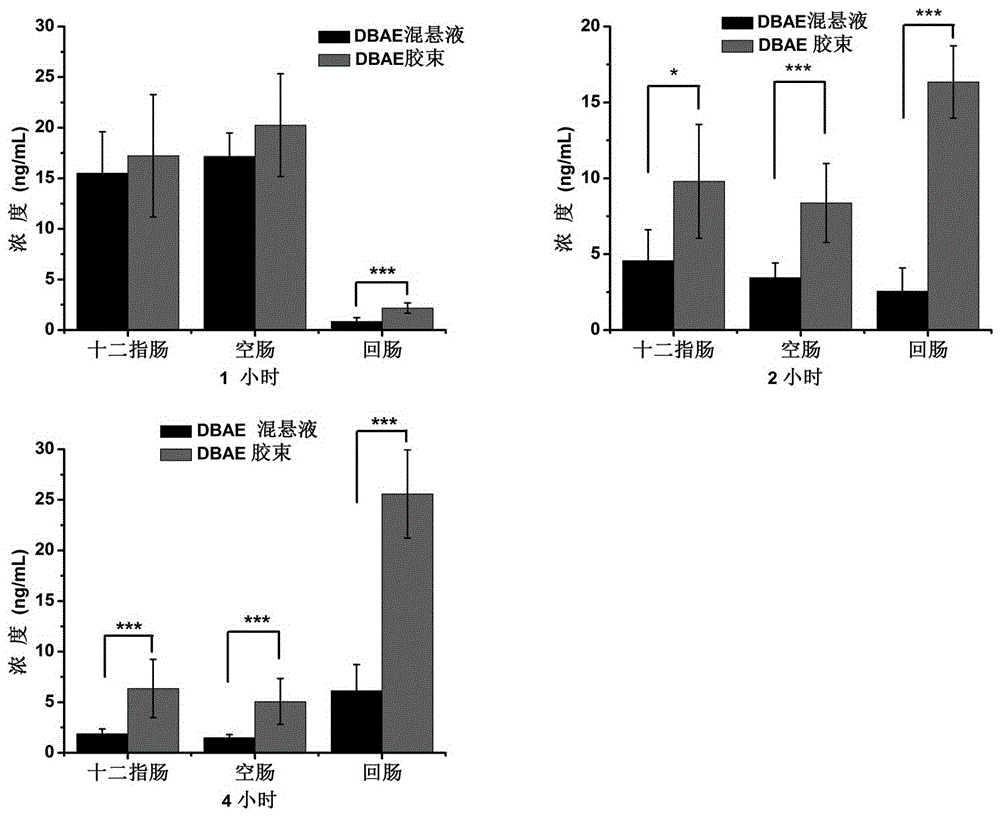
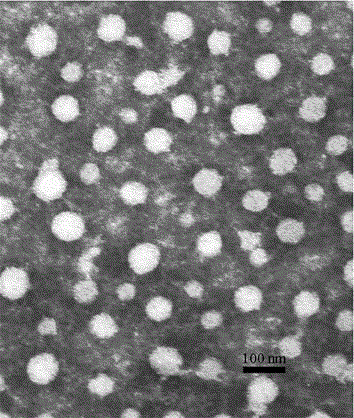
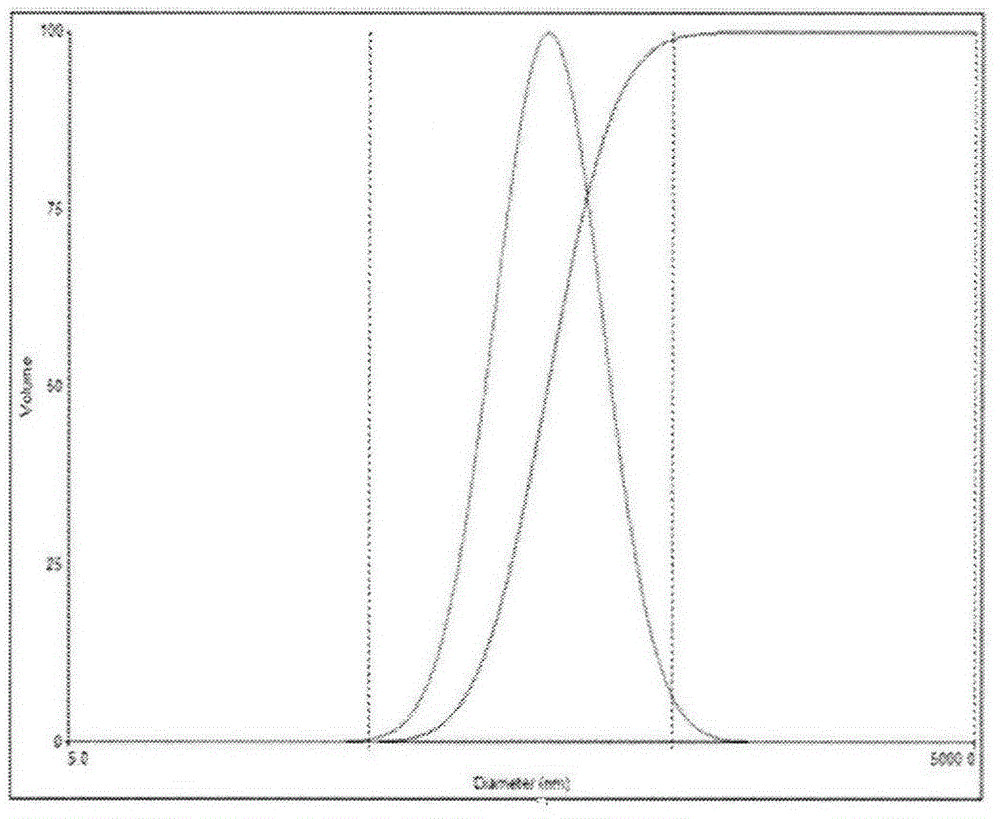
Dabigatran etexilate nano mixed micelle and preparation method thereof
ActiveCN105997868AImprove solubilityPromote dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsMixed micelleDabigatran ethyl ester
The invention provides dabigatran etexilate nano mixed micelle and a preparation method thereof. A film dispersion method is adopted for preparing the soluplus / VitaminE-TPGS nano mixed micelle of the dabigatran etexilate, and the particle size is within 70-100 nm.
Owner:SICHUAN UNIV
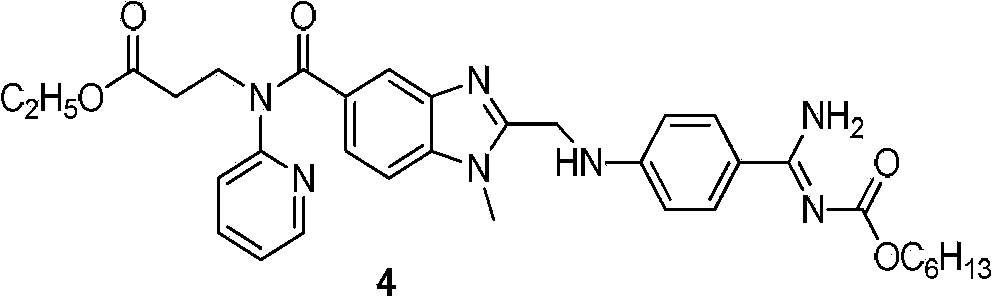
Dabigatran preparation method
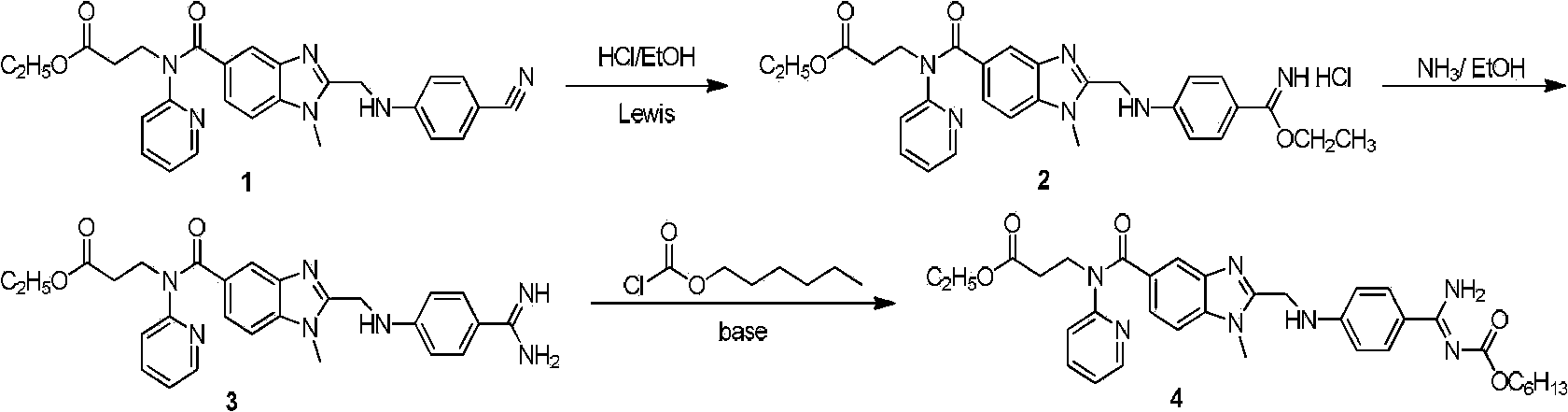
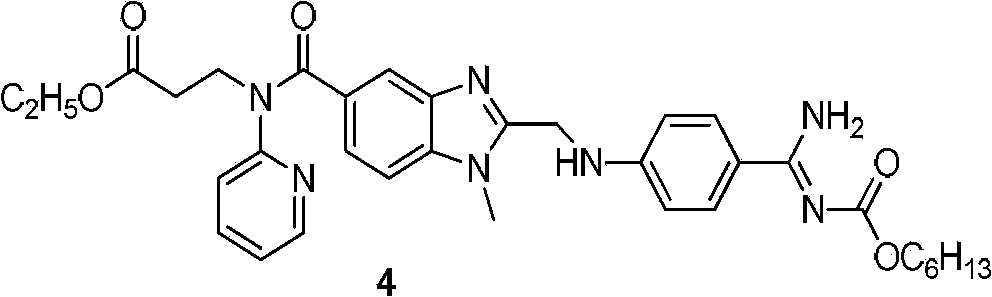
The present invention relates to a dabigatran preparation method, which is characterized in that in a saturated hydrochloric acid ethanol solution, a compound represented by a formula 1 is subjected to alcoholysis under catalysis of a Lewis acid to obtain a compound represented by a formula 2, the compound represented by a formula 2 reacts with ammonia to obtain a compound represented by a formula 3, and the compound represented by the formula 3 reacts with hexyl chloroformate in the presence of an alkali to obtain a compound represented by a formula 4, wherein the compound represented by the formula 4 is dabigatran.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
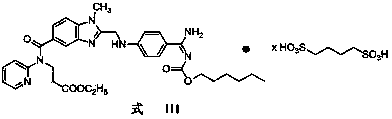
Butanedisulfonic acid dabigatran etexilate and preparation method and application thereof
ActiveCN103864756AEasy to prepareQuality improvementOrganic active ingredientsSulfonic acids salts preparationActive componentDabigatran ethyl ester
The invention discloses a butanedisulfonic acid dabigatran etexilate and a preparation method thereof, a pharmaceutical composition with butanedisulfonic acid dabigatran etexilate as an active component, and an application of the butanedisulfonic acid dabigatran etexilate and the pharmaceutical composition in preparing drugs for prevention or treatment of diseases caused by thrombus or embolism. The butanedisulfonic acid dabigatran etexilate is simple in preparation method, excellent in quality and suitable for industrialized production, moreover, has good stability, drug efficacy and security, and is an excellent pharmaceutical form of dabigatran etexilate.
Owner:海思科制药(眉山)有限公司
Preparation method for dabigatran etexilate intermediate and intermediate compound
The invention discloses a preparation method for a dabigatran etexilate intermediate and an intermediate compound. The preparation method of the dabigatran etexilate intermediate 4 comprises the following step: in a protic organic solvent, reacting a compound 3 with a C1-C3 alkanol solution of methylamine. In the formula of the compound 3, X is chlorine, bromine or iodine. The invention also discloses the intermediate compound 3 and a preparation method of the intermediate compound 3. The preparation method for the dabigatran etexilate intermediate is simple and easy to operate, the yield is high, the product is easy to purity, and the preparation method is suitable for industrial production. The formula of the preparation method for the dabigatran etexilate intermediate is shown in the specification.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
Process for the preparation of benzimidazole derivatives and its salts
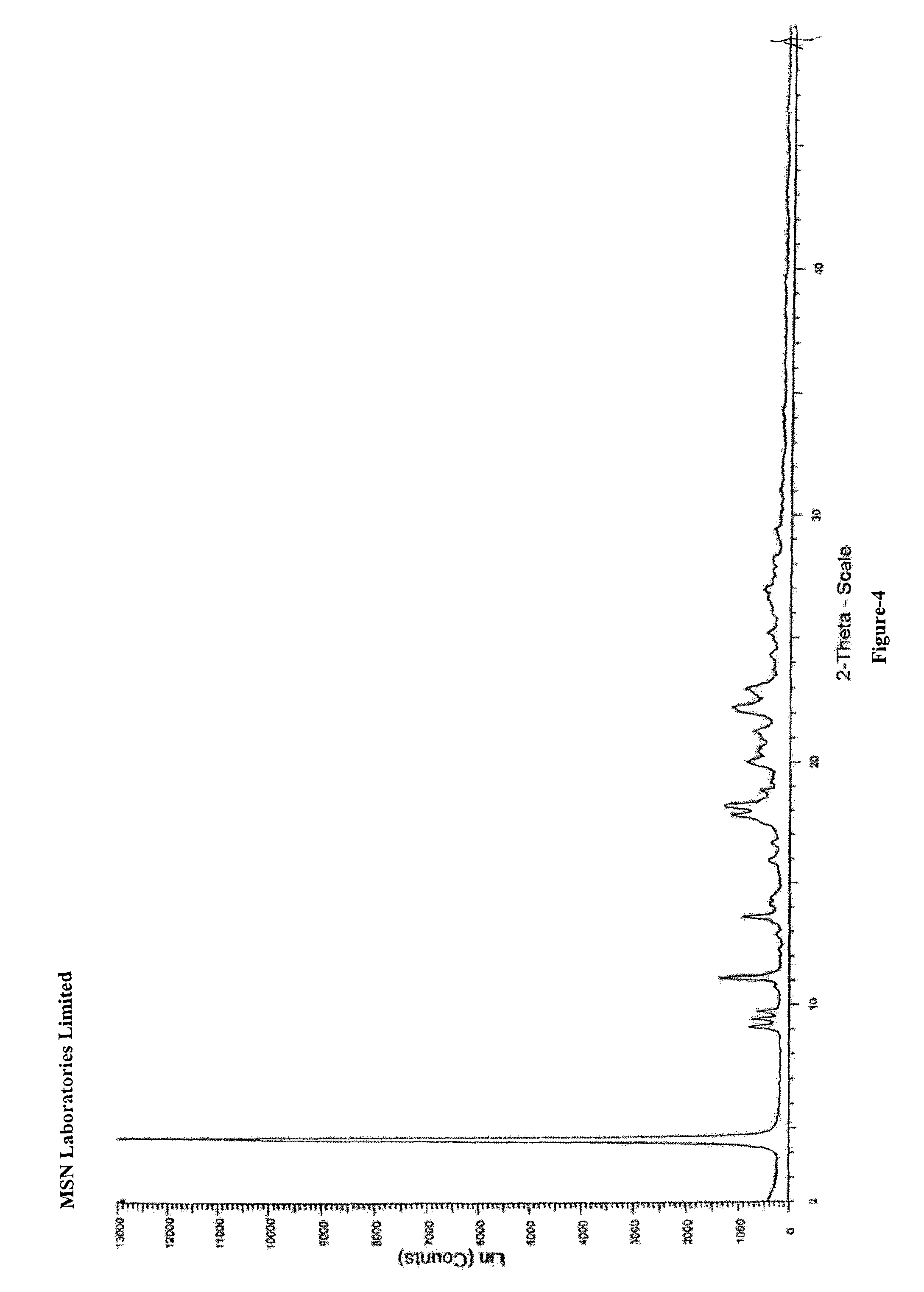
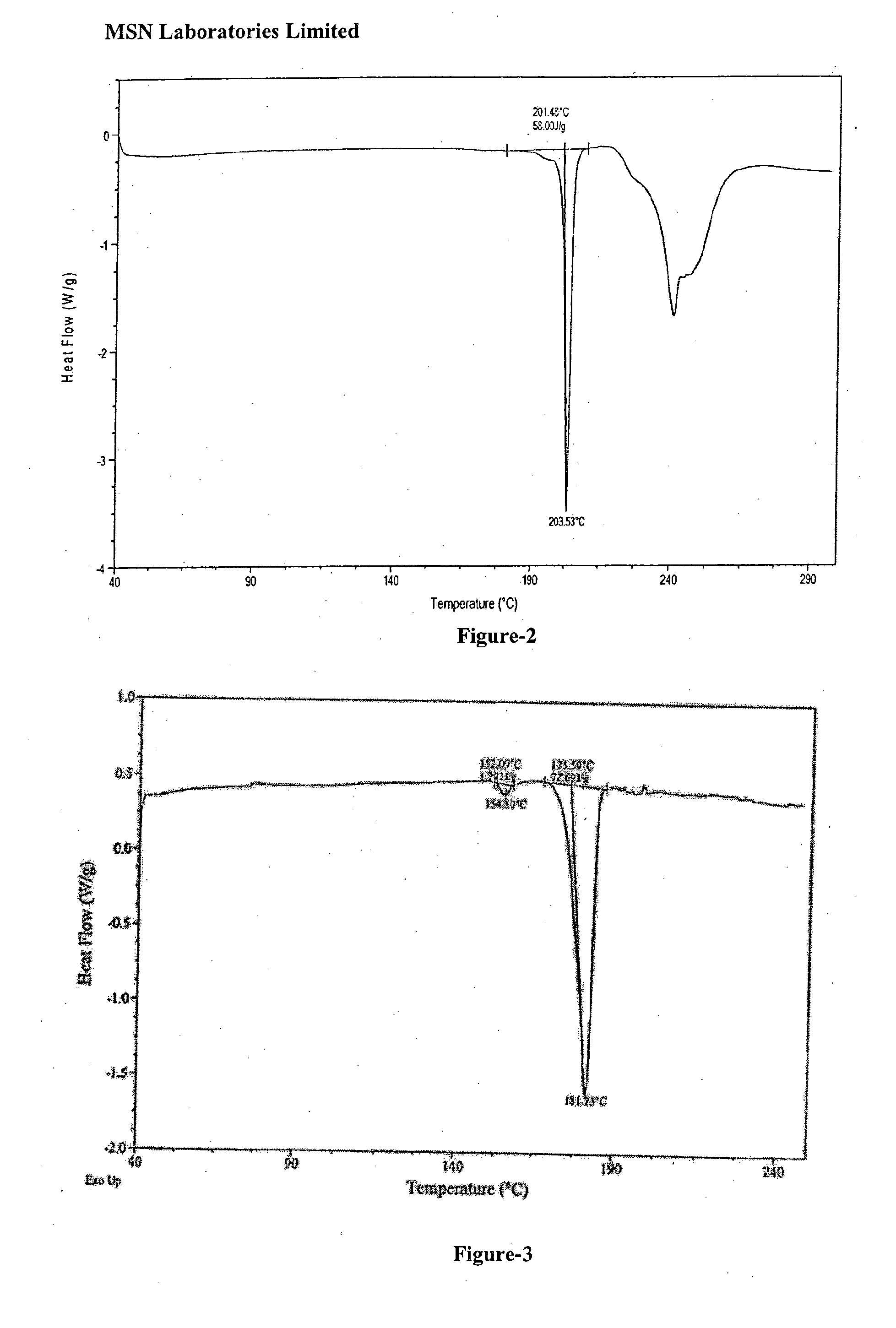
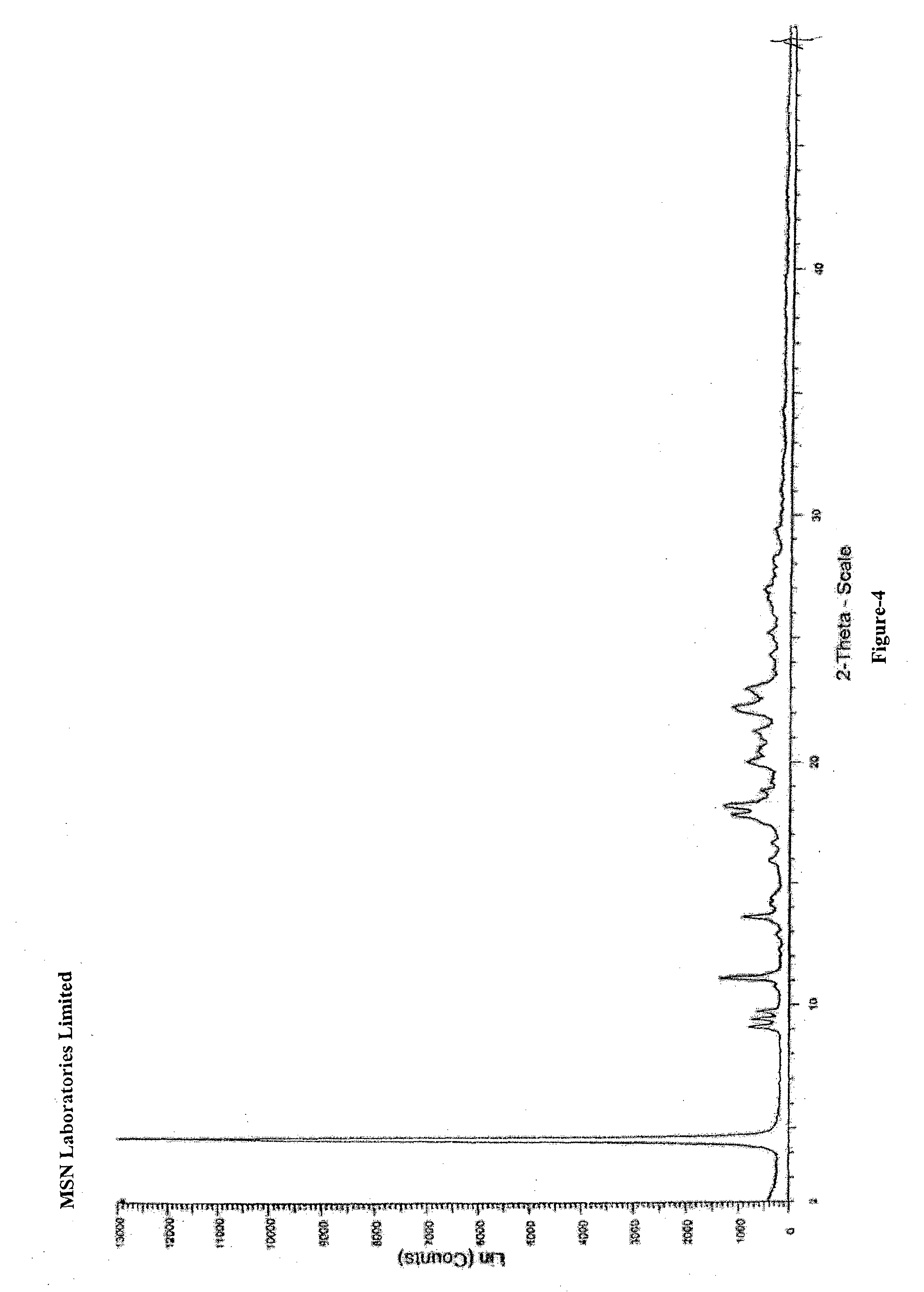
An dabigatran etexilate intermediate of Formula-6a, and the use in the preparation of dabigatran etexilate thereof.
Owner:MSN LAB PTE LTD
Process for the preparation of benzimidazole derivatives and its salts
InactiveUS20140148601A1Simple processMild reaction conditionsOrganic chemistryBlood disorderBenzimidazole derivativeDabigatran ethyl ester
An dabigatran etexilate intermediate of Formula-6a, and the use in the preparation of dabigatran etexilate thereof.
Owner:MSN LAB PTE LTD
Pradaxa-containing microemulsion preparation
The invention relates to the technical field of medicine preparations, and particularly relates to a pradaxa-containing microemulsion preparation and a preparation method thereof. The pradaxa-containing microemulsion preparation is prepared by combination of a certain proportion of a surfactant, a cosurfactant, an oil phase, a water phase and a moderate amount of a stabilizer, a suspension aid, a sweetener and a preservative, the prepared pradaxa-containing microemulsion preparation is oil-in-water type, the particle size is in the range of 200-400 nm, appearance is clear transparent uniform, and stability is good. The microemulsion preparation, compared with an oral solid preparation, has the advantages of low production cost, simple preparation process and the like, after oral administration, dissolution of pradaxa in the gastrointestinal tract is increased, the bioavailability is improved, and the pradaxa-containing microemulsion preparation has the good market prospect.
Owner:CHINA PHARM UNIV
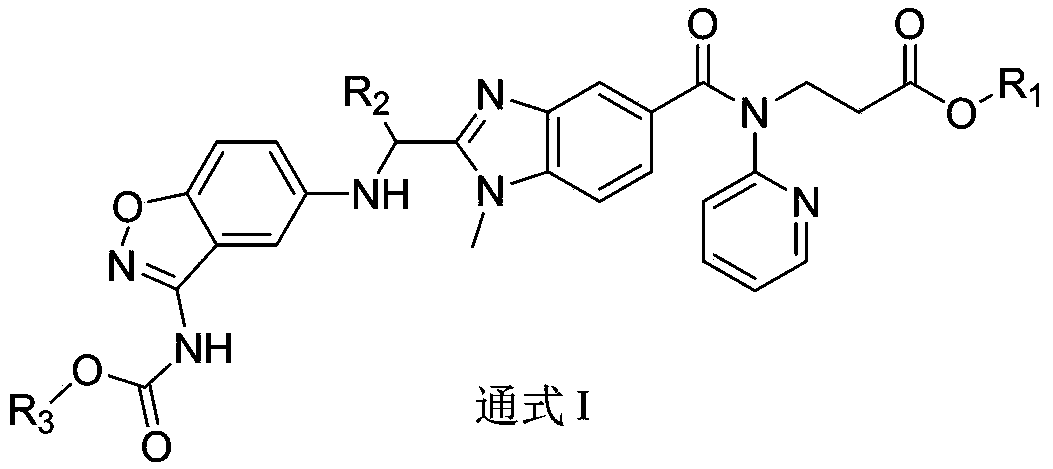
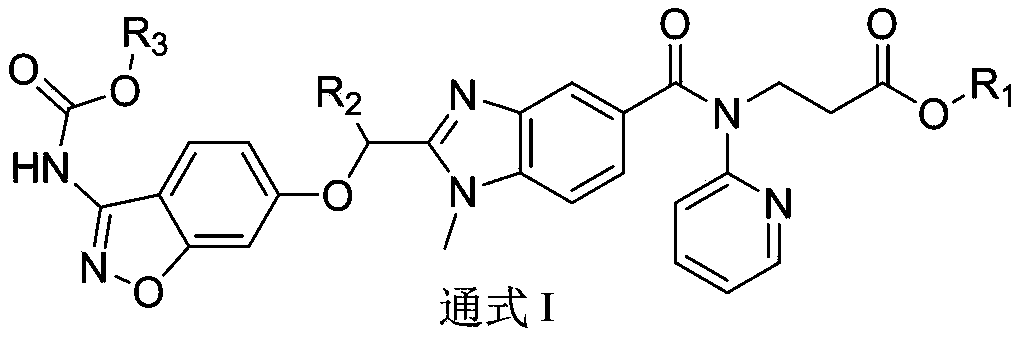
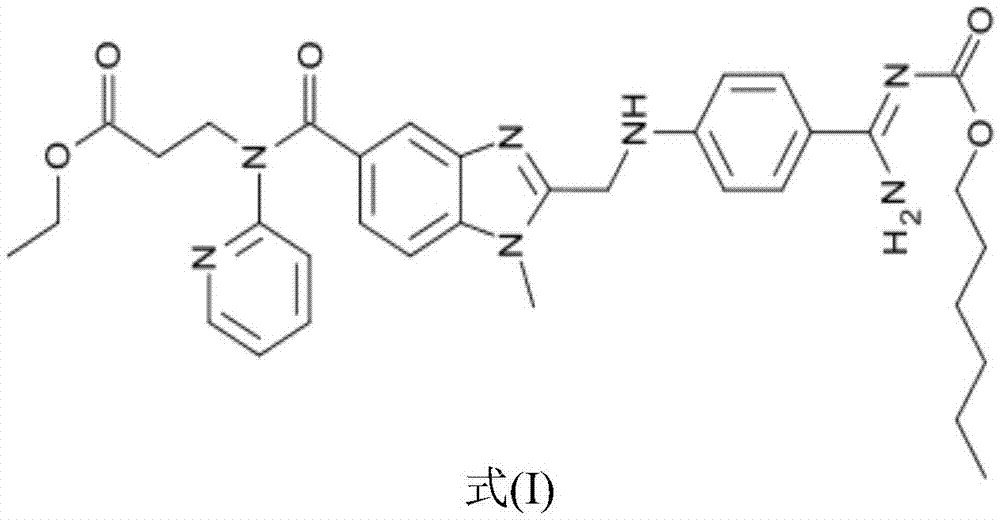
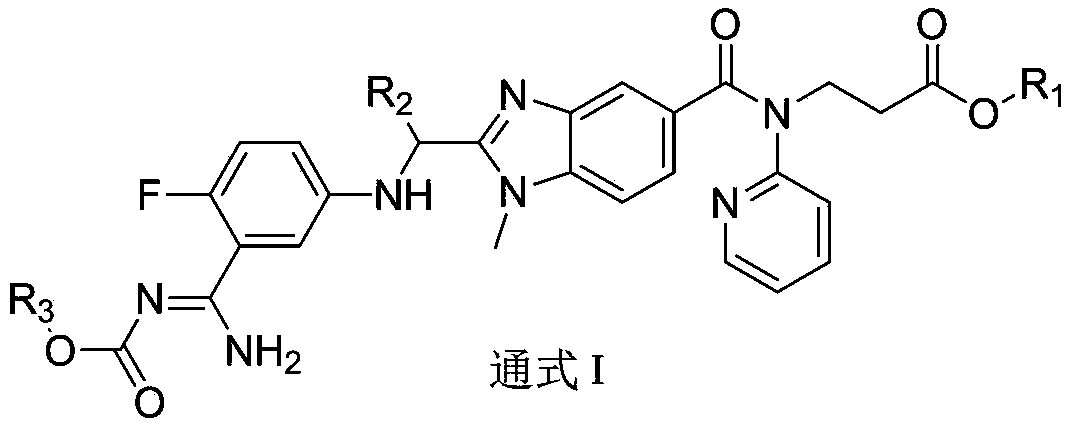
Dabigatran derivative used as prodrug, and preparation method and application thereof
InactiveCN103420985AOrganic active ingredientsOrganic chemistryBenzimidazole derivativeActive component
The invention relates to a preparation method of a benzimidazole derivative shown as a general formula I, wherein X, R, R1, R2 and R3 are defined as in the specification. The invention relates to a Dabigatran derivative shown as the general formula I and non-toxic pharmaceutically acceptable salts thereof, and a pharmaceutical composition containing these compounds as active components, and application of the compounds and the pharmaceutical composition as thrombin inhibitors.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Production process of pradaxa mesylate
The invention discloses a production process of pradaxa mesylate. The production process comprises the following steps: (1) preparing an intermediate PR-I; (2) preparing an intermediate PR-II; (3) preparing pradaxa PR-III; (4) refining the pradaxa PR-III; and (5) preparing pradaxa mesylate. The production process is mild in reaction condition, simple in reaction route, convenient in operation, high in selectivity, and capable of shortening the production period; and the obtained pradaxa intermediate is low in water content, the prepared pradaxa mesylate is high in yield and purity, and the maximum impurity is low in impurity content; and the production process is less in emission of three wastes, environmentally friendly, free from requiring the columnar chromatography purification, suitable for the industrialized production, capable of avoiding the requirement of palladium-on-carbon high-pressure hydrogenation on equipment and capable of reducing the risk.
Owner:JIANGXI GUOYAO PHARMA LLC
Method for synthesizing dabigatran etexilate intermediate
The invention provides a method for synthesizing a dabigatran etexilate intermediate, comprising: esterifying 3-nitro-4-methylaminobenzoic acid as a starting material with methanol to generate 3-nitro-4-methyl-(methylamino)benzoate, and carrying out catalytic hydrogenation reduction to obtain 3-amino-4-methyl-(methylamino)benzoate; carrying out closed-ring reaction to generate 1-methyl-2-chloromethylbenzimidazole-5-methyl formate; subjecting 1-methyl-2-chloromethylbenzimidazole-5-methyl formate to substitution reaction with 4-cyanoaniline to generate 1-methyl-2-(4-cyanophenylamino)methylbenzimidazole-5-methyl formate, and hydrolyzing to obtain 1-methyl-2-(4-cyanophenylamino)methylbenzimidazole-5-formic acid; amidating to generate a target product.The target product is synthesized through a converging synthetic process, reactive operations are simple, and the method is easy for industrial production.
Owner:江西胜富化工有限公司
Preparation method of N-substituted phenyl glycine
ActiveCN103992241ASimple unit operationLow equipment requirementsCarboxylic acid nitrile preparationOrganic compound preparationGlyoxylic acidGlycine
The invention discloses a preparation method of novel N-substituted phenyl glycine (dabigatran ester intermediate). The preparation method of the novel N-substituted phenyl glycine comprises the following processes: carrying out condensation on glyoxylic acid and cheap and available substituted phenylamine (1) which is taken as a starting raw material to obtain imide; meanwhile, carrying out hydrogenation reduction to obtain N-(substituted phenyl) glycine. The preparation method of the novel N-substituted phenyl glycine has the advantages that the synthetic route is not reported before, raw materials are cheap and easily available; unit operation is easy, and equipment requirement is low, so that the preparation method of the novel N-substituted phenyl glycine is applicable to industrial production.
Owner:ABA CHEM CORP
Method for producing an intermediate product of dabigatran etexilate
The invention relates to a process for preparing the compound of formula 1a valuable intermediate product in the synthesis of the pharmaceutical active substance dabigatran etexilate.
Owner:BOEHRINGER INGELHEIM INT GMBH
Dabigatran etexilate pharmaceutical composition for injection, preparation method and uses thereof
PendingCN109010249AExcellent kinetic curveIncrease exposureOrganic active ingredientsPharmaceutical delivery mechanismDrugs regulationsProper treatment
The present invention relates to a dabigatran etexilate pharmaceutical composition for injection, a preparation method and uses thereof, wherein the dabigatran etexilate pharmaceutical composition comprises: (1) an active substance dabigatran etexilate represented by a formula I-2, (2) a pharmaceutically acceptable solvent, and optionally (3) other pharmaceutically acceptable excipients. Accordingto the present invention, the test results show that various indexes of the dabigatran etexilate pharmaceutical composition meet the relevant drug regulations, and the safety, the resolubility and the long-term stability are good; the pharmacokinetic test results show that the dabigatran etexilate pharmaceutical composition can maintain the proper blood drug level and the proper treatment duration time in the body after administration, and the pH value is neutral so as to greatly reduce the stimulating effect on gastrointestinal tract; and the preparation method has characteristics of simplepreparation process, convenience, feasibility, good repeatability and low production cost, and is suitable for industrial mass production.
Owner:SHANGHAI MEIYUE BIOTECH DEV
Oral drug composition of dabigatran etexilate and preparation method thereof
InactiveCN108261409AReduce the impactHigh yieldOrganic active ingredientsPharmaceutical non-active ingredientsOrganic acidCellulose
The invention provides an oral drug composition of dabigatran etexilate and a preparation method thereof, belongs to the field of pharmaceutic preparation, aims at providing oral preparation of the dabigatran etexilate which has the advantages of simple technology, easy implementation, lower cost, better active material dissolution and easy absorption and a preparation method for the oral preparation. The oral drug composition comprises (a) medicated particles composed of active material ingredients, adhesive, disintegrating agent and flow aid, wherein the active material ingredients are dabigatran etexilate or acceptable salt in pharmacy; (b) isolated organic acid crystals, wherein an isolating layer is made from an empty capsule I or an isolating material of acceptable cellulose-based auxiliary materials in pharmacy; a preferred isolating layer is made from the empty capsule I; and the medicated particles and the isolated organic acid crystals are evenly mixed in proportion and filled into an empty capsule II.
Owner:QILU PHARMA
Industrial preparation method of dabigatran
ActiveCN106543144AReduce recrystallizationSimple processOrganic chemistryPurification methodsDistillation
The invention discloses an industrial preparation method of dabigatran, and belongs to the field of medicinal chemistry, wherein the preparation method sequentially comprises a condensation reaction, a closed cyclization reaction, a Pinner reaction, and other steps. According to the present invention, the hydrogen chloride / alcohol / ester solution is prepared by using acyl chloride and alcohol as raw materials, such that the problems of corrosion on equipment, high hidden safety danger, environmental pollution and the like caused by the use of hydrogen chloride gas in the prior art are solved; and the reactions in various steps are subjected to the industrial-scale-based optimization, the unnecessary distillation, extraction and re-crystallization process is reduced, the process is simplified, the purification method of the final product dabigatran is improved, the purification efficiency is increased in the case of the ensuring of the process yield and the product quality, the process reproducibility is good, the preparation cost is low, and the method is the ideal industrial preparation method of the dabigatran.
Owner:CHENGDU LIKAI CHIRAL TECH
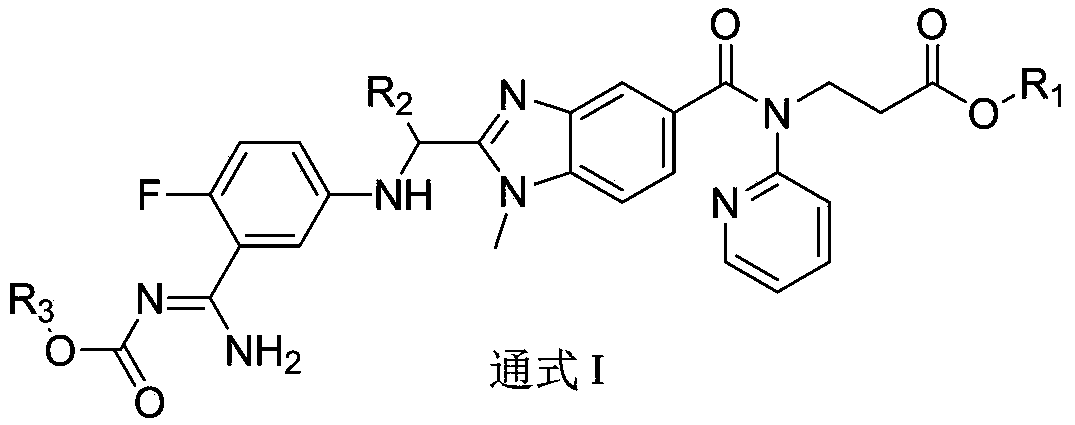
Dabigatran derivative, and preparation method and application thereof
InactiveCN103420983AOrganic active ingredientsOrganic chemistryBenzimidazole derivativeActive component
The invention relates to a preparation method of a benzimidazole derivative shown as a general formula I, wherein R1, R2 and R3 are defined as in the specification. The invention relates to a Dabigatran derivative shown as the general formula I and non-toxic pharmaceutically acceptable salts thereof, and a pharmaceutical composition containing these compounds as active components, and application of the compounds and the pharmaceutical composition as thrombin inhibitors.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of dabigatran etexilate intermediate
The invention discloses a preparation method of a dabigatran etexilate intermediate. The preparation method of the dabigatran etexilate intermediate 2 comprises that in an aprotic organic solvent, a compound 1 and a C1-C3 alkyl alcohol solution of methylamine undergo a reaction to produce the dabigatran etexilate intermediate, wherein X represents chlorine, bromine or iodine. The preparation method of the dabigatran etexilate intermediate has simple processes, can be operated easily, has a high reaction rate and a high yield, can be purified easily and can produce the product having high purity.
Owner:SHANGHAI INST OF PHARMA IND +2
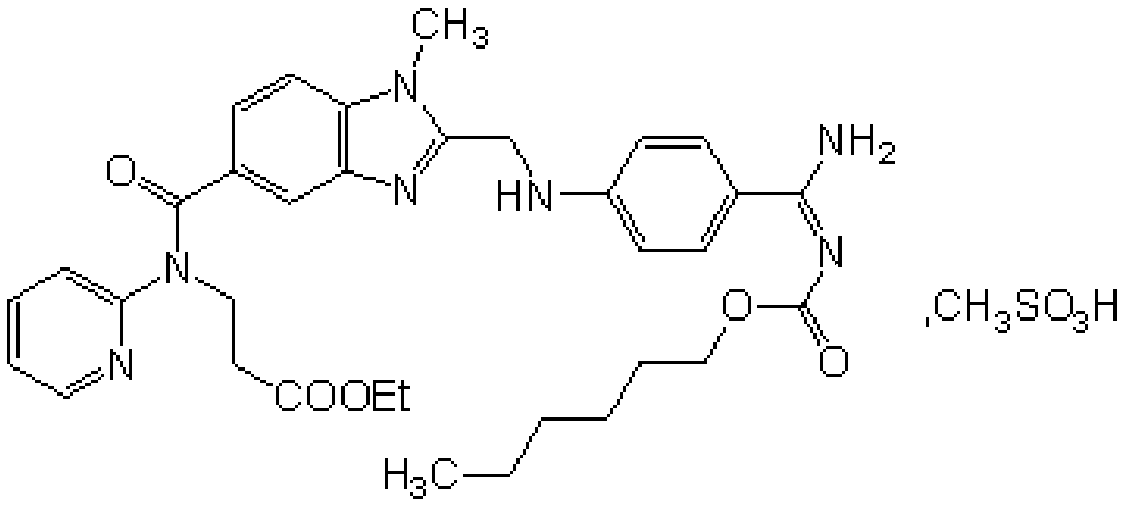
Method for synthesizing and preparing pradaxa formamidine intermediates
InactiveCN105294651AReduce dosageReduce time for dangerous operationsOrganic chemistryPurification methodsDistillation
The invention relates to a method for synthesizing and preparing pradaxa formamidine intermediates. A concrete compound refers to 3-(2-(4-guanyl-phenylamino)methyl)-1-methyl-N-(pyridine-2-yl)-1H-benzo[d]imidazole-5-acylamino) alanine ethyl ester hydrochloride. The compound has the main purpose of preparing anticoagulants of methylsulfonic acid pradaxa formamidine. The method has the advantages that a solvent reducing method is adopted; the consumption of HCl / NH3 gas is reduced; in addition, the HCl solution is subjected to pressure reduction distillation under the relative low temperature condition; the distillation time is shortened; the requirements on the pressure resistance performance and the anti-corrosion performance of equipment are lowered; the impurity content is reduced. The invention also adopts a novel purification method; the solvent loss is reduced; the product purity is improved; the product cost is reduced.
Owner:YANTAI DONGCHENG PHARMA GRP
Preparing method of dabigatran etexilate mesylate crystal form I
InactiveCN104725360AHigh crystal purityQuality improvementOrganic chemistry methodsMedicinal chemistryDabigatran Etexilate Mesylate
An embodiment of the invention discloses a preparing method of dabigatran etexilate mesylate crystal form I. The method includes the steps of adding dabigatran etexilate into acetone solution before heating for solution, and adding methylsulfonic acid to generate dabigatran etexilate mesylate; stirring the dabigatran etexilate mesylate in ester solvent or ether solvent under heating so that the dabigatran etexilate mesylate with low crystal form I (containing little crystal form II or part of semihydrate) is converted into the dabigatran etexilate mesylate with high content of the crystal form I. The dabigatran etexilate mesylate crystal form I prepared by the method is high in purity, lower in water content and more stable in quality.
Owner:CHONGQING TOPTECH PHARMA TECH
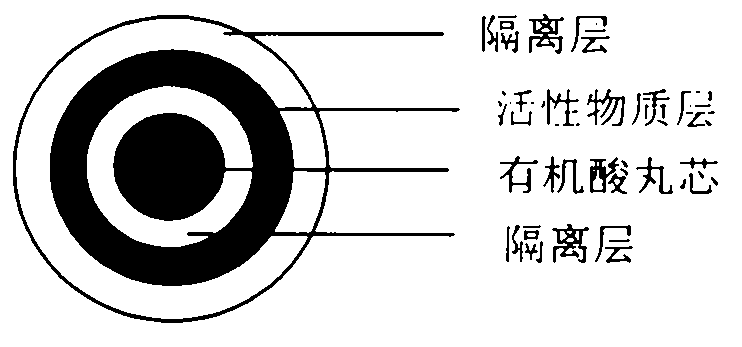
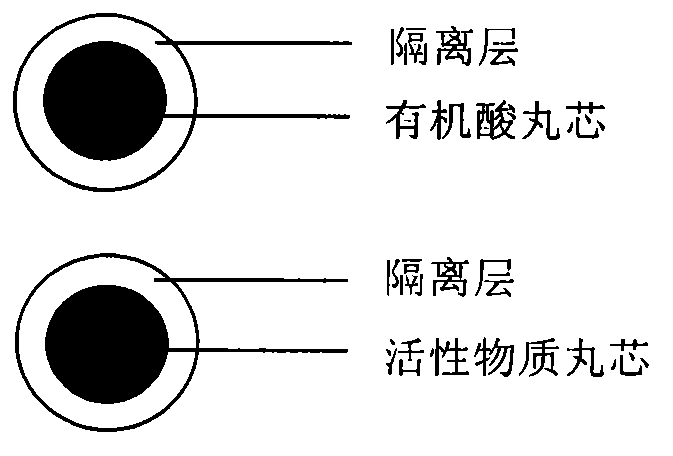
Pellet medicine composition containing pradaxa or salt and hydrate thereof
InactiveCN104434882ASimple preparation processEasy to operateOrganic active ingredientsGranular deliverySolubilityOrganic acid
The invention discloses a medicinal combination containing pradaxa or salt and hydrate thereof. The medicinal combination comprises an active substance pellet core material containing an active ingredient pradaxa or pharmaceutically acceptable salt or hydrate thereof and filler and / or adhesive, an organic acid pellet core material containing organic acid and filler and / or adhesive, and an isolation layer which is arranged outside the active substance pellet core material and the organic acid pellet core material as shown in a figure I. By means of screening, the pradaxa active substance and organic acid are respectively prepared into pellet cores, and the pellet cores are respectively coated with the isolation layer, and the two pellets are prepared in a proportion to form the pradaxa oral medicine composition. The process is stable and controllable, and the preparation has good solubility and dissolution rate.
Owner:YANTAI DONGCHENG PHARMA GRP
Pharmaceutical composition and preparation method thereof
ActiveCN106924256AGood storage stabilityEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsBULK ACTIVE INGREDIENTSurface modification
The invention relates to a pharmaceutical composition for oral administration, a preparation method and an application of the pharmaceutical composition as well as a modifying method of acid pharmaceutical adjuvants, wherein the pharmaceutical composition consists of a) the pharmaceutically acceptable surface-modified acid pharmaceutical adjuvants, and b) dabigatran etexilate, or pharmaceutically acceptable salt or a hydrate; and the modifying method comprises the following steps: preparing a pharmaceutically acceptable modifying alkaline substance water solution, and adding the water solution to powder particles of the acid pharmaceutical adjuvants, so that a neutral salt layer is each formed on the surface of the powder particles of the acid pharmaceutical adjuvants, so that hydrotropy can be performed by virtue of the acid adjuvants in a preparation prescription of the dabigatran etexilate, and meanwhile, the dabigatran etexilate can be prevented from being broken up by the acid adjuvants. The technology is also applicable to surface modification of alkaline pharmaceutical adjuvants, so that a neutral salt layer is each formed on the surfaces of the alkaline pharmaceutical adjuvants; therefore, the alkaline pharmaceutical adjuvants are prevented from being broken up by pharmaceutical active ingredients.
Owner:SHENZHEN PHARMACIN CO LTD
Pradaxa dropping pill and preparation method thereof
InactiveCN104523616AHas a wetting effectEvenly dispersedOrganic active ingredientsPill deliveryMedicineAnticoagulant
The invention discloses a pradaxa dropping pill serving as an anticoagulant and a preparation method of the pradaxa dropping pill. The preparation method comprises the steps of with pradaxa as a raw material, adding a matrix according to a prescription, and uniformly mixing to obtain a mixed material; heating the mixed material to be molten, and uniformly stirring to obtain a mixture; placing the mixture into a special pill dropping machine; and dropping pills into a condensing agent at a proper speed to condense to form the pradaxa dropping pill. The pradaxa dropping pill is high in bioavailability, capable of rapidly releasing drugs, high in effect taking speed and drug stability and convenient to take; in addition, the consumption of auxiliary materials is reduced, the production process is simple, the preparation method is easy to operate, the weight difference is small, and the production cost is reduced; and no dust is generated, and labor protection can be favorably realized.
Owner:TIANJIN KANGRUI PHARMA
Preparation method of high-purity dabigatran etexilate
InactiveCN106349221AReduce lossesEconomical purification processOrganic chemistryChloroformateAminolysis
The invention discloses a preparation method of high-purity dabigatran etexilate, prepared by subjecting ethyl 3-[[[2-[[(4-cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazole-5-yl]carbonyl]pyridine-2-ylamino]propanoate to alcoholysis and aminolysis to obtain a compound 4, and subjecting the compound to esterification reaction with hexyl chloroformate; recrystallization is performed after the esterification reaction; the recrystallization employs a recrystallization solvent that is ethyl acetate, isopropyl acetate or n-butyl acetate. The recrystallization solvent can decrease the content of the byproduct compound 7 in dabigatran etexilate to 1% and below, particularly to 0.5% and below for ethyl acetate, with the loss of dabigatran etexilate less than 5%; the purifying process is economical and practical and is suitable for industrial large-scale production.
Owner:CHANGZHOU SUNLIGHT PHARMA
Dabigatran derivative used as prodrug, and preparation method and application thereof
InactiveCN103420984AOrganic active ingredientsOrganic chemistryBenzimidazole derivativeActive component
The invention relates to a preparation method of a benzimidazole derivative of a general formula I, wherein R1, R2 and R3 are defined in the specification. The invention relates to a Dabigatran derivative of the general formula I and non-toxic pharmaceutically acceptable salts thereof, and a pharmaceutical composition containing these compounds as active components, and application of the compounds and the pharmaceutical composition as thrombin inhibitors.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Method for preparing dabigatran amidated impurities
InactiveCN105566296AHigh puritySimple methodOrganic chemistryChromatographic separationPropanoic acid
The invention relates to the technical field of organic synthesis, in particular to a method for preparing dabigatran amidated impurities. The method includes subjecting a raw material, 4-aminobenzamidine-carbamic acid N-hexylester, to hydrolysis reaction to obtain a hydrolysate, and subjecting the hydrolysate and 3-[(3-amino-4-methyl amino benzoyl) pyridine-2-amino] ethyl propionate to condensation reaction under the action of a catalyst to obtain the dabigatran amidated impurities. The method has the advantages that final products, namely the dabigatran amidated impurities, can be obtained by the two-step method, the method is simple and easy to operate, high-purity composites can be obtained without chromatographic separation, and high-purity impurity comparison products can be provided for quality control of dabigatran drugs.
Owner:BENGBU BBCA MEDICINE SCI DEV
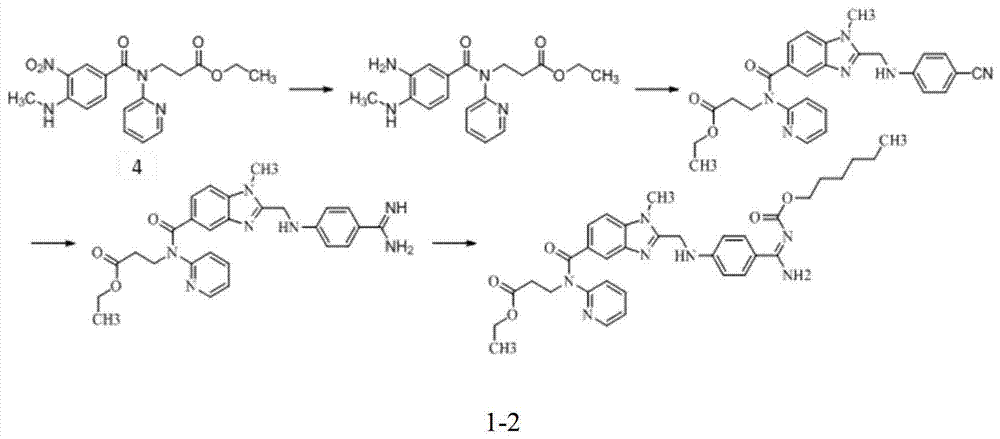
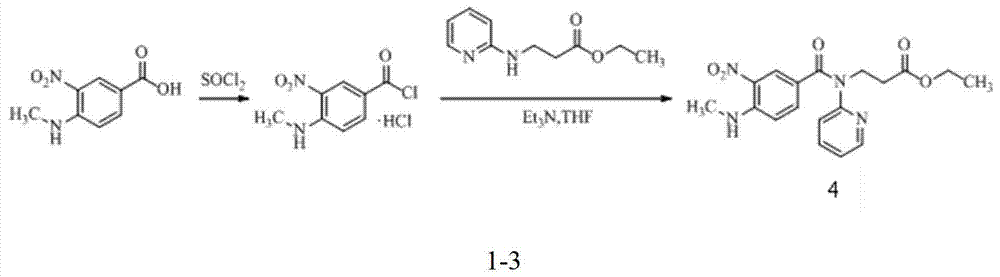
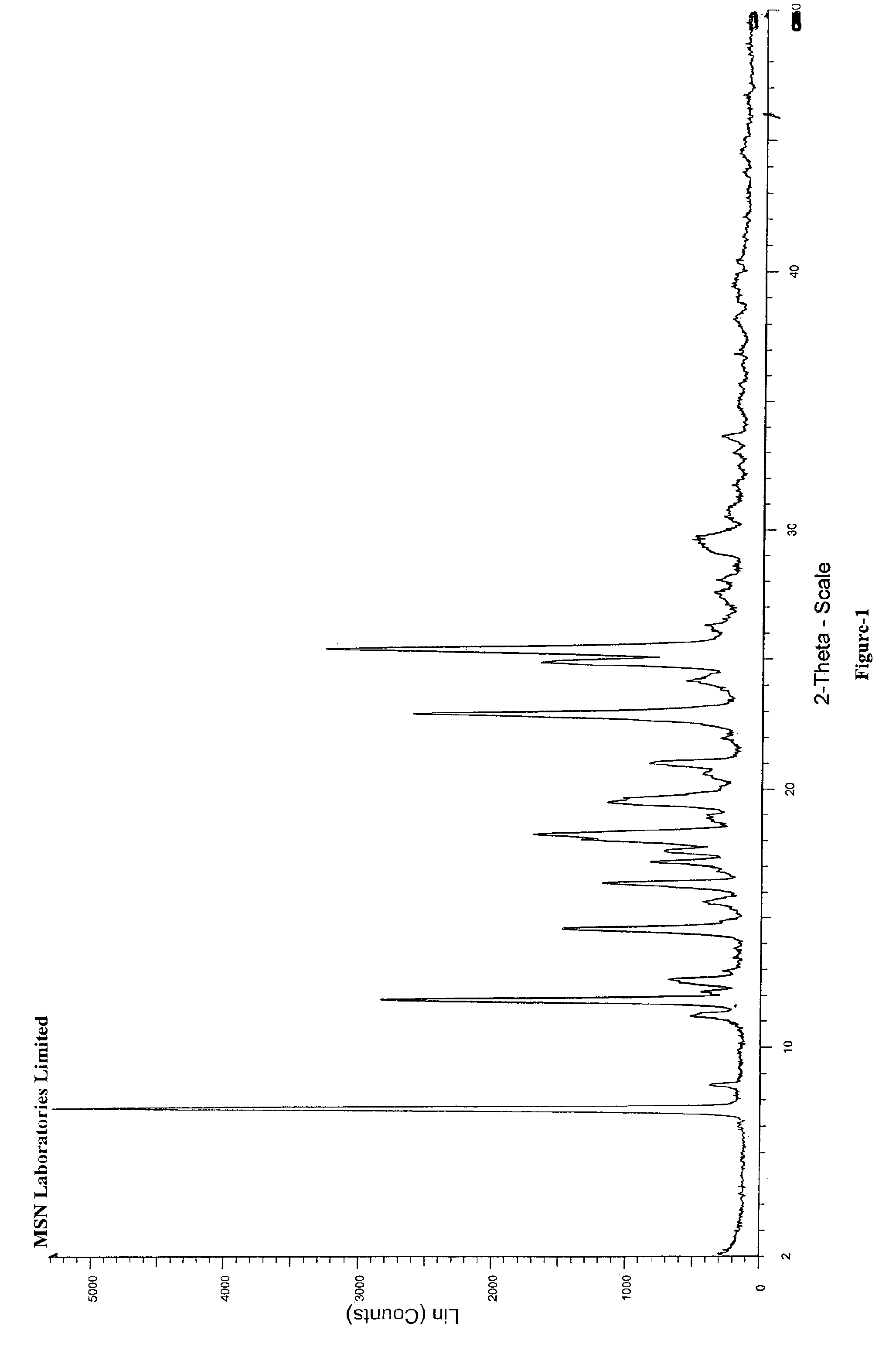
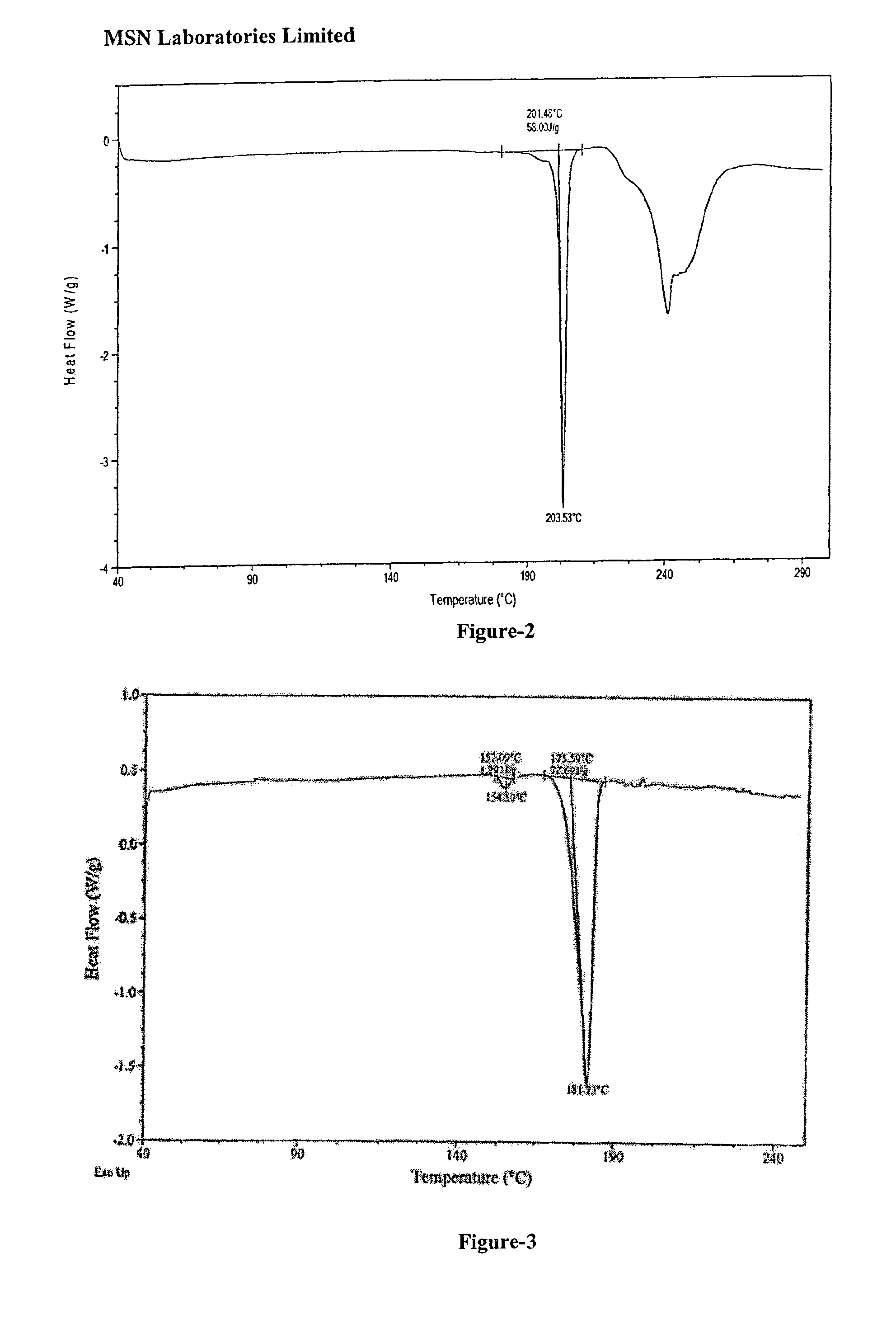
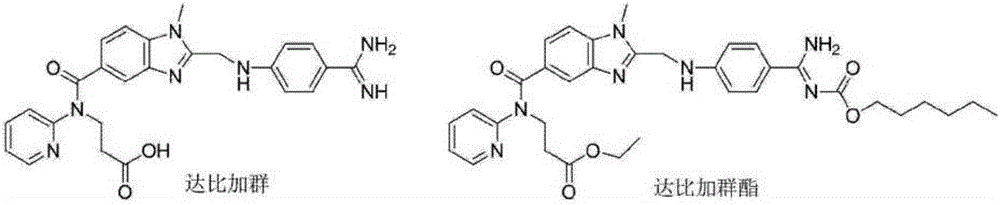
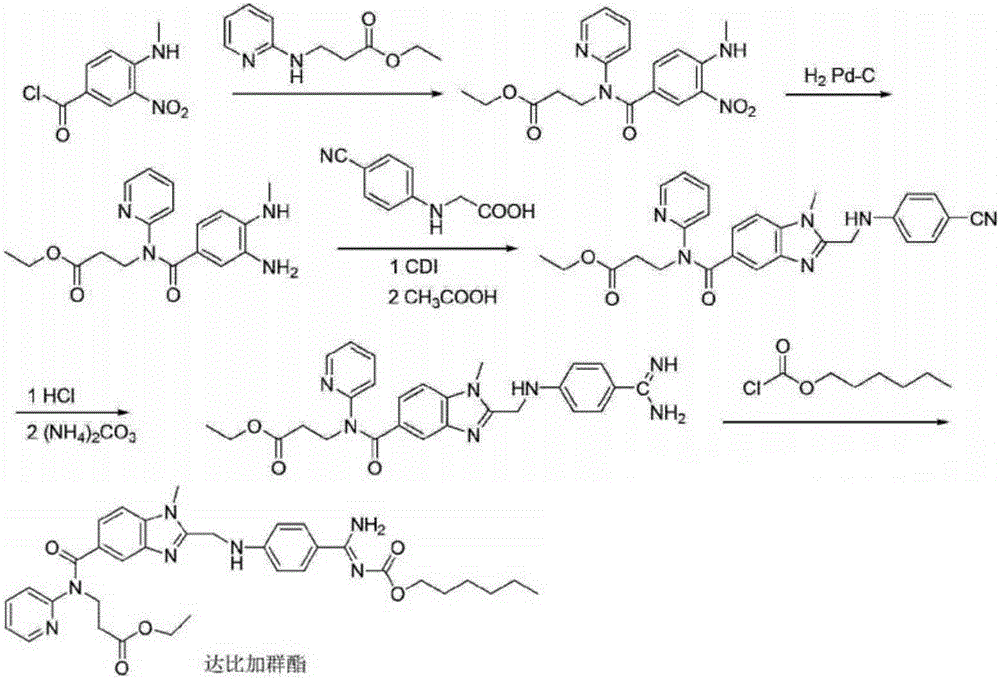
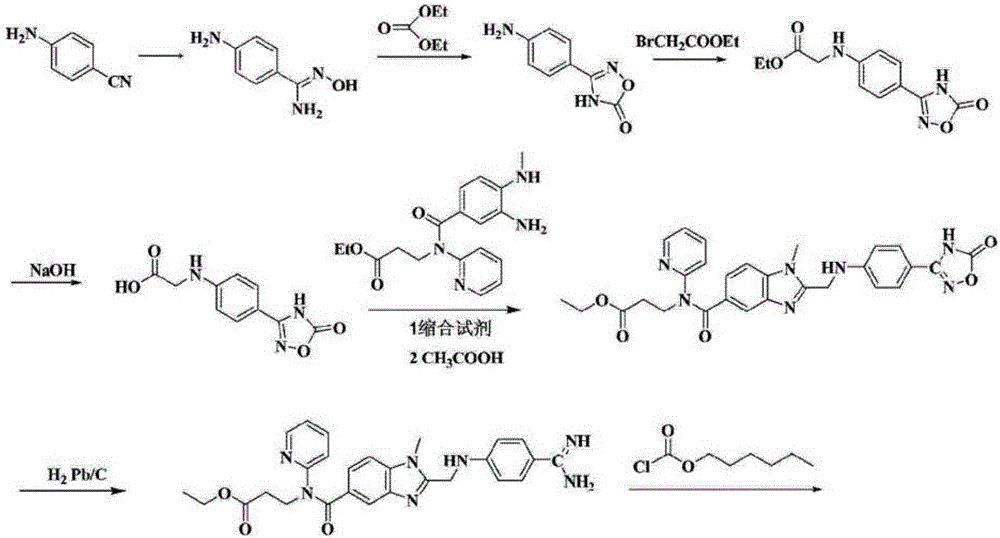
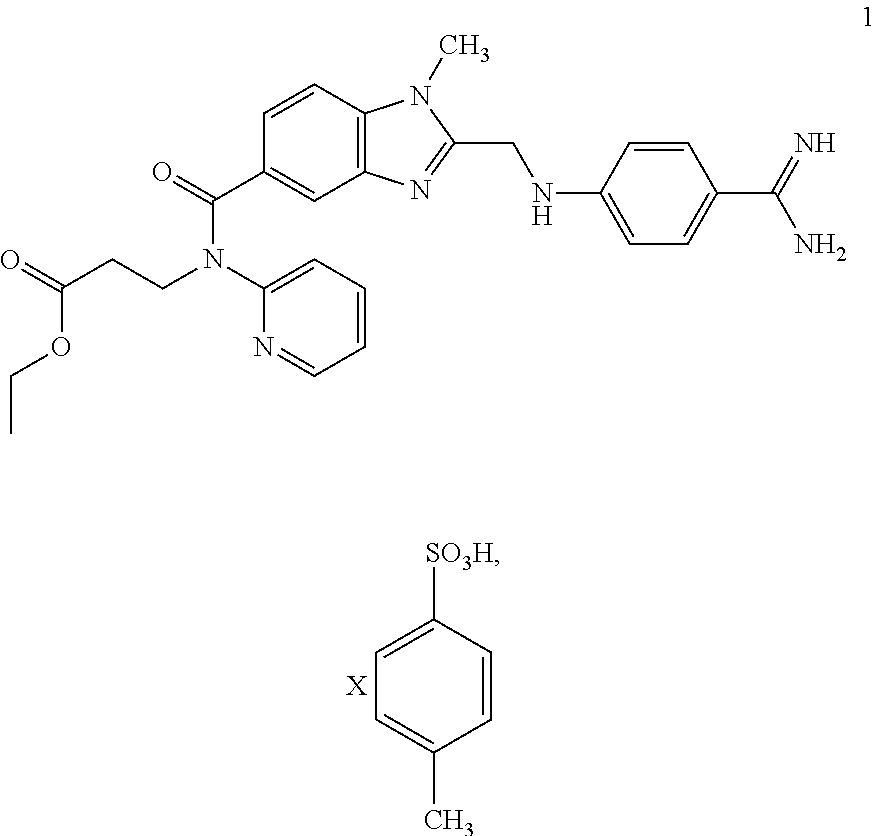
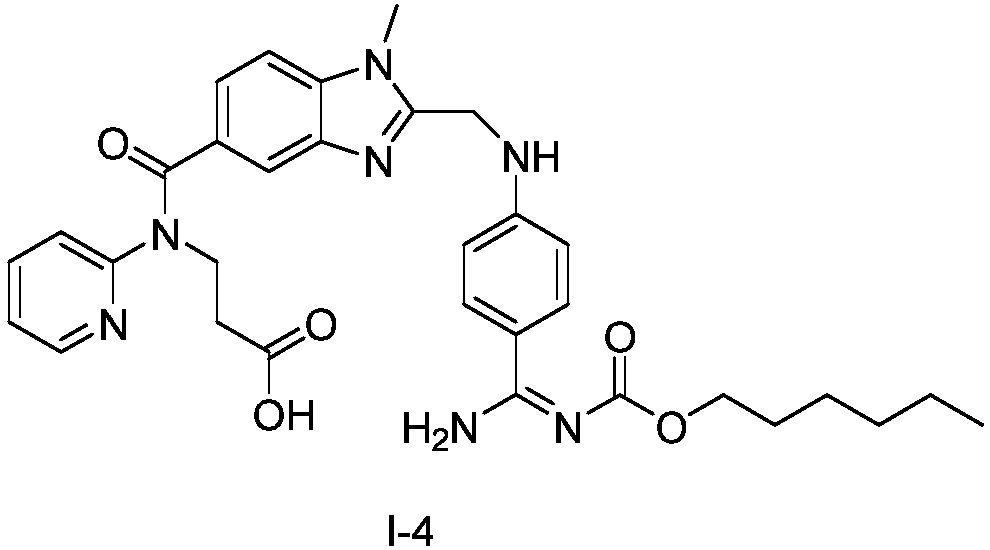
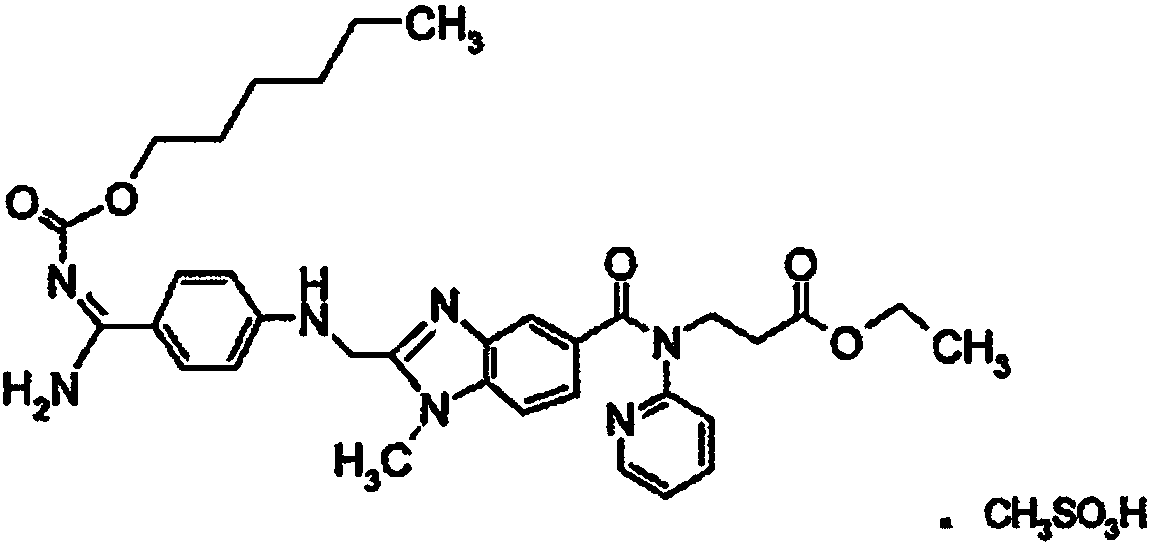
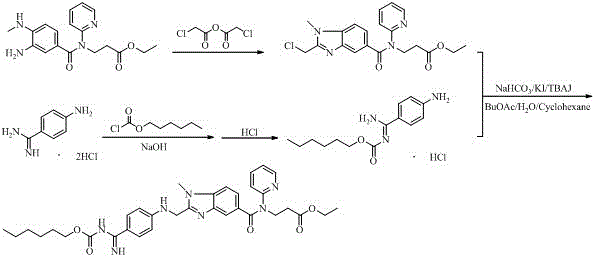
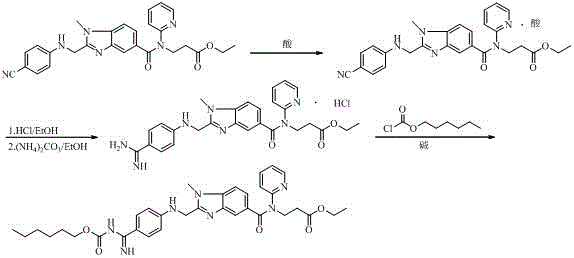
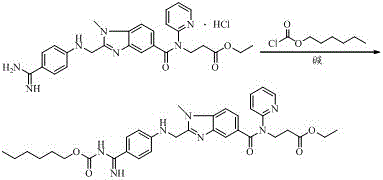
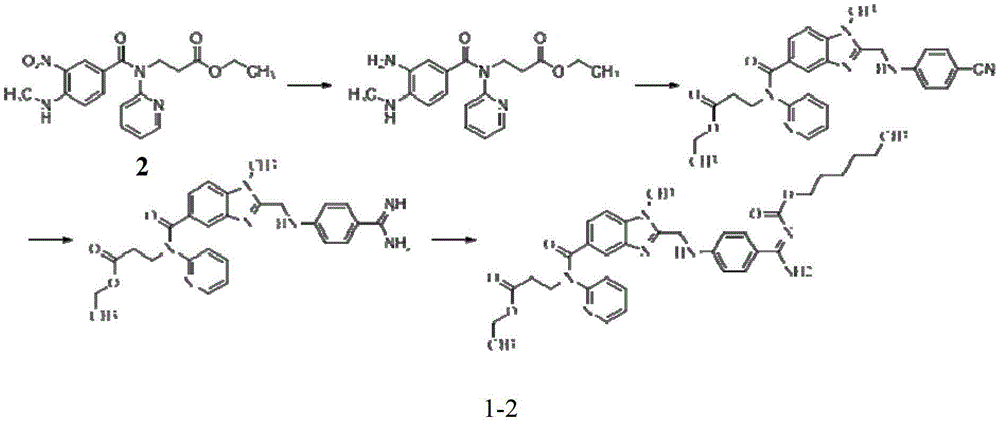
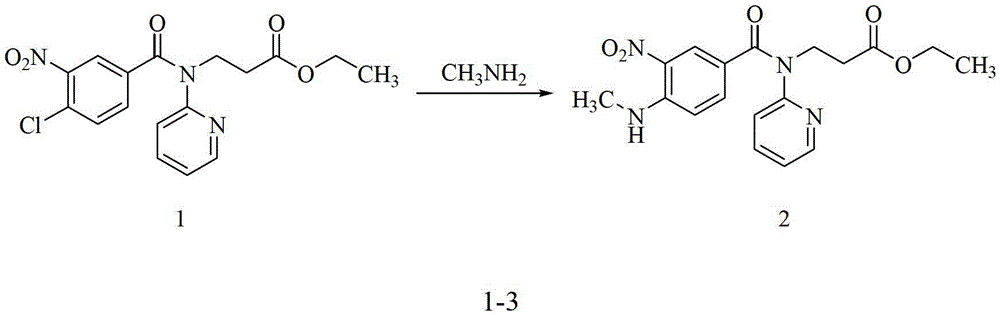
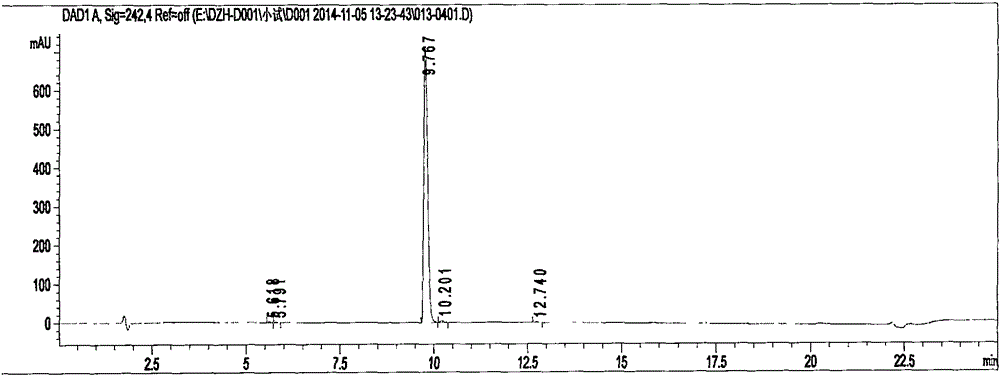
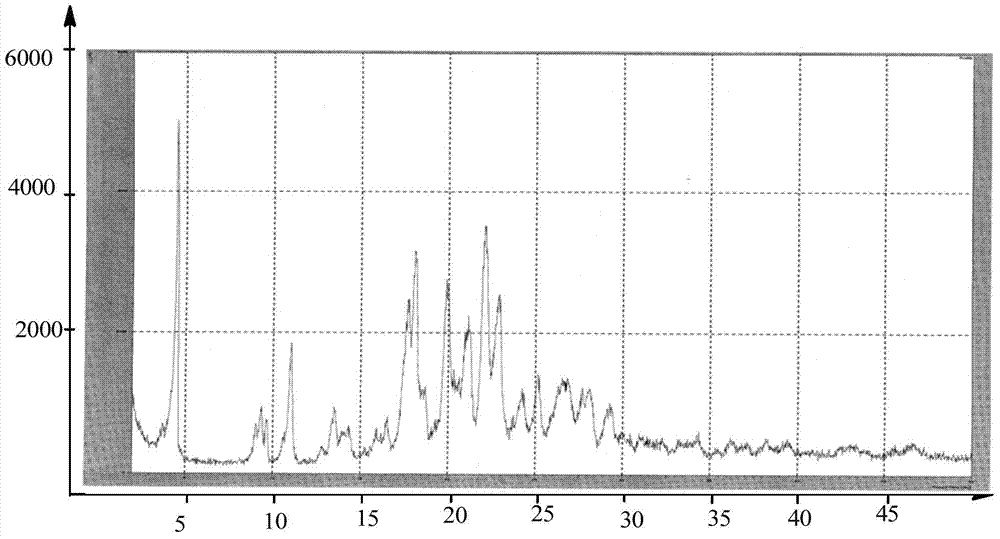
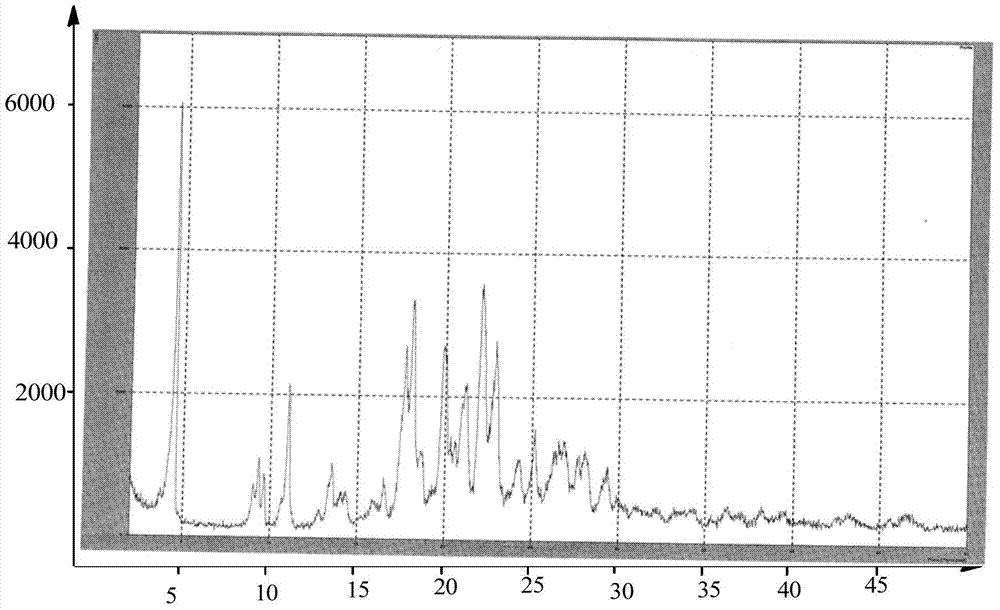
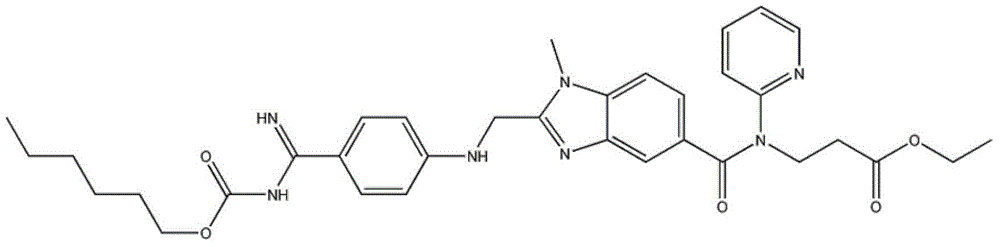
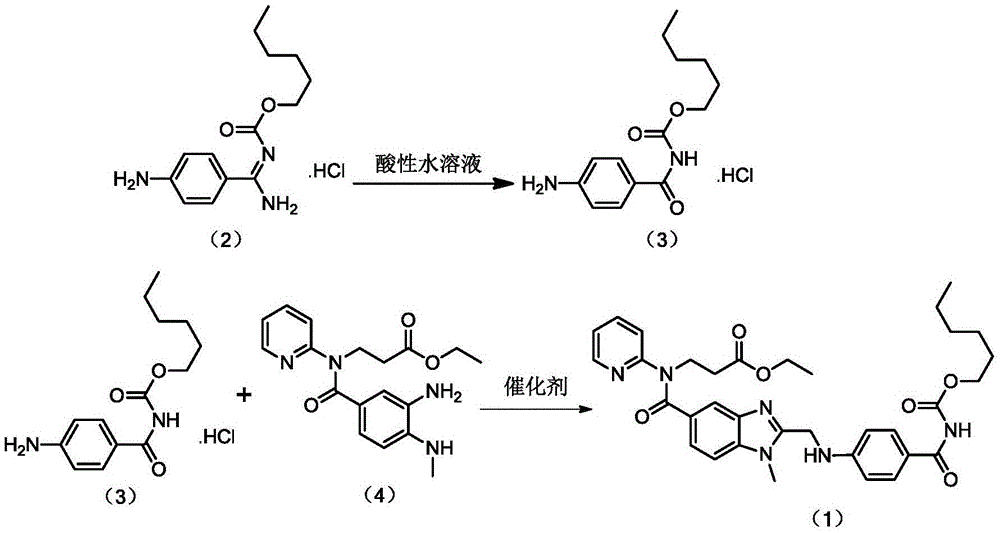
Preparation methods of 3-[N-(2-pyridyl)-3-amino-4-methylamino benzamido]-ethyl propionate
InactiveCN103539730AReduce processing operationsReduce manufacturing costOrganic chemistryEthyl propionateEthyl ester
The invention discloses two methods of preparing a dabigatran etexilate intermediate 3-[N-(2-pyridyl)-3-amino-4-methylamino benzamido]-ethyl propionate (I).
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
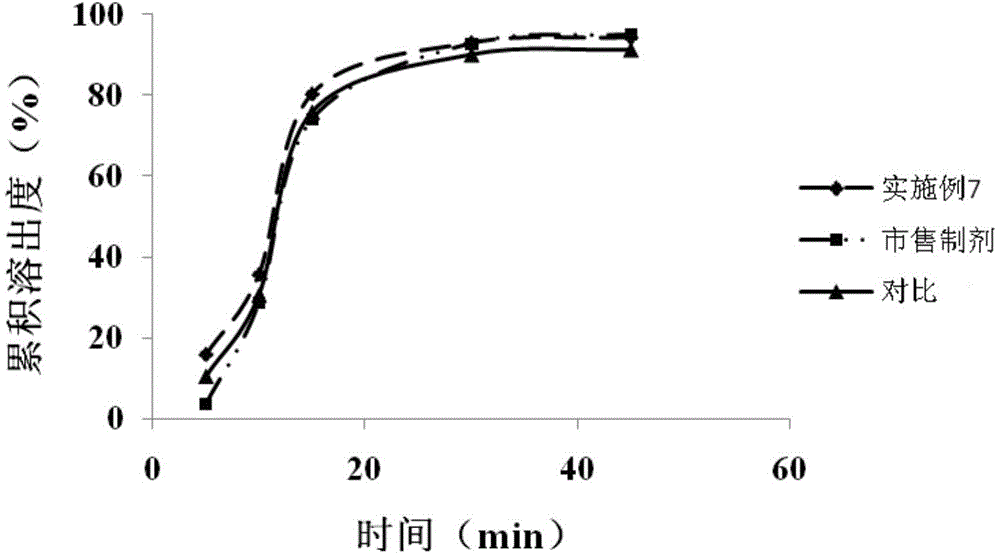
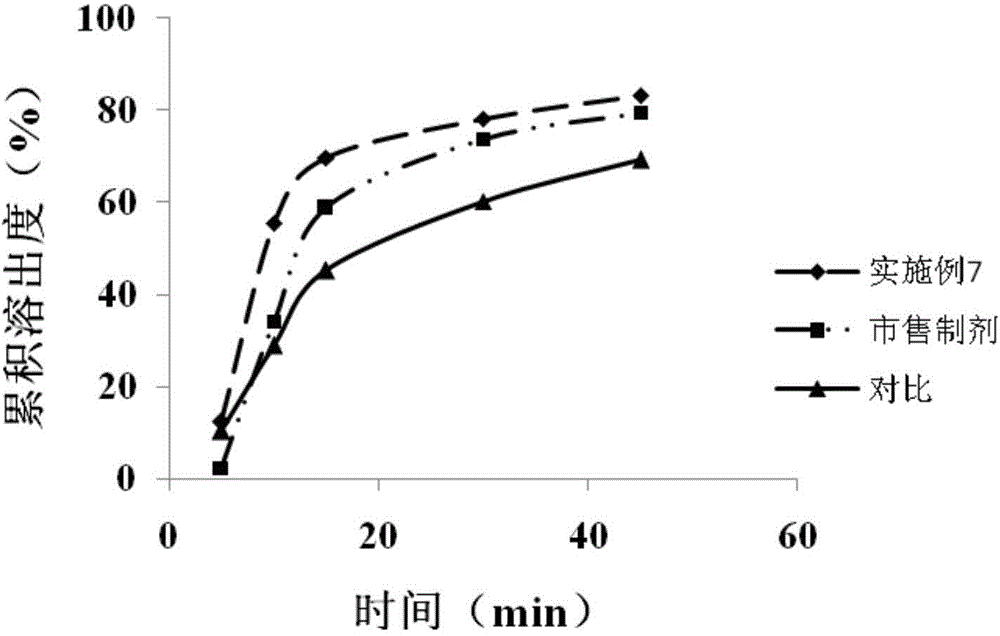
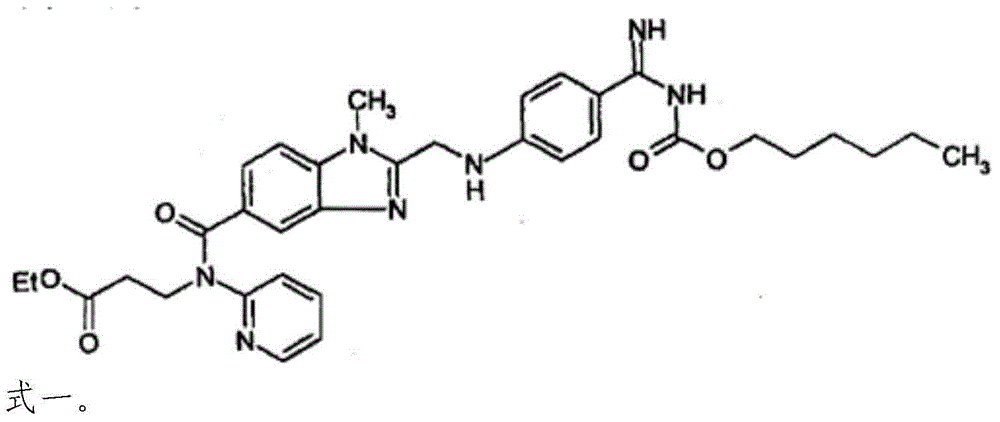
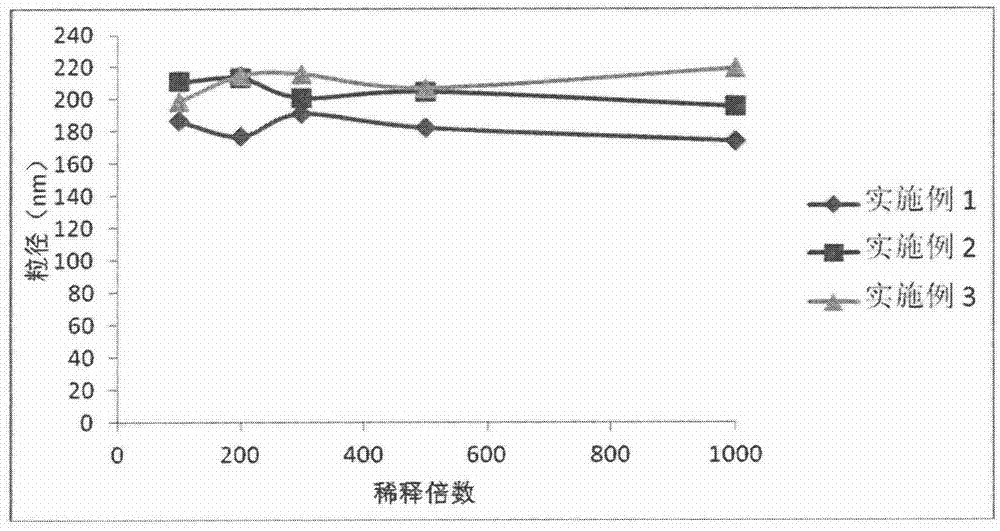











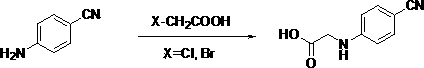

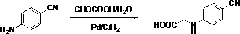












![Preparation methods of 3-[N-(2-pyridyl)-3-amino-4-methylamino benzamido]-ethyl propionate Preparation methods of 3-[N-(2-pyridyl)-3-amino-4-methylamino benzamido]-ethyl propionate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/90b29b4e-c1af-4d5d-b5ea-a6293cb5ddc1/BSA00000748925600011.PNG)
![Preparation methods of 3-[N-(2-pyridyl)-3-amino-4-methylamino benzamido]-ethyl propionate Preparation methods of 3-[N-(2-pyridyl)-3-amino-4-methylamino benzamido]-ethyl propionate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/90b29b4e-c1af-4d5d-b5ea-a6293cb5ddc1/BSA00000748925600012.PNG)
![Preparation methods of 3-[N-(2-pyridyl)-3-amino-4-methylamino benzamido]-ethyl propionate Preparation methods of 3-[N-(2-pyridyl)-3-amino-4-methylamino benzamido]-ethyl propionate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/90b29b4e-c1af-4d5d-b5ea-a6293cb5ddc1/BSA00000748925600021.PNG)