Dabigatran etexilate mesylate oral solid preparation and preparation method thereof
A technology for dabigatran etexilate mesylate and dabigatran etexilate, which is applied in the directions of pill delivery, pharmaceutical formulation, coating, etc. The effect of reducing coating steps and simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
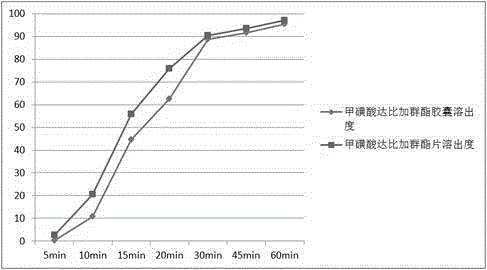
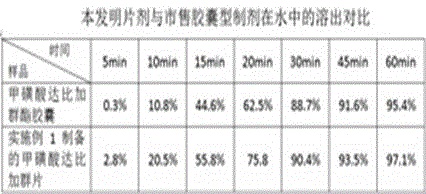
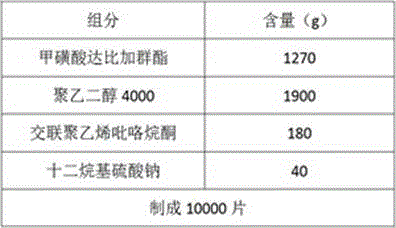
[0031] Example 1 prescription and preparation process
[0032]
[0033] Preparation:
[0034] 1) Weigh the main drug and various auxiliary materials required for the prescription, and pass the main drug through a 100-mesh sieve for use;
[0035] 2) Take a prescription amount of polyethylene glycol and heat it to 50~60℃ to make it into a molten liquid;
[0036] 3) Under slow stirring, add the prescription amount of dabigatran etexilate mesylate and 40% of the prescription amount of disintegrant in batches. After the addition is complete, stir slowly for 15~30min, and stir vigorously for 30~60min to disintegrate the drug. The solution is evenly dispersed. The speed of slow stirring is 150~300 rpm, and the speed of vigorous stirring is 5000~10000 rpm;
[0037] 4) Place the molten liquid prepared in step 3) under -20°C and rapidly cool down and solidify for 2~3 hours to obtain a solidified product;
[0038] 5) The solidified product obtained in step 4) is crushed at 5~10°C, passed through ...
Embodiment 2
[0043] Embodiment 2 prescription and preparation process
[0044] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com