Method for preparing dabigatran amidated impurities
A technology of dabigatran etexilamide and dabi, which is applied in the field of preparing dabigatran etexilate amidation impurities, can solve the problems of complex operation and no impurity synthesis method, and achieve the effect of simple method and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
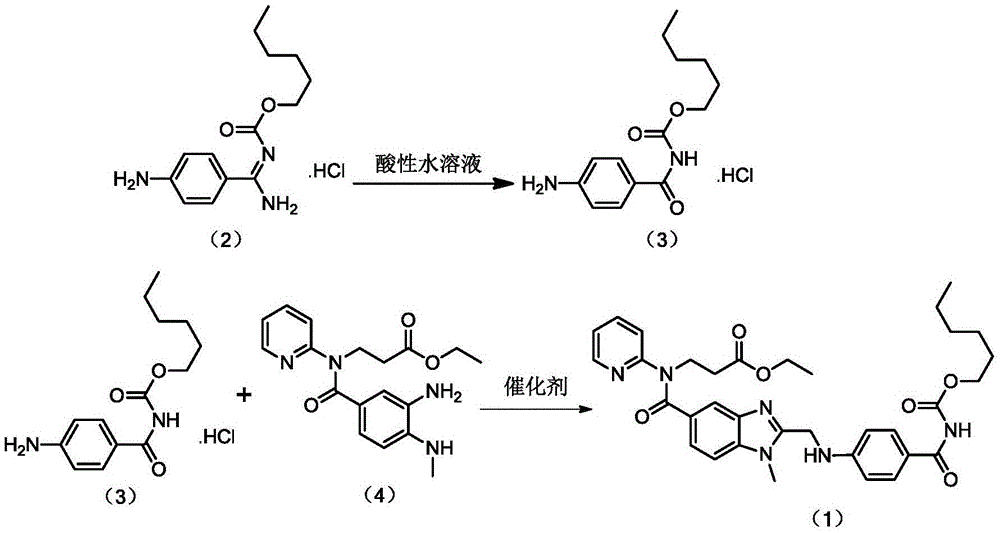
[0024] Embodiment 1 prepares compound (3)
[0025] Add 10g (33.36mmol) of compound (2) and 400g 0.1mol / L hydrochloric acid to a 1000mL reaction flask, heat to 60°C with stirring, keep at 60°C for 5h after dissolution, cool down and stir for crystallization. Suction filtration, washing with 20 mL of cold water, and vacuum drying at 45° C. for 4 hours gave 7.6 g of a yellow solid, yield 75.92%, HPLC: 98.72%.
Embodiment 2
[0026] Embodiment 2 prepares compound (3)
[0027] Add 10g (33.36mmol) of compound (2) and 400g of 0.1mol / L sulfuric acid to a 1000mL reaction flask, heat to 60°C under stirring, keep at 60°C for 5h after dissolution, cool down and stir for crystallization. Suction filtration, washing with 20 mL of cold water, and vacuum drying at 45°C for 4 hours gave 7.4 g of a yellow solid, yield 73.92%, HPLC: 98.54%.
Embodiment 3
[0028] Embodiment 3 prepares compound (3)
[0029] Add 10g (33.36mmol) of compound (2) and 300g 0.1mol / L hydrochloric acid to a 1000mL reaction flask, heat to 50°C with stirring, keep at 50°C for 5h after dissolution, cool down and stir for crystallization. Suction filtration, washing with 20 mL of cold water, and vacuum drying at 45°C for 4 hours gave 6.9 g of a yellow solid, yield 68.93%, HPLC: 97.36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com