Nonaqueous electrolyte material of fluorosulfonylimide lithium and application thereof
A technology of lithium fluorosulfonimide and non-aqueous electrolyte, applied in the field of non-aqueous electrolyte materials, can solve the problems of unsatisfactory flame retardant effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
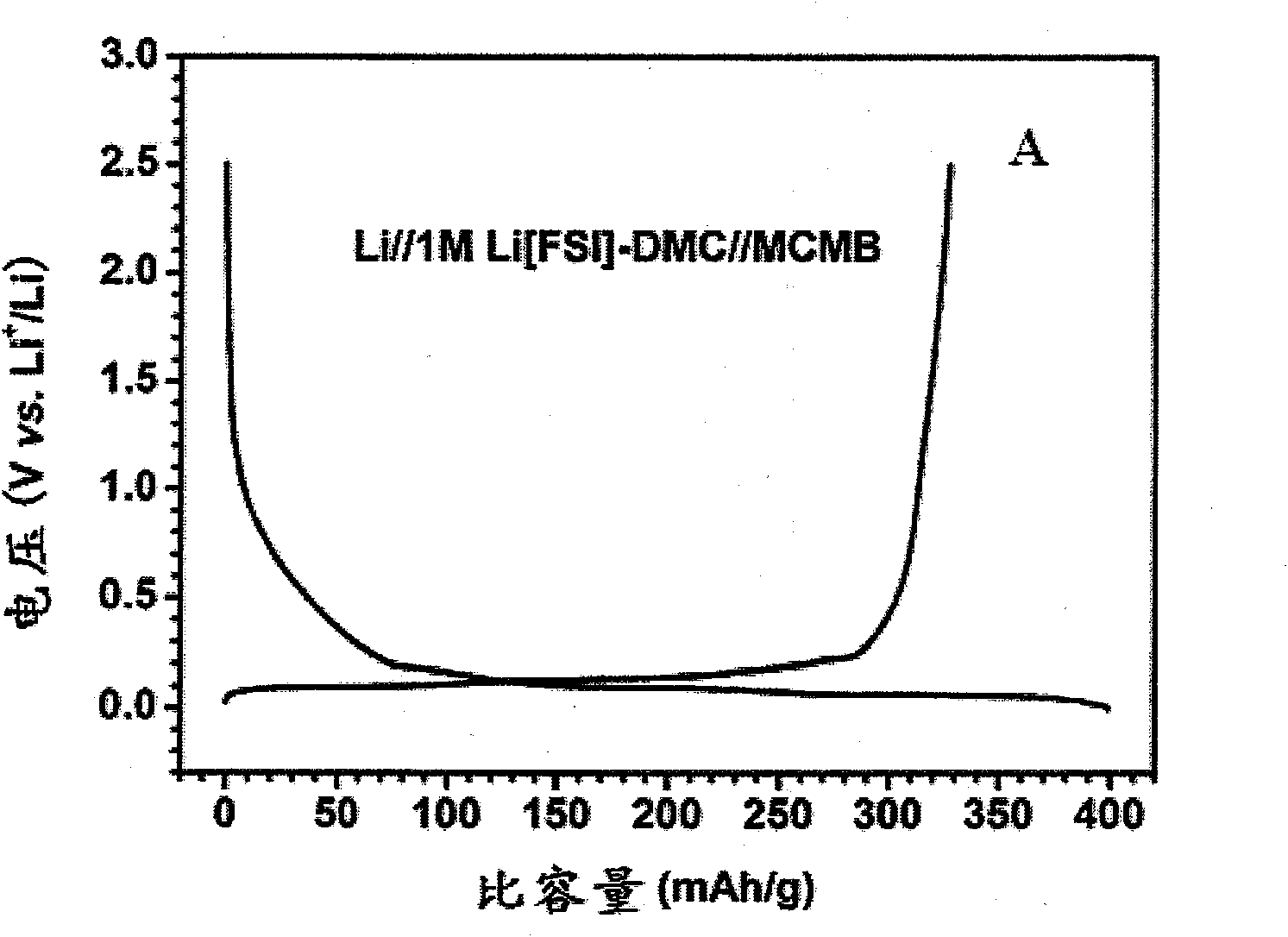
[0057] Electrolyte solution preparation: the conductive salt component A lithium bis(fluorosulfonyl)imide (Li[FSI]) was vacuum-dried, and the organic solvent component B dimethyl carbonate (DMC) was dried and placed in a vacuum glove box ( water content is less than 1ppm). Weigh 18.7g Li[FSI] into a beaker, slowly add DMC several times under magnetic stirring to prepare an electrolyte solution with a molar concentration of 1.0M, and store it in a sealed seal until use.
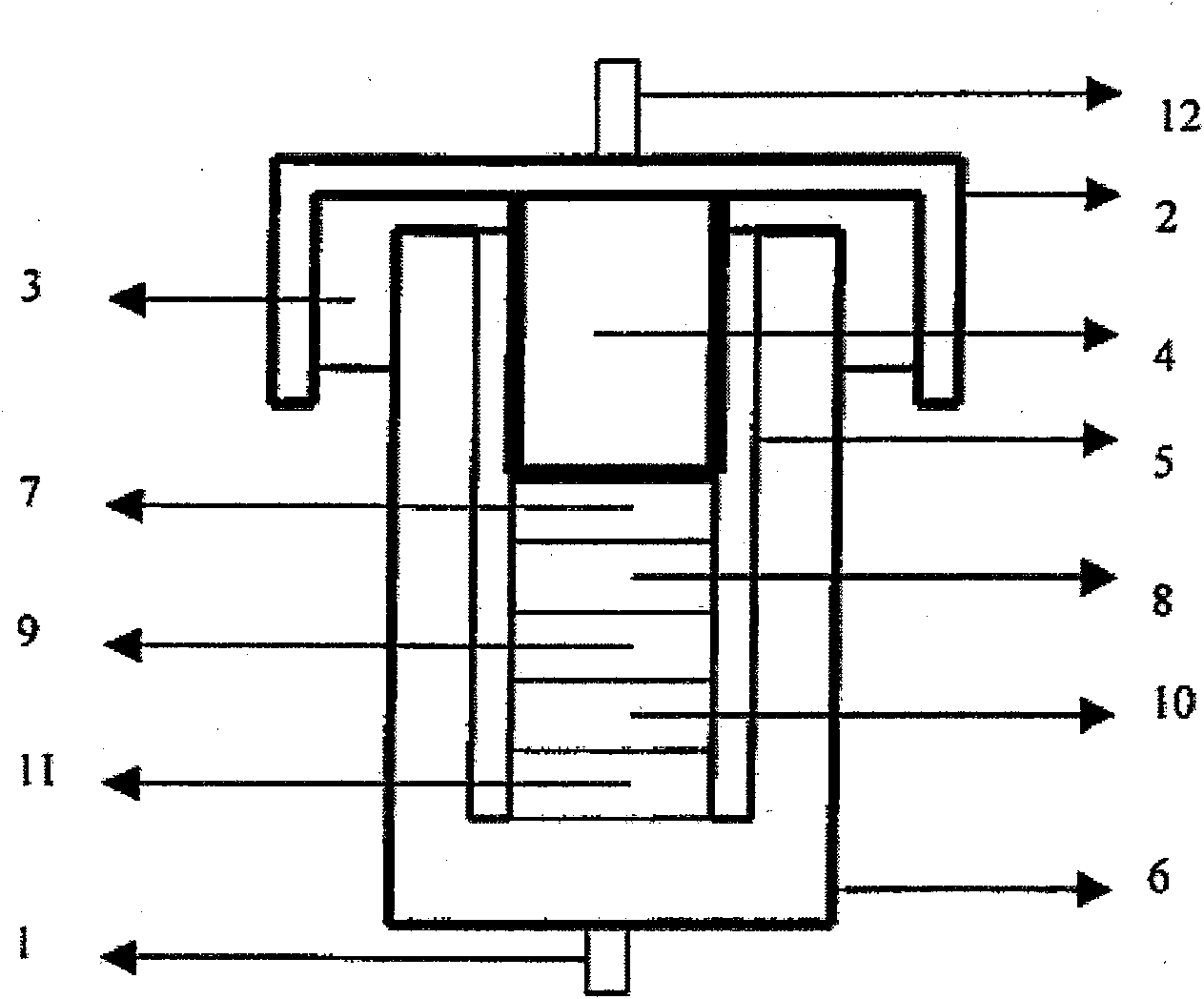
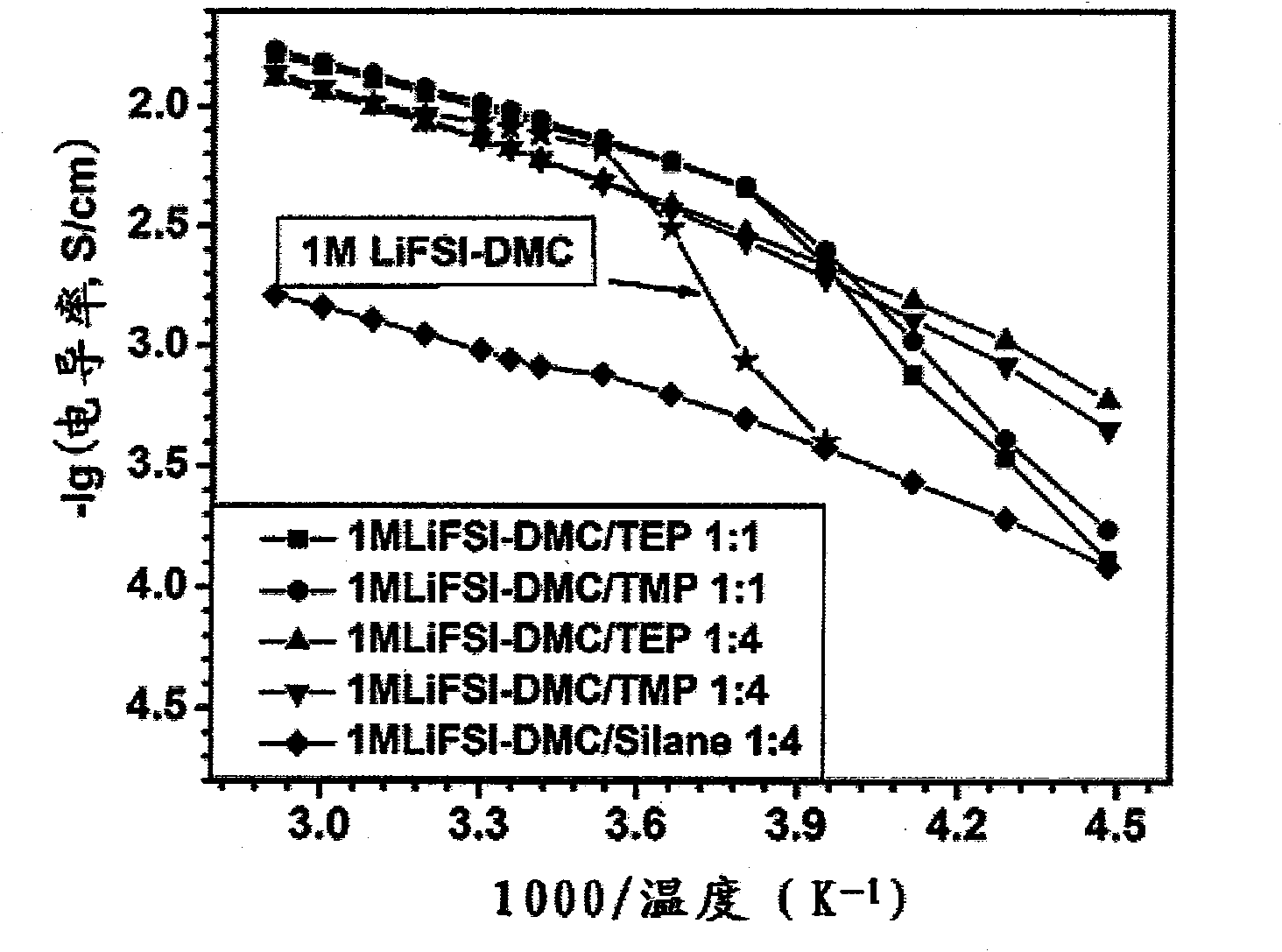
[0058] Conductivity measurement: Add the above electrolytic solution dropwise into a glass conductivity cell with platinum electrodes at both ends, use a GDW6005 high and low temperature test box to control the temperature, measure the impedance spectrum (5Hz-13MHz) with an HP4192 impedance spectrometer, and obtain the temperature range Conductivity for -80°C to 60°C. The conductivity measured at -80°C was 0.2mS / cm, the conductivity at 25°C was 9.2mS / cm, and the conductivity at 60°C was 14.6mS / cm. The electr...
Embodiment 2
[0065] Electrolyte solution preparation: Conductive salt component A lithium bis(fluorosulfonyl)imide (Li[FSI]) was vacuum-dried, organic solvent component B DMC and ethyltriethoxysiloxane ((CH 3 CH 2 O) 3 SiC 2 h 5 ) (abbreviated as silane) dried and placed in a vacuum glove box (water content less than 1ppm). Weigh 18.7g Li[FSI] in a beaker, under magnetic stirring, slowly add DMC and sliane in a mixed solvent (DMC:silane=1:4, volume ratio) several times to prepare an electrolyte with a molar concentration of 1M solution, sealed and stored for later use.
[0066] Conductivity measurement: Add the above electrolytic solution dropwise into a glass conductivity cell with platinum electrodes at both ends, use a GDW6005 high and low temperature test box to control the temperature, measure the impedance spectrum (5Hz-13MHz) with an HP4192 impedance spectrometer, and obtain the temperature range Conductivity for -80°C to 60°C. The measured conductivity at -80°C is 0.03mS / cm, ...
Embodiment 3
[0069] The preparation of the electrolyte solution: the conductive salt component A lithium bis(fluorosulfonyl)imide (Li[FSI]) was vacuum-dried, the DMC of the organic solvent component B, and trimethyl phosphate (TMP) were dried and put into In a vacuum glove box (water content less than 1ppm). Weigh 18.7g Li[FSI] in a beaker, under magnetic stirring, slowly add in the mixed solvent of dimethyl carbonate (DMC) and TMP (DMC:TMP=1:1, volume ratio) in several times slowly, be formulated into Electrolyte solution with a molar concentration of 1.0M, sealed and stored for later use.
[0070] Conductivity measurement: Add the above electrolytic solution dropwise into a glass conductivity cell with platinum electrodes at both ends, use a GDW6005 high and low temperature test box to control the temperature, measure the impedance spectrum (5Hz-13MHz) with an HP4192 impedance spectrometer, and obtain the temperature range Conductivity for -80°C to 60°C. The conductivity measured at -8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com