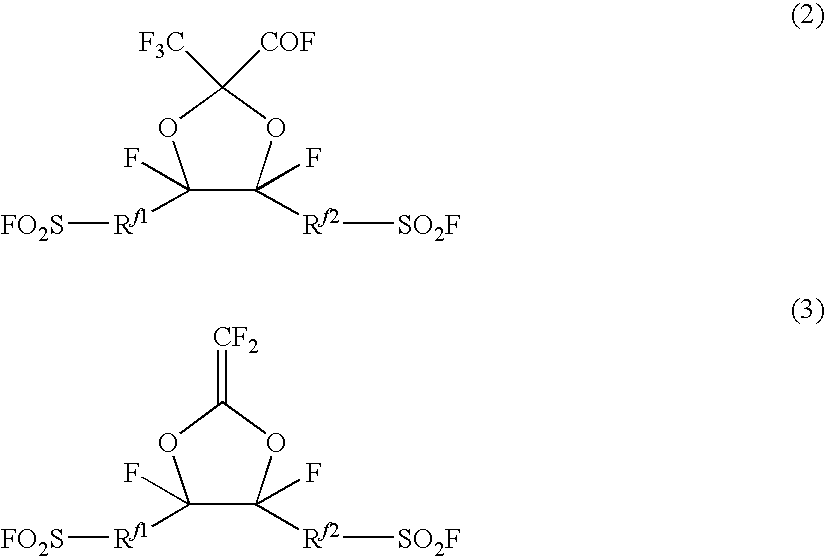
Fluorosulfonyl group-containing monomer and its polymer, and sulfonic acid group-containing polymer
a technology of fluorosulfonyl group and polymer, which is applied in the field of fluorosulfonyl group-containing monomer and its polymer, and sulfonyl group-containing polymer, can solve the problems of low molecular weight of copolymer, insufficient mechanical strength and durability of low molecular weight, and inapplicability, etc., to achieve high molecular weight, high polymerization reactivity, and easy to
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0080]
[0081]A 5 L 4-necked flask was equipped with a thermometer, a Dimroth condenser and a stirrer. Under an atmosphere of nitrogen, 1,800 ml of diglyme was added. Then, AgF (593 g, 4.68 mol) was added with stirring. A reactor was equipped with a dropping funnel, and the reactor was cooled in an ice bath until its inner temperature became at most 10° C. While maintaining the inner temperature of at most 10° C., sulfone (10b) (843 g, 4.68 mol) was dropped from a dropping funnel over a period of 2 hours, followed by stirring for 1 hour in a water bath.
[0082]Again, the reactor was cooled in an ice bath, and while maintaining the inner temperature of at most 10° C., trans-1,3-dibromo-2-butene (10a-1) (500 g, 2.34 mol) dissolved in 500 g of diglyme was dropped from a dropping funnel over a period of 1.5 hours. After the dropping, stirring was continuously carried out for 11 hours. When the crude liquid of the reaction was subjected to a GC analysis, it was confirmed that the reaction wa...
example 2
[0087]
[0088]A 5 L 4-necked flask was equipped with a thermometer, a Dimroth condenser and a stirrer. Under an atmosphere of nitrogen, the compound (1-1) (475 g, 1.05 mol), 2,800 g of 1,2-dichloroethane and 352 g of m-chloroperbenzoic acid (m-CPBA) (purity >65%) were added, and reflux was carried out for 7 hours. When the mixture was subjected to a GC analysis, the degree of conversion was 36.4%. 1,029 g of 1,2-dichloroethane and 352 g of M-CPBA (purity >65%) were further added into a reactor, and reflux was carried out for 31 hours. The degree of conversion was 94.6%.
[0089]The crude liquid of the reaction was filtrated to recover 5,132 g. It was washed twice with a saturated sodium carbonate aqueous solution and with 4.8 mol / l of a sodium chloride aqueous solution, and it was subjected to liquid separation to obtain 4,859 g of a crude liquid of the reaction.
[0090]Such a crude liquid was dried over sodium sulfate and filtrated, and then it was concentrated by an evaporator and dried ...
example 3
[0093]
Acetonide Formation
[0094]A 1 L 4-necked flask was equipped with a thermometer, a stirrer and a Dean-Stark trap. Under an atmosphere of nitrogen, 71.32 g (152.3 mmol) of the compound (11-1), 263 g of dried acetone, 290 g of dried toluene, 58.77 g (152.2 mmol) of a compound (15-1) and BF3.OEt2 (2.67 g, 18.8 mmol) were sequentially added.
[0095]The reactor was heated in an oil bath and heated to the inner temperature of 90° C. under a normal pressure to distill 300 ml of the solvent. Then, stirring was carried out for 4 hours at the inner temperature of 100° C. The degree of conversion analyzed by gas chromatograph was 97%.
Ketal Exchange
[0096]The Dean-Stark trap was removed from the flask and a simple distillation device was attached thereto. It was heated to the inner temperature of 90° C. at 33 kPa to distill 239 g of toluene. Into the reactor, BF3.OEt2 (1.33 g, 9.37 mmol) was added, and a reaction was carried out at 40 kPa at the inner temperature of 90° C. for 2.5 hours. Furth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Reactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com