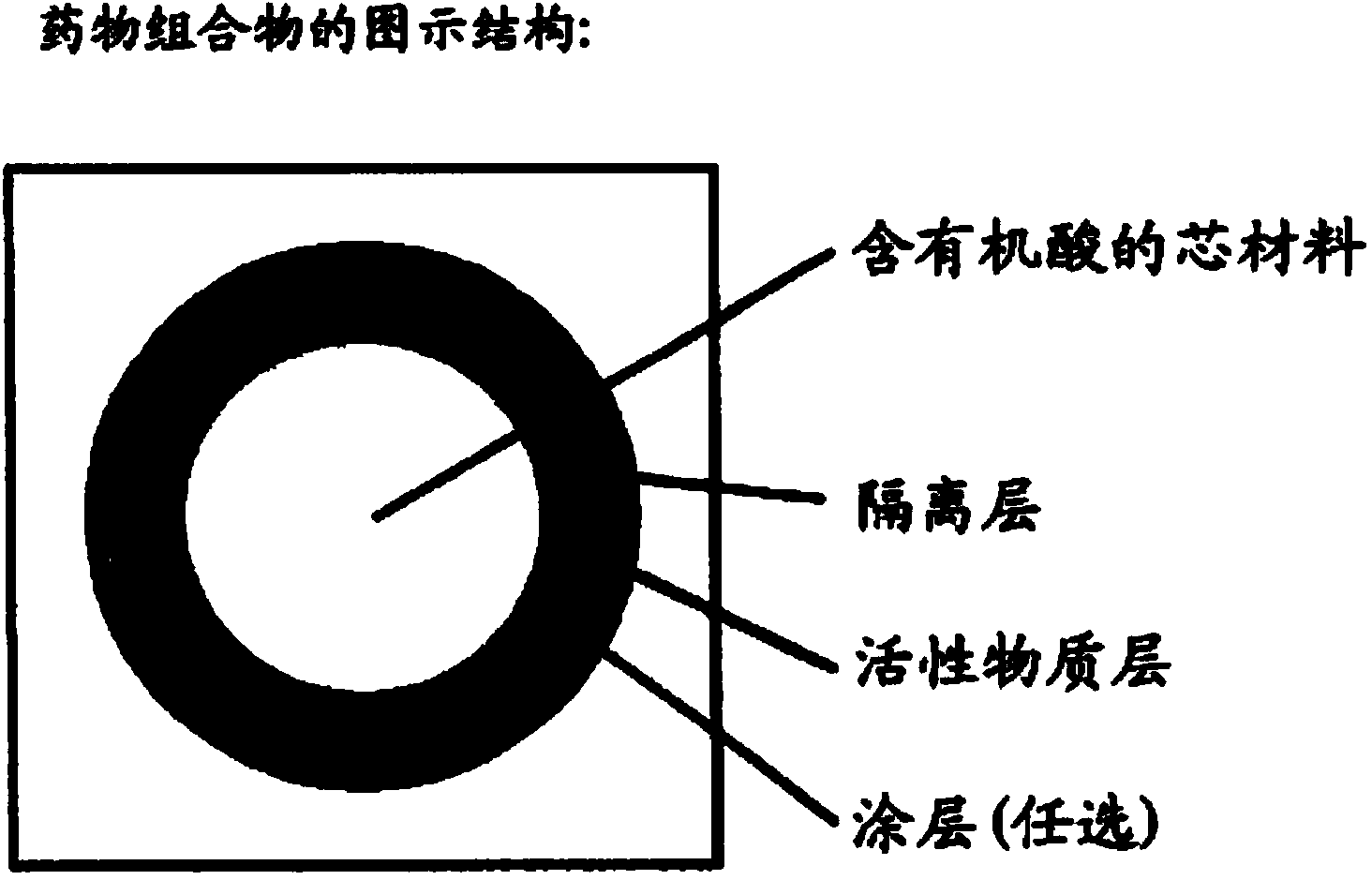
Multi-layer tablets containing dabigatran etexilate mesylate
A technology of dabigatran etexilate mesylate and dabigatran etexilate is applied in the field of multi-layer tablets to achieve the effects of prolonging storage time, solving disintegration risk and improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: a kind of monolayer tablet containing dabigatran etexilate, tablet core prescription is:
[0023]
[0024] In the table: MCC102 is microcrystalline cellulose 102, CC-Na is croscarmellose sodium, HPMCE5 is hypromellose E5, MS is magnesium stearate.
[0025] Single-layer core preparation method:
[0026] (1) Weigh the prescribed amount of tartaric acid, dabigatran etexilate mesylate, lactose, MCC102, CC-Na, HPMCE5, add the same amount through a 60-mesh sieve and mix three times, add magnesium stearate, and mix by hand for 30 seconds .
[0027] (2) A 14.3x7.5mm shallow arc die is used to press the tablet, and the control pressure is 2000lbs.
[0028] Coating prescription and process:
[0029] The isopropanol solution of hydroxypropyl cellulose (HPC) was used for coating, and the solid content was 5%. Control coating weight gain was 5%. Obtain single-layer coated tablets.
Embodiment 2
[0030] Embodiment 2: the multi-layer tablet containing dabigatran etexilate, this tablet core prescription is:
[0031]
[0032] Double-layer tablet core preparation method:
[0033] (1) Preparation of tartaric acid layer
[0034] Weigh the prescribed amount of tartaric acid, lactose, MCC102, CC-Na, HPMCE5, add the same amount through a 60-mesh sieve and mix three times, add magnesium stearate, and mix by hand for 30 seconds.
[0035] (2) Preparation of drug-containing layer
[0036] Weigh the prescribed amount of dabigatran etexilate mesylate, lactose, MCC102, HPMCE5, CC-Na, add equal amounts through a 60-mesh sieve and mix three times, add magnesium stearate, and mix by hand for 30 seconds.
[0037] (3) A 14.3x7.5mm shallow arc punching die is used to press the tablet, and the control pressure is 2000lbs.
[0038] Coating prescription and process:
[0039] The isopropanol solution of hydroxypropyl cellulose (HPC) was used for coating, and the solid content was 5%. Co...
Embodiment 3
[0041] Embodiment 3: the multi-layer tablet containing dabigatran etexilate, this tablet core prescription is:
[0042]
[0043]
[0044] Three-layer tablet core preparation method:
[0045] (1) Preparation of tartaric acid layer
[0046] Weigh the prescribed amount of tartaric acid, lactose, MCC102, CC-Na, HPMCE5, add the same amount through a 60-mesh sieve and mix three times, add magnesium stearate, and mix by hand for 30 seconds.
[0047] (2) Preparation of drug-containing layer
[0048] Weigh the prescribed amount of dabigatran etexilate mesylate, lactose, MCC102, HPMCE5, CC-Na, add equal amounts through a 60-mesh sieve and mix three times, add magnesium stearate, and mix by hand for 30 seconds.
[0049] (3) Preparation of isolation layer
[0050] Weigh the prescribed amount of HPMCE5 and MCC102 and add them through a 60-mesh sieve and mix three times, add magnesium stearate, and mix by hand for 30 minutes.
[0051] (4) A 14.3x7.5mm shallow arc punching die is use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com