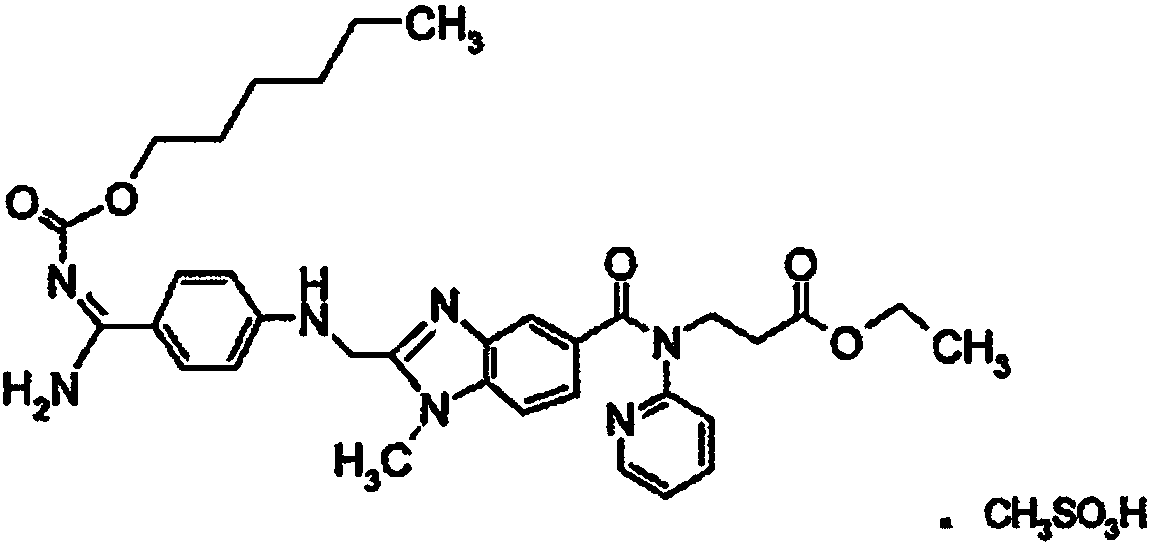
Oral drug composition of dabigatran etexilate and preparation method thereof
A composition and drug technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of difficult quality control of multi-component pellets, many process steps, and high production cost, and achieves simplified human processing and simplified process. , the effect of reducing the influence of excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
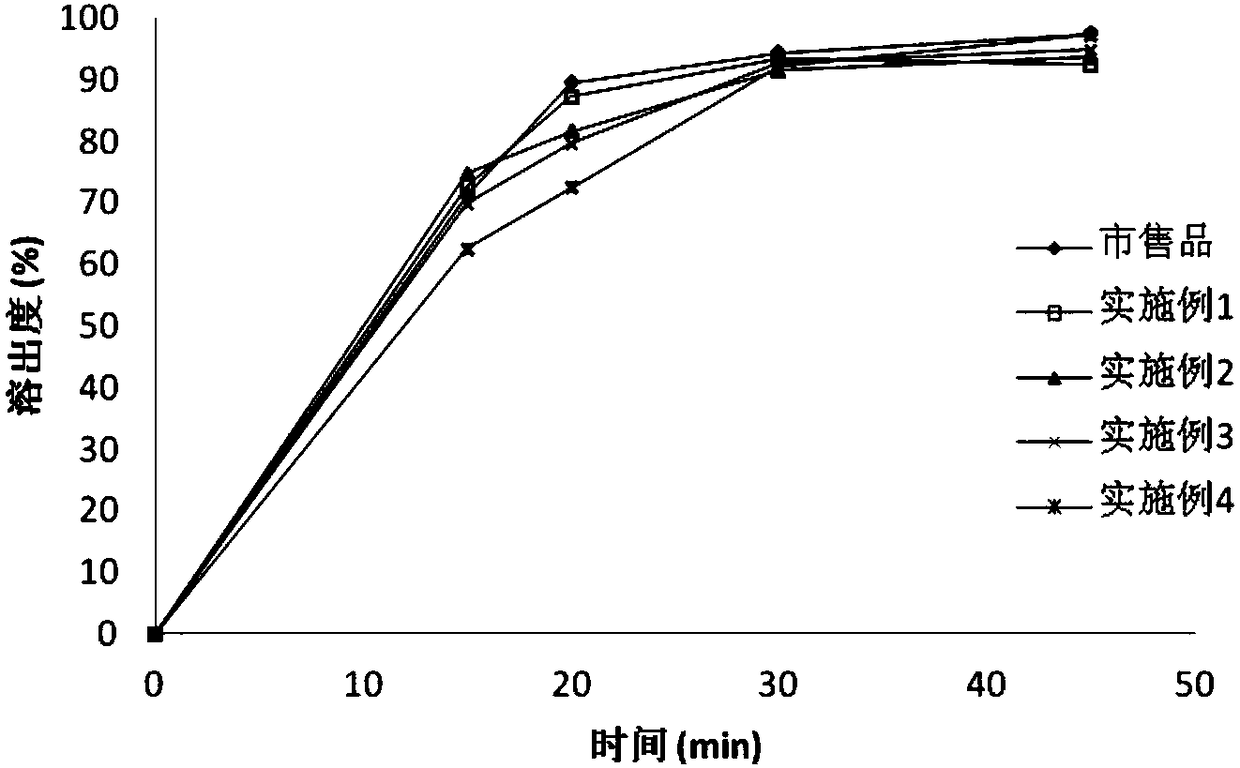
Image
Examples
Embodiment 1
[0040]
[0041] a) Preparation of drug-containing granules
[0042] composition:
[0043]
[0044]Add the prescribed amount of active substances, hydroxypropyl cellulose, and low-substituted hydroxypropyl cellulose into the mixing granulator, and mix for 5 minutes. After mixing, continue stirring, add the prescribed amount of isopropanol, and stir for 2 minutes to obtain The granules are added to the fluidized bed and dried until the water content is below 0.7%. Pass through a 30-mesh sieve or granulate with a granulator.
[0045] Add colloidal silicon dioxide and mix well.
[0046] b) Isolation of tartaric acid crystals
[0047] composition:
[0048] 129.9 parts by weight of tartaric acid
[0049] Gelatin Empty Capsule No. 4 Capsule
[0050] Fill the prescribed amount of tartrate crystals into a size 4 capsule.
[0051] c) Capsule filling
[0052] Pack the drug-containing granules and tartaric acid-containing gelatin capsules into No. 1 hypromellose hollow capsu...
Embodiment 2
[0054]
[0055] a) Preparation of drug-containing granules
[0056] composition:
[0057]
[0058] Add the prescribed amount of active substances, hydroxypropyl cellulose, and low-substituted hydroxypropyl cellulose into the mixing granulator, and mix for 5 minutes. After mixing, continue stirring, add the prescribed amount of isopropanol, and stir for 2 minutes to obtain The granules are added to the fluidized bed and dried until the water content is below 0.7%. Pass through a 30-mesh sieve or granulate with a granulator.
[0059] Add colloidal silicon dioxide and mix well.
[0060] b) Isolation of tartaric acid crystals
[0061] composition:
[0062] 110 parts by weight of tartaric acid
[0063] Gelatin empty capsule No. 5 capsule
[0064] Fill the prescribed amount of tartaric acid crystals into a size 5 capsule.
[0065] c) Capsule filling
[0066] Pack the drug-containing granules and tartaric acid-containing gelatin capsules into No. 1 hypromellose hollow c...
Embodiment 3
[0068]
[0069] a) Preparation of drug-containing granules
[0070] composition:
[0071]
[0072] Add the prescribed amount of active substances, hydroxypropyl cellulose, and low-substituted hydroxypropyl cellulose into the hopper mixer, and mix for 10 minutes at 15 rpm. After mixing, add them to the dry granulator for granulation. The pore size of the granulation screen is selected. 2.0mm, and then use a granulator with a pore size of 1.2mm for granulation.
[0073] Add colloidal silicon dioxide and mix well.
[0074] b) Isolation of citric acid crystals
[0075] composition:
[0076] 110 parts by weight of tartaric acid
[0077] Gelatin empty capsule No. 5 capsule
[0078] Fill the prescribed amount of citric acid crystals into a size 5 capsule.
[0079] c) Capsule filling
[0080] Pack the drug-containing granules and the gelatin capsule containing citric acid into No. 1 hypromellose hollow capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com