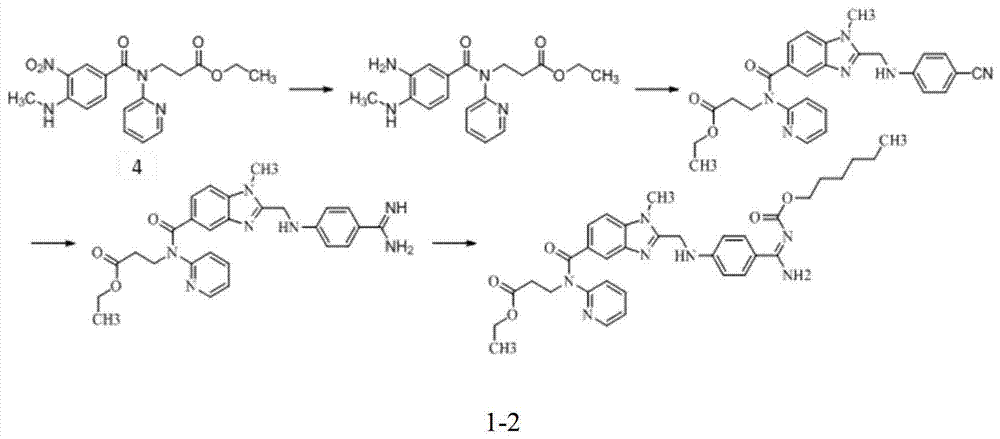
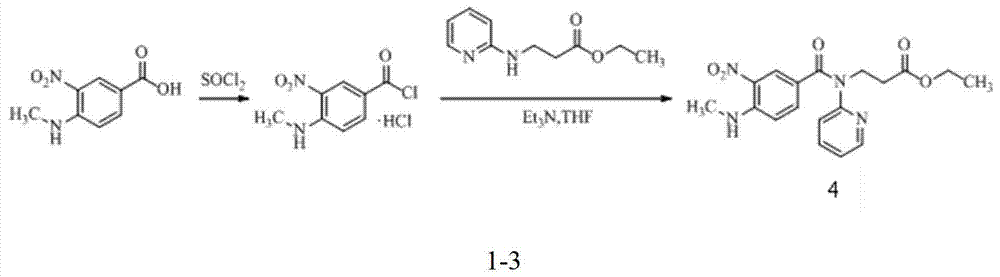
Preparation method for dabigatran etexilate intermediate and intermediate compound
A technology of dabigatran etexilate and compounds, applied in the field of drug synthesis, can solve the problems of high preparation difficulty, many side reactions, and difficult purification, and achieve the effects of simple preparation method, easy purification, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of 3-nitro-4-chlorobenzoyl chloride (compound 2)
[0056]
[0057] Add 3-nitro-4-chlorobenzoic acid (10.0g, 49.6mmol), N,N-dimethylformamide (0.3ml, 3.6mmol) and toluene (50ml) into the reaction kettle, heat to 70°C, add Thionyl chloride (4.3ml, 59.5mmol), continue to heat and reflux for 30min, distill off thionyl chloride and solvent under reduced pressure to obtain a light yellow oil (compound 2), add dichloromethane (60ml) to dissolve, and directly use Next reaction.
Embodiment 2
[0059] Synthesis of ethyl 3-[(3-nitro-4-chlorobenzoyl)(pyridin-2-yl)amino]propanoate (compound 3)
[0060]
[0061] Add ethyl 3-(pyridin-2-yl-amino)propionate (9.6g, 49.6mmol), triethylamine (13.8ml, 99.2mmol) and dichloromethane (20ml) into the reaction kettle, dropwise add the The dichloromethane solution of the obtained compound 2. After the addition, stir at room temperature for 1h. The reaction solution was washed with water, dried over anhydrous sodium sulfate and filtered. The filtrate was evaporated to dryness, and the remaining solid was purified by column chromatography to obtain compound 3 (16.3 g, yield 87.0%). mp63~65℃; ESI-MS(m / z): 378[M+H] + , 400[M+Na] + ; 1 H-NMR (DMSO-d 6 , 400MHz) δ: 1.19 (t, 3H), 2.70 (t, 2H), 3.95 (q, 2H), 4.20 (t, 2H), 7.22 (d, 2H), 7.25 (t, 1H), 7.44 (dd , 1H), 7.66 (d, 1H), 7.75 (m, 1H), 7.92 (d, 1H), 8.35 (dd, 1H). HPLC purity 98.5%.
Embodiment 3
[0063] Synthesis of ethyl 3-[(4-methylamino-3-nitrobenzoyl)(pyridin-2-yl)amino]propanoate (compound 4)
[0064]
[0065] Compound 3 (16.3g, 47.15mmol) and ethanol (60.0ml) were added to the reaction kettle, the temperature was raised to 40°C, 27.0%-32.0% methylamine in ethanol solution (16.3ml) was slowly added dropwise, and stirred for 2h. The reaction solution was evaporated to dryness, and the remaining solid was purified by column chromatography to obtain compound 4 (13.6 g, yield 84.6%). mp86~88℃; 1 H-NMR (DMSO-d 6 , 400MHz) δ: 1.11(t, 3H), 2.66(t, 2H), 2.91(t, 3H), 3.96(q, 2H), 4.18(t, 2H), 6.83(d, 1H), 7.08(d , 1H), 7.21 (m, 1H), 7.32 (dd, 1H), 7.69 (m, 1H), 7.93 (d, 1H), 8.36 (d, 1H), 8.43 (dd, 1H). HPLC purity 97.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com