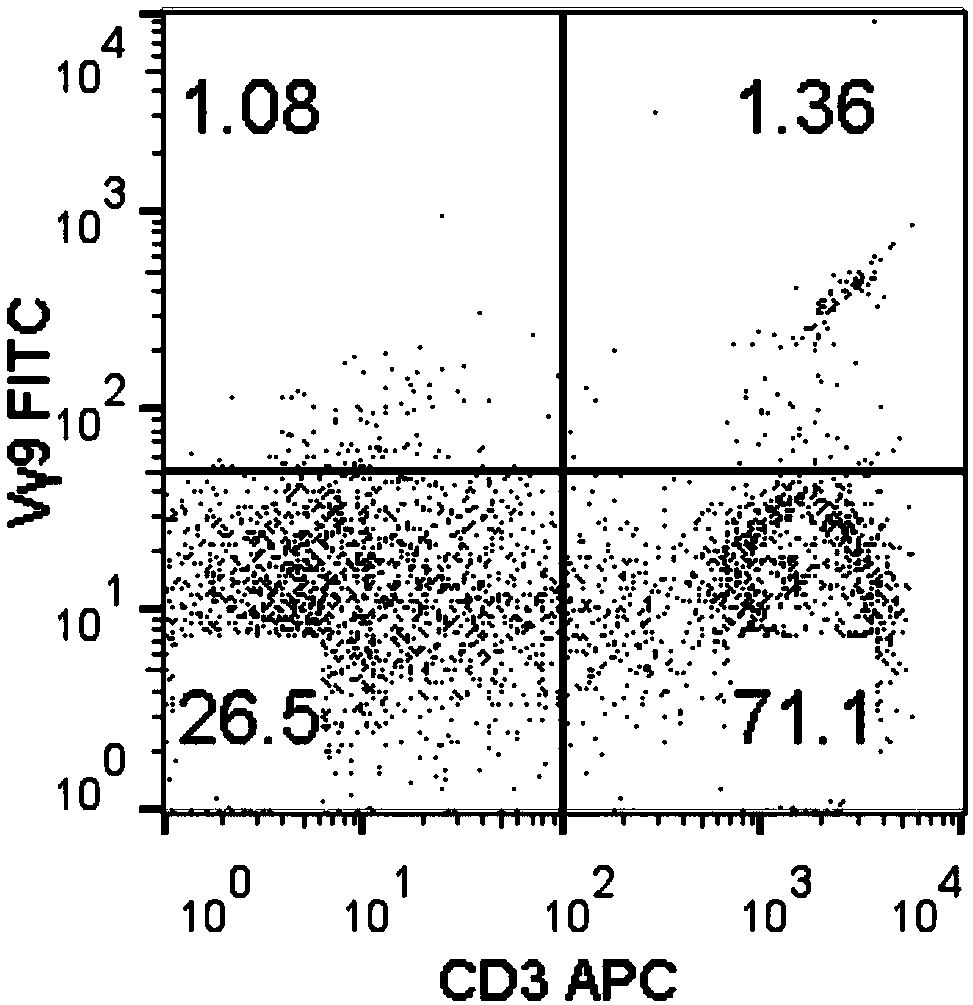
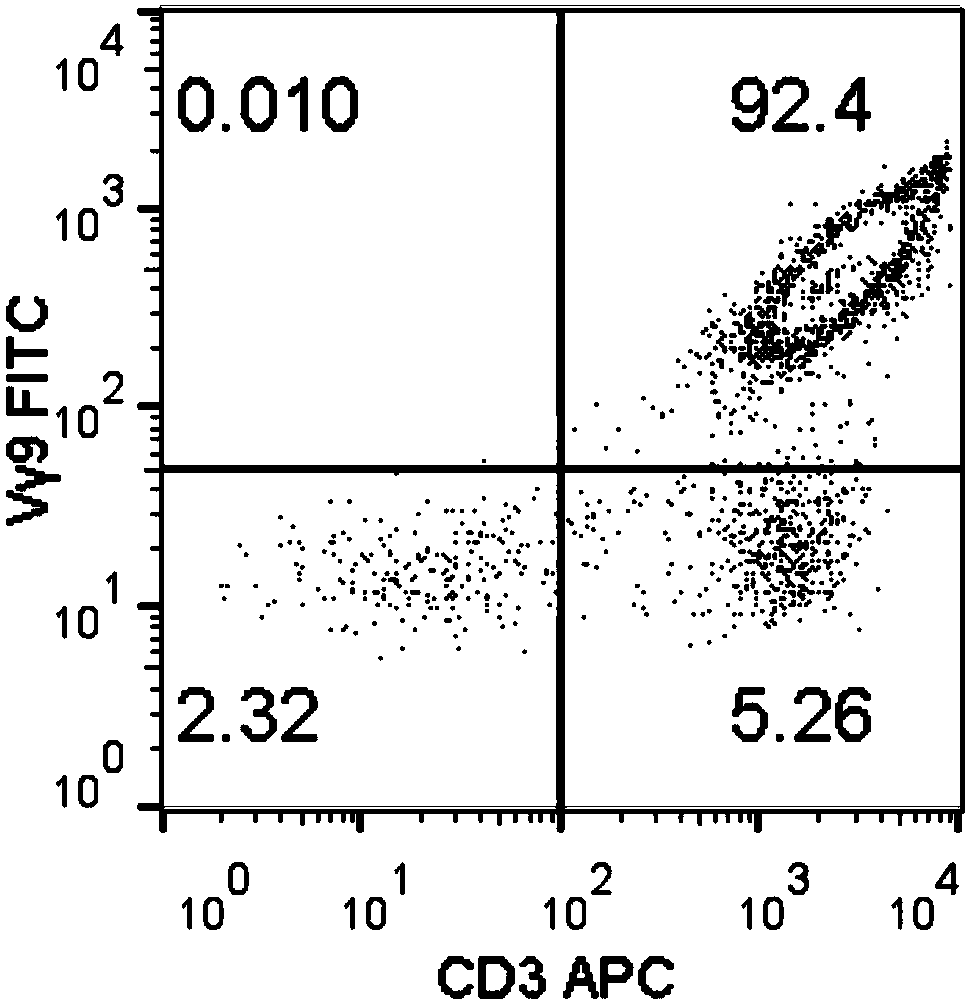
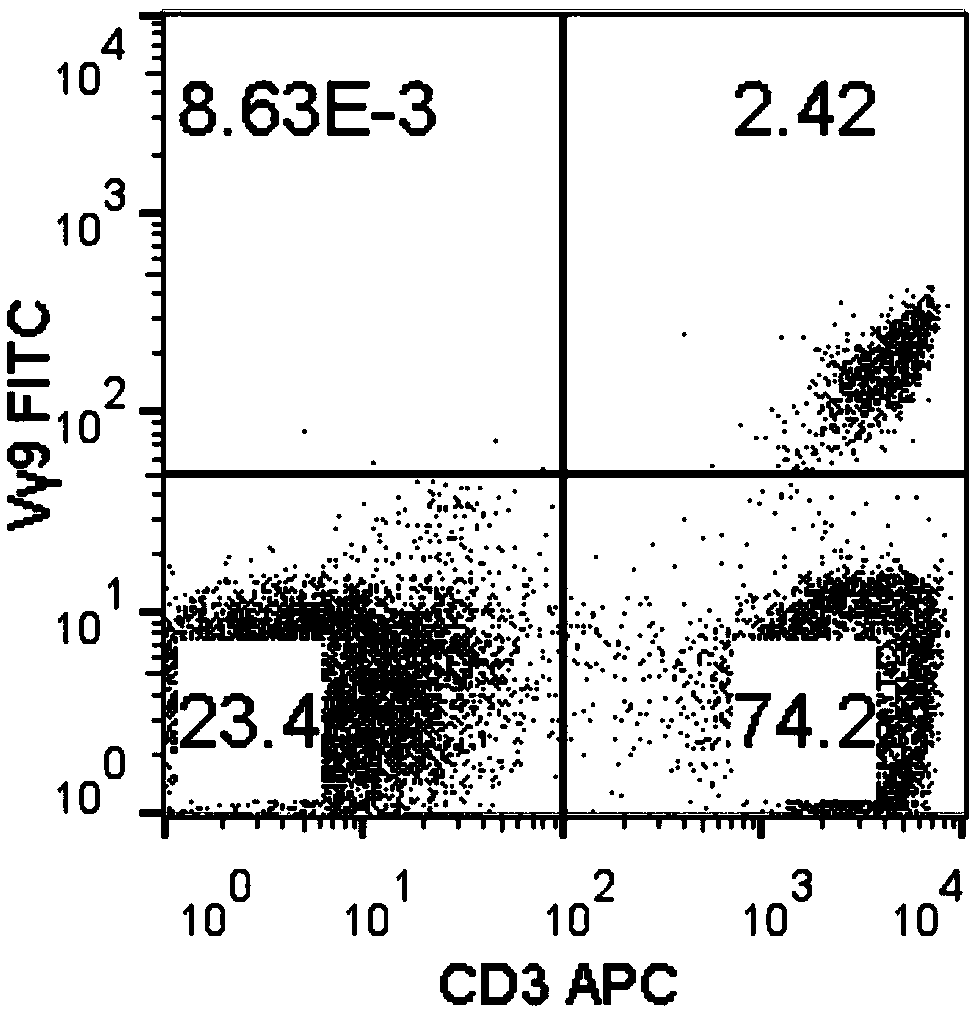
Method for amplifying killing activity gamma-delta T cell by induction in vitro
A technology of killing activity and cells, applied in the direction of animal cells, vertebrate cells, cell culture active agents, etc., to meet the needs of clinical applications, simple operation steps, and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for inducing and expanding γδT cells in vitro, comprising the steps of:
[0027] (1) Preparation of PBMCs:
[0028] A. Use heparin anticoagulated vacuum blood collection tube to extract 50mL peripheral blood from a healthy volunteer, centrifuge at 3000rpm room temperature for 10min, put the plasma into a new 50mL centrifuge tube, and place it in a constant temperature incubator at 56°C for 30min to inactivate complement.
[0029] B. Take 30mL of lymphocyte separation solution and add it evenly to the lymphocyte separation tubes, 15mL in each tube.
[0030] C. Add 40mL of PBS to the centrifuged cell pellet, mix well, slowly add the diluted blood cells into the lymphocyte separation tube containing 15mL of lymphocyte separation medium, and centrifuge at 1800rpm for 20min at room temperature.
[0031] D. After centrifugation, gently remove the buffy coat cells in the middle of the lymphocyte separation tube to two new 50mL centrifuge tubes, then add 10 times the...
Embodiment 2
[0044] A method for inducing and expanding γδT cells in vitro, comprising the steps of:
[0045] (1) Preparation of PBMCs:
[0046] A. Use a heparin anticoagulated vacuum blood collection tube to extract 50mL peripheral blood from another healthy volunteer, centrifuge at 3000rpm room temperature for 10min, put the plasma into a new 50mL centrifuge tube, and place it in a constant temperature incubator at 56°C 30min to inactivate complement.
[0047] B. Take 30mL of lymphocyte separation solution and add it evenly to the lymphocyte separation tubes, 15mL in each tube.
[0048] C. Add 40mL of PBS to the centrifuged cell pellet, mix well, slowly add the diluted blood cells into the lymphocyte separation tube containing 15mL of lymphocyte separation medium, and centrifuge at 1800rpm for 20min at room temperature.
[0049] D. After centrifugation, gently remove the buffy coat cells in the middle of the lymphocyte separation tube to two new 50mL centrifuge tubes, then add 10 times...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com