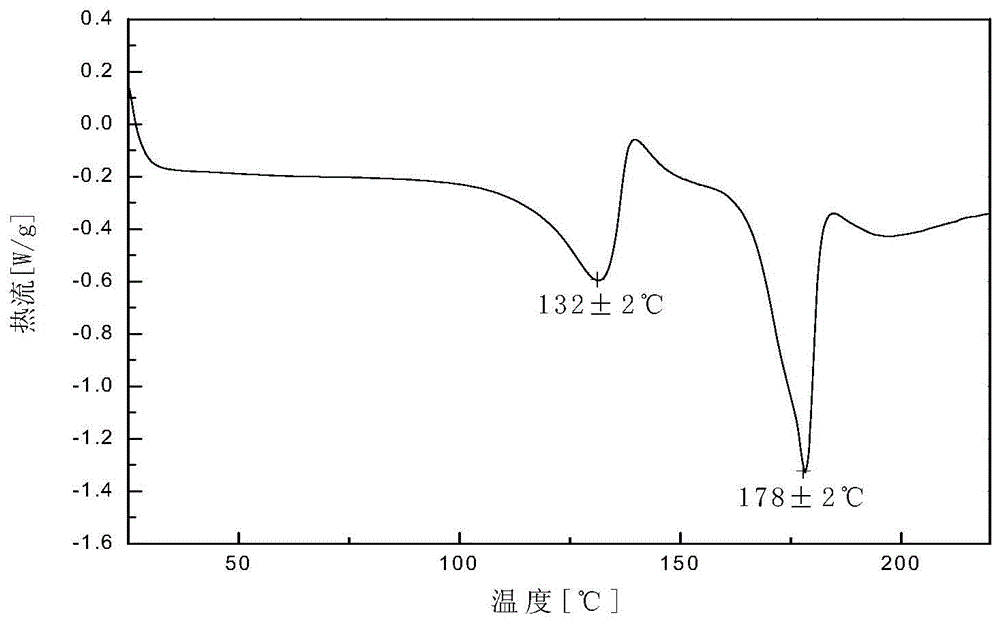
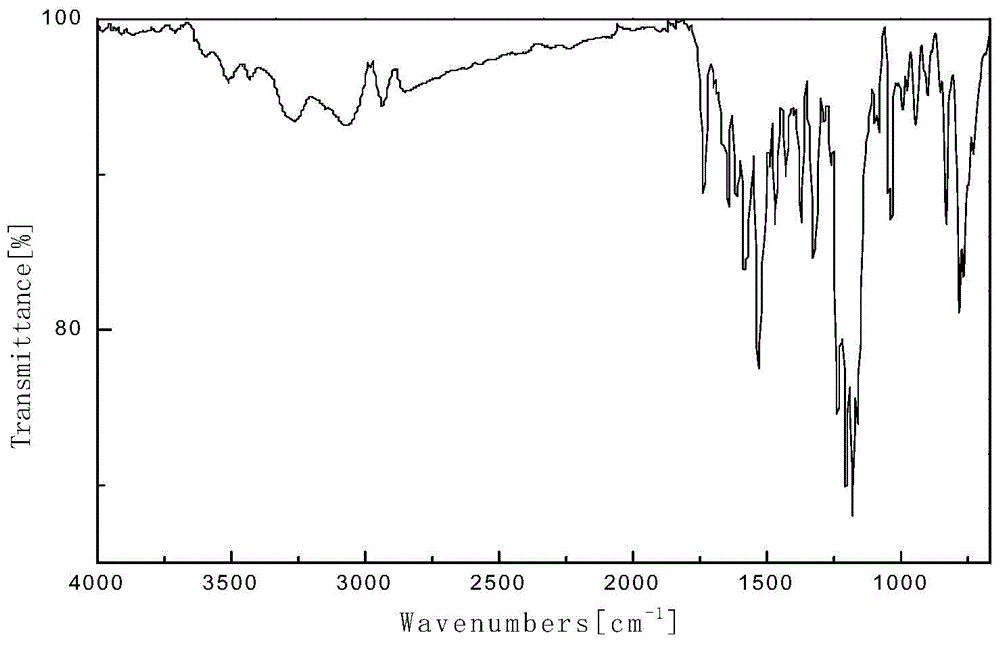
Crystal V of dabigatran etexilate mesylate and preparation method thereof
A technology of dabigatran etexilate mesylate and crystals, which is applied in the field of new dabigatran etexilate mesylate crystals and its preparation, can solve problems such as the harmful effects of drug reproducibility, and is suitable for technological production , short time-consuming, high-purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add 0.2033 g of dry dabigatran etexilate mesylate into 0.5 mL of methanol to form a suspension, stir at 15°C until completely dissolved, and then slowly cool the solution to 5°C under stirring (the solution has no crystallization phenomenon), constant temperature, 4 mL of butyl acetate was added dropwise to the solution within 10 minutes to obtain a suspension, the crystal slurry was vacuum filtered, and the product was dried to constant weight at 35°C under normal pressure to obtain dabigatran mesylate in V crystal form ester.
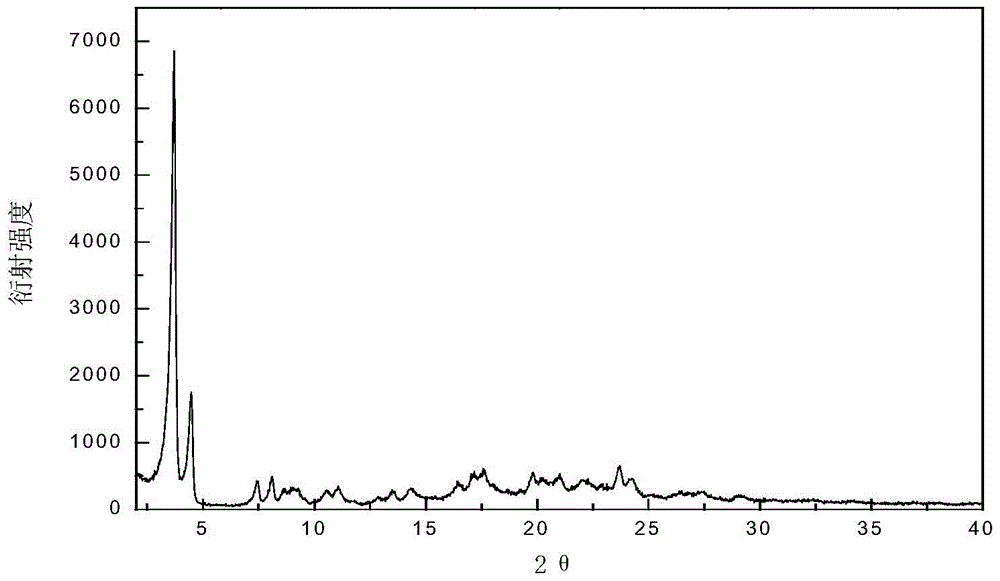
[0058] The X-ray powder diffraction pattern of the crystal obtained in this example is 3.7±0.2, 4.5±0.2, 7.4±0.2, 8.1±0.2, 8.6±0.2, 9.3±0.2, 10.5±0.2, 11.0±0.2 at the diffraction angle 2θ , 12.8±0.2, 13.5±0.2, 14.3±0.2, 16.4±0.2, 17.2±0.2, 17.6±0.2, 19.8±0.2, 20.2±0.2, 21.0±0.2, 22.0±0.2, 23.0±0.2, 23.7±0.2, 24.2 There are characteristic peaks at ±0.2, 25.2±0.2, 26.5±0.2, 27.4±0.2, and 29.0±0.2 degrees. The specific spectrum is as follows fig...
Embodiment 2
[0068] Add 0.2013g of dry dabigatran etexilate mesylate into 0.4mL ethanol to form a suspension, stir at 10°C until the solids are completely dissolved, then slowly cool the solution to 0°C under stirring (the solution has no crystallization), Constant temperature, add 4 mL of butyl acetate dropwise to the solution within 5 minutes to obtain a suspension, vacuum filter the slurry, and dry the product at 45°C under normal pressure to constant weight to obtain Dabiga methanesulfonate in V crystal form Group esters.
[0069] The crystal product obtained in this example has a purity of 99.32%, and the characterization data are the same as those in Example 1.
Embodiment 3
[0071] Add 0.1998g of dry dabigatran etexilate mesylate into 0.35mL of n-pentanol to form a suspension, stir at 15°C until the solids are all dissolved, then slowly cool the solution to 5°C under stirring (the solution has no crystallization phenomenon ), constant temperature, 3mL of butyl acetate was added dropwise to the solution within 10 minutes to obtain a suspension, the slurry was vacuum filtered, and the product was dried to constant weight at 30°C under normal pressure to obtain the V crystal form methanesulfonic acid Bigatran etexilate.
[0072] The crystal product obtained in this example has a purity of 99.41%, and the characterization data are the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com