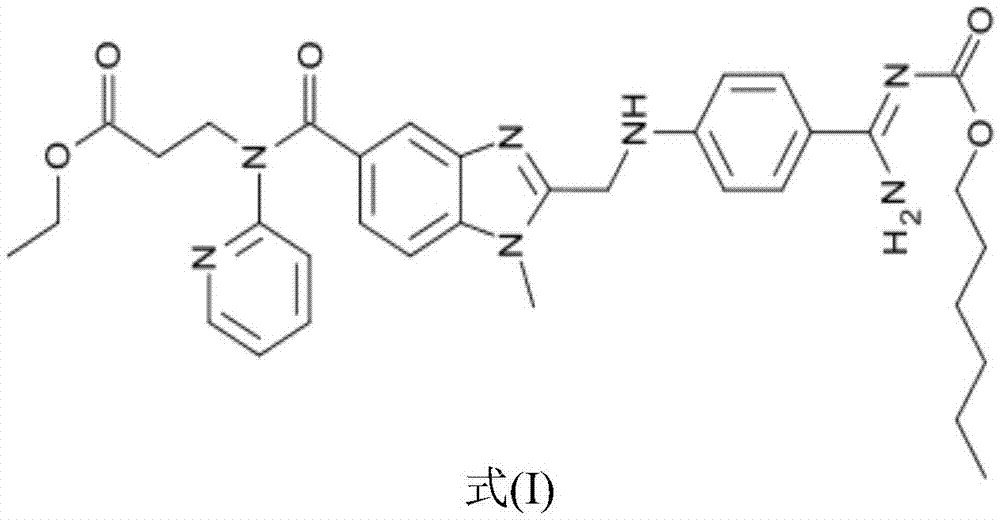
Pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the field of pharmaceutical compositions, can solve the problems of complex process operation, high production cost, uneven drug application, etc., and achieve the effect of simple process operation, low production cost and improved storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
[0039] recipe one
[0040]
[0041] Control group formula:
[0042] Dabigatran etexilate mesylate 173.0mg
[0043] Tartaric acid 177mg
[0044] Total 350mg
[0045] Dabigatran etexilate mesylate, tartaric acid, and sodium carbonate are all commercially available raw materials.
[0046] According to formula 1, dissolve a certain amount of sodium carbonate in 20mL of water, add tartaric acid powder particles with a particle size of 0.4-1.5mm to the aqueous solution of sodium carbonate under stirring, and mix the tartaric acid powder particles Put into drying equipment such as a drying box or a fluidized bed to dry to obtain modified tartaric acid powder particles.
[0047] The modified tartaric acid powder granules and the dabigatran etexilate mesylate in the formula one are mixed uniformly and packed into hydroxypropyl methylcellulose (HPMC) capsules, and the capsules are packed into high-density polyethylene (HDPE) bottles , HDPE built-in desiccant, after storage for 1...
no. 2 example
[0053] recipe two
[0054]
[0055] According to formula 2, dissolve a certain amount of sodium carbonate in 20mL of water, add tartaric acid powder particles with a particle size of 0.4-1.5mm to the aqueous solution of sodium carbonate under stirring, and mix the tartaric acid powder particles Put into drying equipment such as a drying box or a fluidized bed to dry to obtain modified tartaric acid powder particles.
[0056] Then dabigatran etexilate mesylate is mixed with the above-mentioned modified tartaric acid powder particles, further mixed with other pharmaceutical excipients, and packed into HPMC capsules to make a pharmaceutical preparation product.
no. 3 example
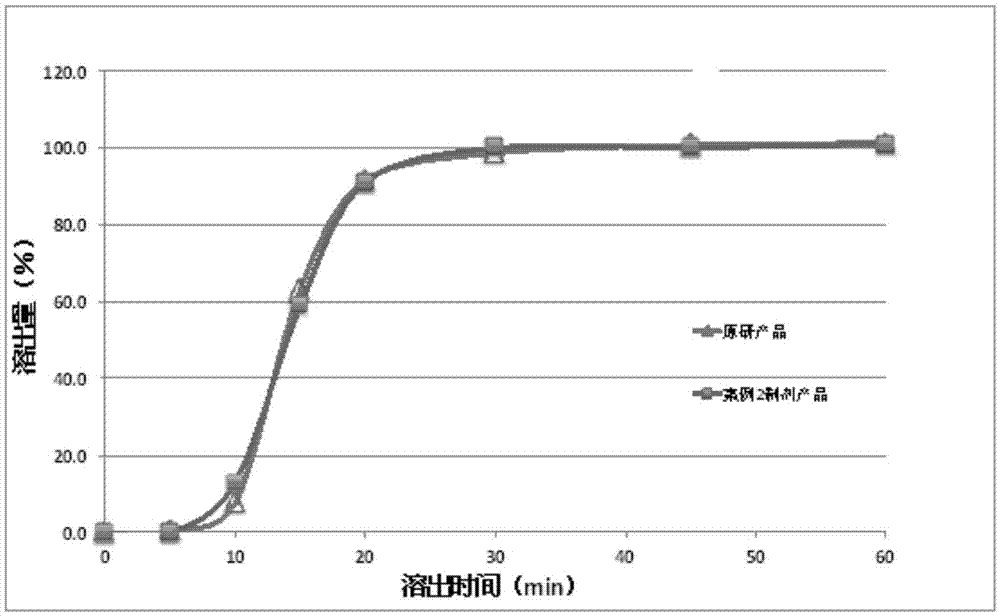
[0058] The dabigatran etexilate mesylate is mixed with modified tartaric acid powder particles, further mixed with other pharmaceutical excipients, and packed into HPMC capsules to make a pharmaceutical preparation product. figure 2 Dissolution results of capsules prepared with different ratios of dabigatran etexilate mesylate API and modified tartaric acid. Dissolution uses the basket method specified in the Pharmacopoeia at 100 rpm, the solvent is water, and the temperature is 37°C.
[0059] The results show that the dissolution of the dabigatran etexilate mesylate preparation product after adding the modified tartaric acid is significantly improved than the dissolution of the preparation without adding the modified tartaric acid.
[0060] To sum up, the pharmaceutical composition of the present invention has excellent storage stability, obtains high solubility, and provides expected bioavailability. At the same time, the method for modifying the surface of the pharmaceuti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com