Methods and compositions for reducing the risk associated with the administration of opioid analgesics in patients with diagnosed or undiagnosed respiratory illness
a technology for respiratory illness and opioids, applied in the direction of drug compositions, biocide, heterocyclic compound active ingredients, etc., can solve the problems of increased carbon dioxide retention, respiratory depression, and morphine-related opioids, and achieve the effect of minimizing the risk of developing sleep
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
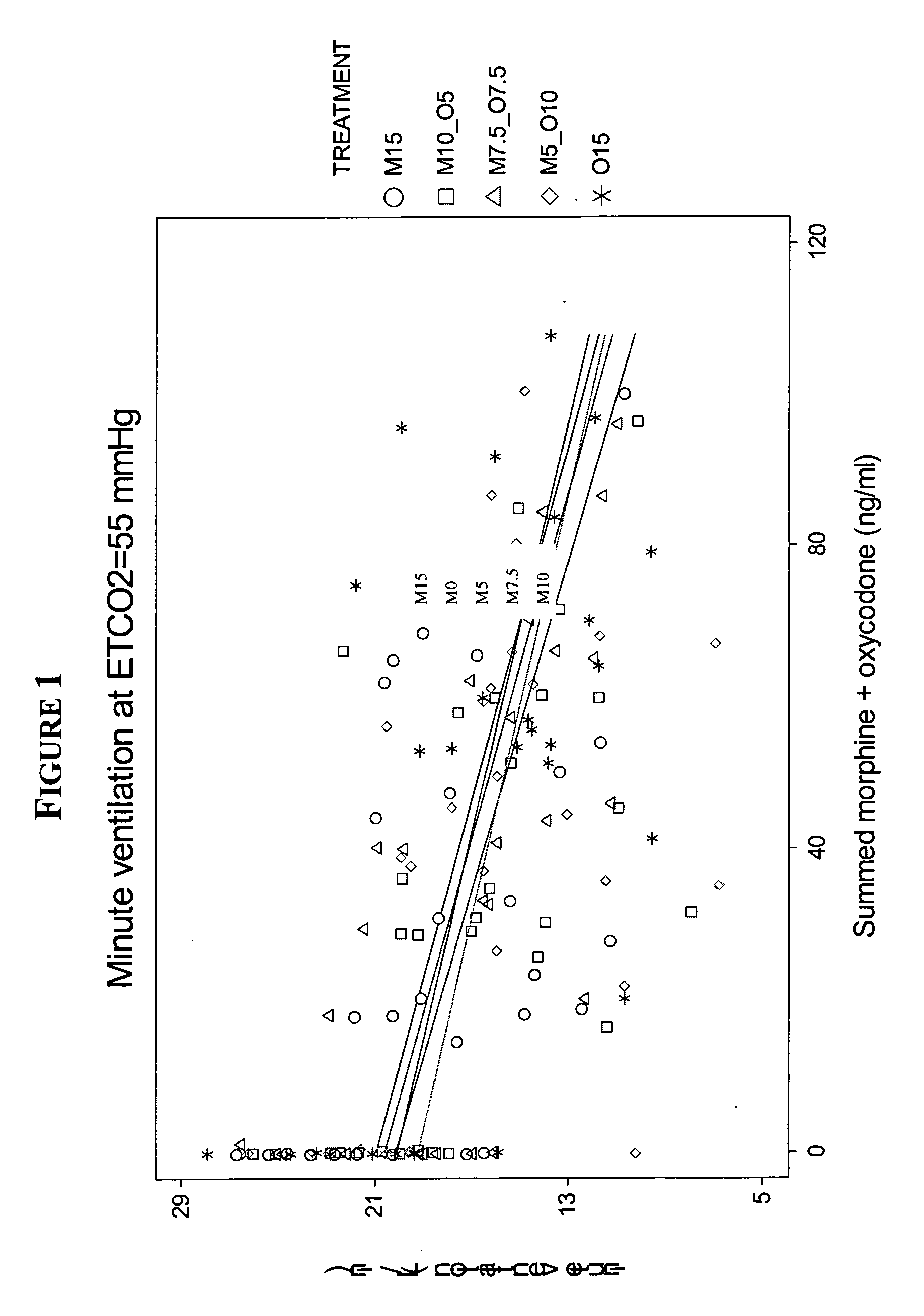
[0060] Effects of Combined Morphine / Oxycodone Formulations on Respiration
[0061] Objective The main objective was to compare the ventilatory responses to hypoxia and hypercarbia following one hour intravenous infusions of (1) morphine 15 mg, or (2) oxycodone 15 mg, or (3) morphine 10 mg+oxycodone 5 mg, or (4) oxycodone 10 mg+morphine 5 mg, or (5) morphine 7.5 mg+oxycodone 7.5 mg. Each infusion was administered over 1 h and was designated by its respective composition as M15, M10_O5, M7.5_O7.5, M5_O10 or O15.
[0062] Design A triple blind placebo-controlled, 5-period, randomised crossover study in 10 males was conducted. On each of the 5 study days for each of the 10 subjects, there was a baseline period, followed by either a 1 h drug or “placebo” i.v. infusion, followed by 1 h “placebo” or drug infusion, followed by a 1 h “washout”. Serial measures of drug effect were accompanied by serial peripheral venous blood sampling. The hypoxemic and hypercapnic ventilatory responses were dete...
example 2
[0066] Analgesia in Patients using Sub-analgesic Combinations of Morphine & Oxycodone in a Ratio of 1:0.66 by Weight
[0067] Objective: to determine the dose of a taste masked oral syrup of morphine and oxycodone in a ratio of 1:0.66 by weight compared to a taste masked syrup containing morphine alone.
[0068] Design A triple-blind (patient, investigator and analyst) randomized, controlled, two-period cross-over study was designed to assess the effectiveness of morphine compared to an oxycodone and morphine mixture. The study was carried out in 21 patients with chronic non-cancer pain. The morphine was formulated to a solution concentration of 5 mg / mL and the combination formulation to a concentration of morphine 1.5 mg / mL and oxycodone 1 mg / mL. The solution strengths were determined so that the expected total dose per day in mL would be similar between the two treatments thereby retaining blinding. Each opioid solution was administered every 4 hours with the 10 pm and 2 am doses bein...
example 3
[0071] Analgesia in Patients using Sub-analgesic Combinations of Morphine & Oxycodeone in a Ratio of 1:2.0 by Weight
[0072] Objective: to determine the dose of a taste masked oral syrup of morphine and oxycodeone in a ratio of 1:2.0 by weight compared to a taste masked syrup containing morphine alone.
[0073] Design & Analysis: a similar design and analysis to that used in Example 1 was followed.
[0074] Results: Analgesic Effects As with Example 2, no significant differences in VAS scores were noted between any of the treatment groups. The effective dose of the 1:2.0 combination versus morphine required to provide analgesia is shown in Table 2. The results show a weight of the combined product to weight or morphine to be 46%. The results was statistically significant (P<0.002).
TABLE 2Comparison of doses required for stabilized pain control.Dose, mgTreatmentPatients(by weight)Morphine2268Combination of2155morphine tooxycodone1:2.0(Morphine +46%Oxycodonedose) / Morphinedose
[0075] In s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com