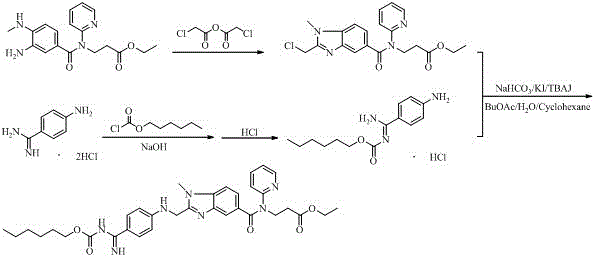
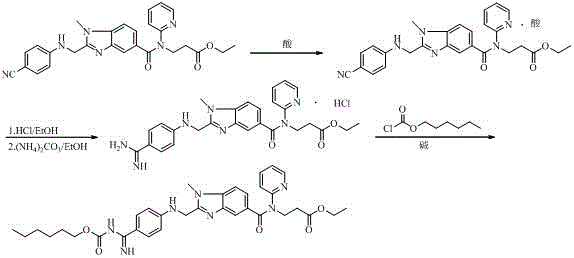
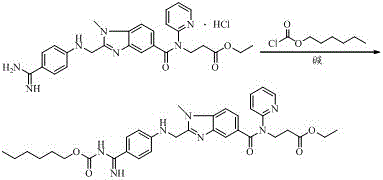
Industrial preparation method of dabigatran
A technology of dabigatran etexilate and compounds, which is applied in the field of industrial preparation of dabigatran etexilate, can solve the problem of difficulty in realizing the industrial scale preparation of dabigatran etexilate and intermediates, the absence of established and perfect quality standards for raw materials and intermediates, Unable to guarantee the stability of the final product quality and other issues, to achieve the effect of mild conditions, less pollution and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] A kind of industrial preparation method of dabigatran etexilate, concrete steps are:
[0069] (1), 3-(3-((2-(4-cyanophenyl)amino)acetamido)-4-(methylamino)-N-(pyridin-2-yl)benzamide) ethyl propionate Preparation of ester (4)
[0070] Pump 200kg of chloroform into the 500L reaction kettle, open the feeding port, and put in 11.3kg of (4-cyanoanilino) acetic acid and 12.2kg of CDI in sequence; ~5 o C, and heat preservation reaction for 3 hours; open the feeding port, slowly drop in 20kg of compound 3 under a slight negative pressure; after the addition is completed, after the refrigerant in the jacket is discharged, pour in tap water and gradually warm up to room temperature, stir and react for 24 hours, then sample HPLC detection, Compound 3 ≤ 1%, compound 4 ≥ 95.0%, stop the reaction; pump in 50kg of water, stir, inject hot water into the jacket for circulation, and recover chloroform (applicable) by vacuum distillation to dryness, and quickly stir the raffinate to dis...
Embodiment 2
[0078] A kind of industrial preparation method of dabigatran etexilate, concrete steps are:
[0079] (1), 3-(3-((2-(4-cyanophenyl)amino)acetamido)-4-(methylamino)-N-(pyridin-2-yl)benzamide) ethyl propionate Preparation of ester (4)
[0080] Pump 200kg of 1,4-dioxane into the 500L reactor, open the feeding port, and put in 11.3kg of (4-cyanoanilino)acetic acid, 13.5kg of EDCI-HCl, and 14.3kg of DMAP in sequence; after feeding, start stirring , the jacket is put into the coolant, and the temperature in the kettle drops to 0-5 o C, and heat preservation reaction for 3 hours; open the feeding port, slowly drop in 20kg of compound 3 under a slight negative pressure; after the addition is completed, after the refrigerant in the jacket is discharged, pour in tap water and gradually warm up to room temperature, stir and react for 24 hours, then sample HPLC detection, Compound 3 ≤ 1%, compound 4 ≥ 95.0%, stop the reaction; pump in 50kg of water, stir, inject hot water into the jacket, ...
Embodiment 3
[0088] A kind of industrial preparation method of dabigatran etexilate, concrete steps are:
[0089] (1) Ethyl 3-(3-((2-(4-cyanophenyl)amino)acetamido)-4-(methylamino)-N-(pyridin-2-yl)benzamide)propionate (4) Preparation
[0090] Pump 1000kg of dichloromethane into the 3000L reactor, open the feeding port, and put in 62kg of (4-cyanoanilino) acetic acid and 62kg of CDI in turn; after feeding, start stirring, put the jacket into the cooling liquid, and the temperature in the kettle will drop to 0 ~5 o C, and heat preservation reaction for 6 hours; open the feeding port, slowly drop into 100kg of compound 3 under a slight negative pressure; after the addition is completed, after the refrigerant in the jacket is discharged, tap water is gradually warmed up to room temperature and stirred for 24 hours, then the sample is detected by HPLC. Compound 3 ≤ 1%, compound 4 ≥ 95.0%, stop the reaction; pump in 250kg of water, stir, inject hot water into the jacket for circulation, distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com