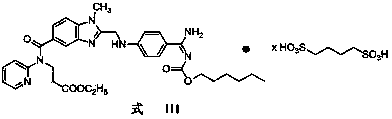
Butanedisulfonic acid dabigatran etexilate and preparation method and application thereof
A technology of dabigatran etexilate and butanedisulfonic acid, applied in the field of medicine, can solve the problems of not clearly providing examples and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
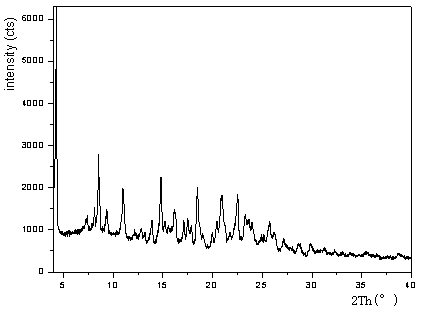
[0090] The preparation of dabigatran etexilate and crystal form A shown in embodiment 1 formula IV
[0091] Add 2.0 g (3.2 mmol) of dabigatran etexilate into a reaction flask containing 20 ml of acetone, control the temperature at about 30°C, and stir to dissolve it. Dissolve 0.35g (1.6mmol) of 1,4-butanedisulfonic acid in 10ml of acetone, add dropwise to the acetone solution of dabigatran etexilate, continue to stir and crystallize after the dropwise addition, filter, and filter the cake with acetone 10ml was washed and dried under vacuum at 40-45°C to obtain the title product.
[0092] With Bruke AV-II 300MHz nuclear magnetic resonance instrument, deuterated dimethyl sulfoxide is used as test solvent, tetramethylsilane is used as internal standard, and the obtained title product has been carried out at room temperature by proton nuclear magnetic resonance spectrum measurement, the result is:
[0093] 1 H-NMR (300MHz, DMSO-d 6 ,δ / ppm): 11.87(s,1H),10.68(s,1H),10.04(s,1H),8...
Embodiment 2
[0099] The preparation of dabigatran etexilate and its crystal form A shown in embodiment 2 formula IV
[0100] Add 2.0 g (3.2 mmol) of dabigatran etexilate into a reaction flask containing 20 ml of acetone, control the temperature at about 30°C, and stir to dissolve it. Dissolve 0.28g (1.3mmol) of 1,4-butanedisulfonic acid in 5ml of acetone, add dropwise to the acetone solution of dabigatran etexilate, continue to stir and crystallize after the dropwise addition, filter, and use acetone for the filter cake 10ml was washed, and vacuum-dried at 45-50°C to obtain the title product.
Embodiment 3
[0101] The preparation of dabigatran etexilate and its crystal form B shown in embodiment 3 formula V
[0102] Add 3.14g (5.0mmol) of dabigatran etexilate into a reaction bottle containing 30ml of tetrahydrofuran, control the temperature at about 30°C, and stir to dissolve it. Disperse 0.79g (3.6mmol) of 1,4-butanedisulfonic acid in 20ml of tetrahydrofuran, add dropwise to the above-mentioned tetrahydrofuran solution of dabigatran etexilate, continue to stir and crystallize after the dropwise addition, filter, and filter the cake with tetrahydrofuran 10ml was washed and dried under vacuum at 40-45°C to obtain the title product.
[0103] With Bruke AV-II 300MHz nuclear magnetic resonance instrument, deuterated dimethyl sulfoxide is used as test solvent, tetramethylsilane is used as internal standard, and the obtained title product has been carried out at room temperature by proton nuclear magnetic resonance spectrum measurement, the result is:
[0104] 1 H-NMR (300MHz, DMSO-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com