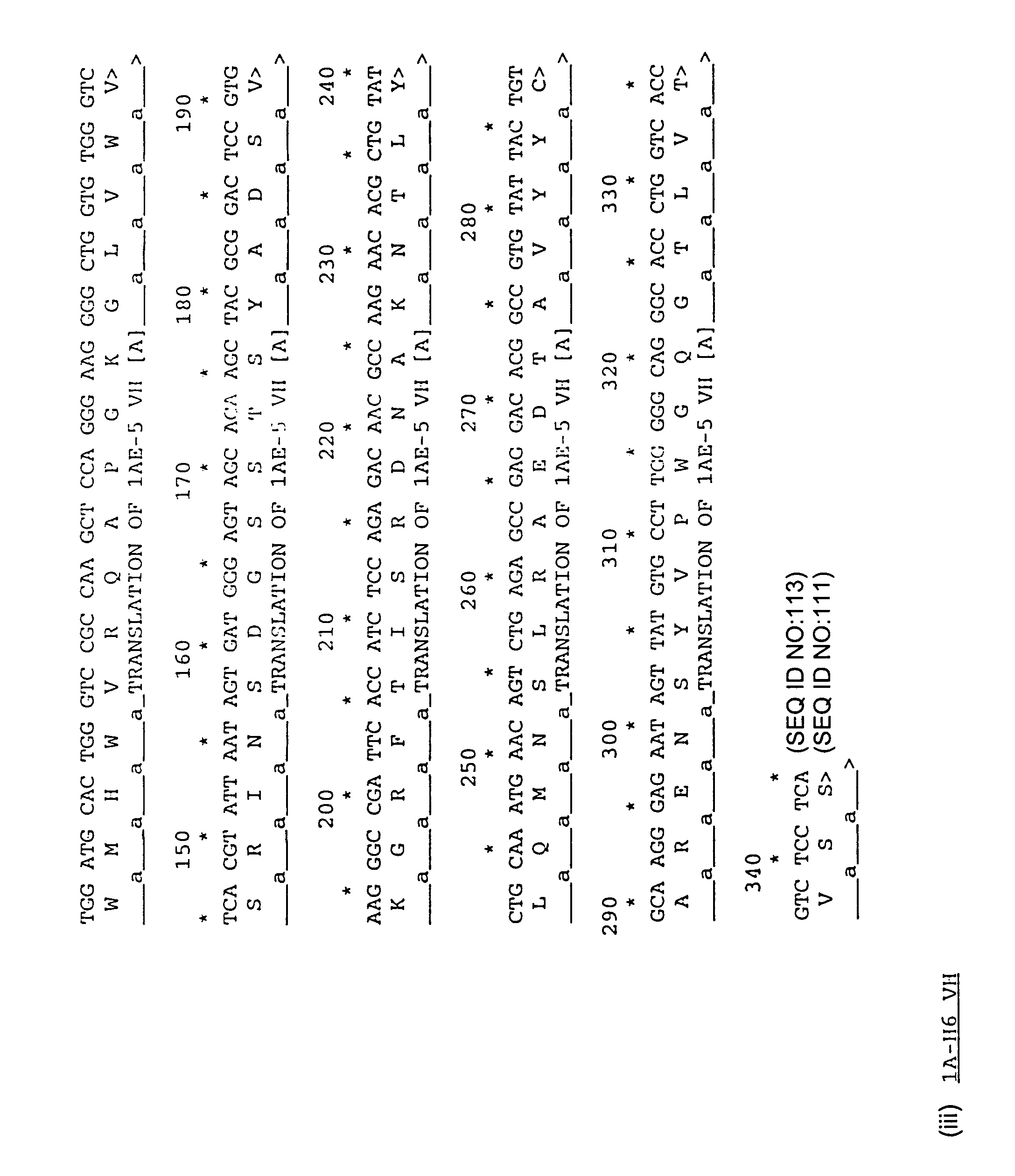
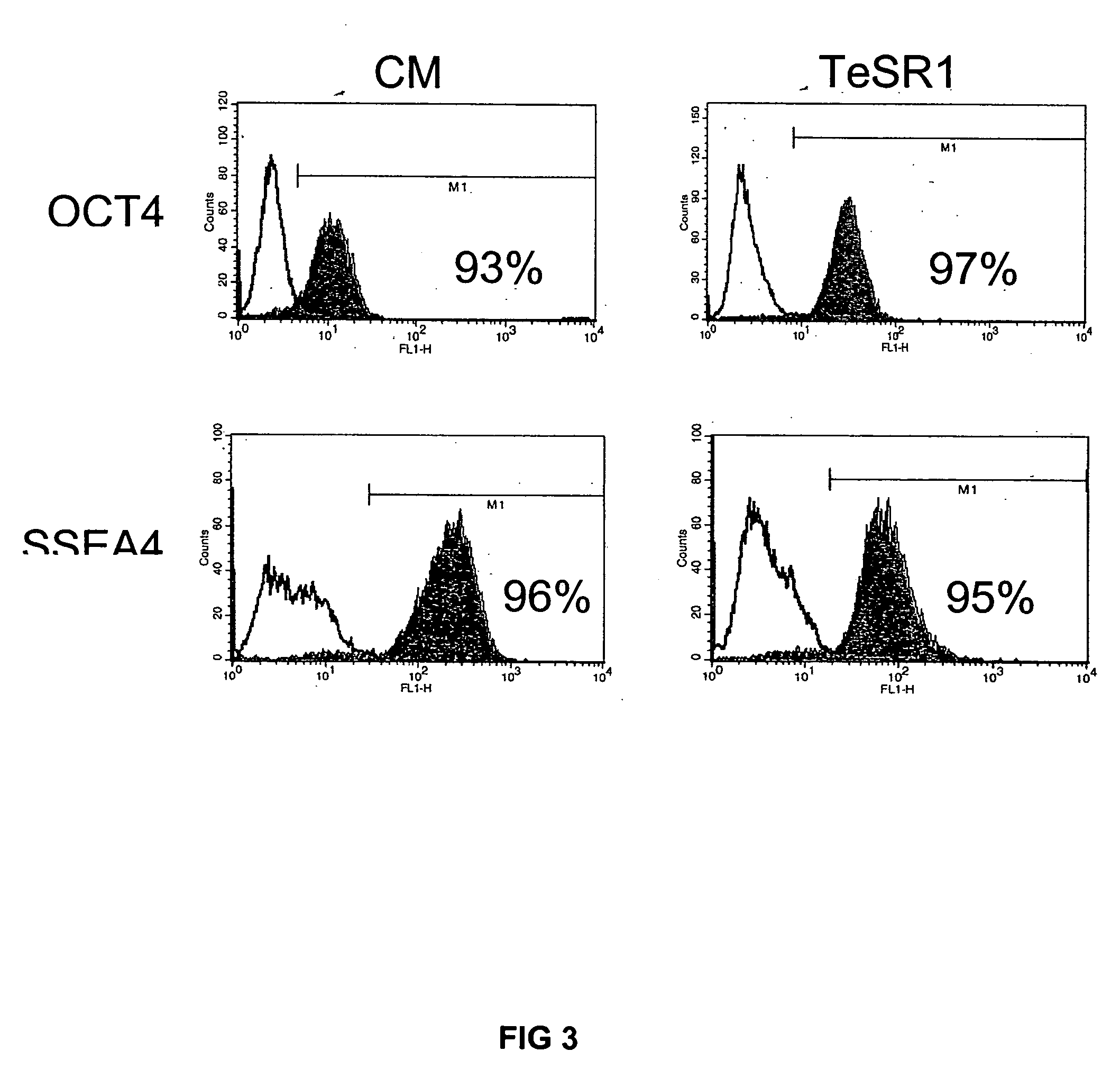
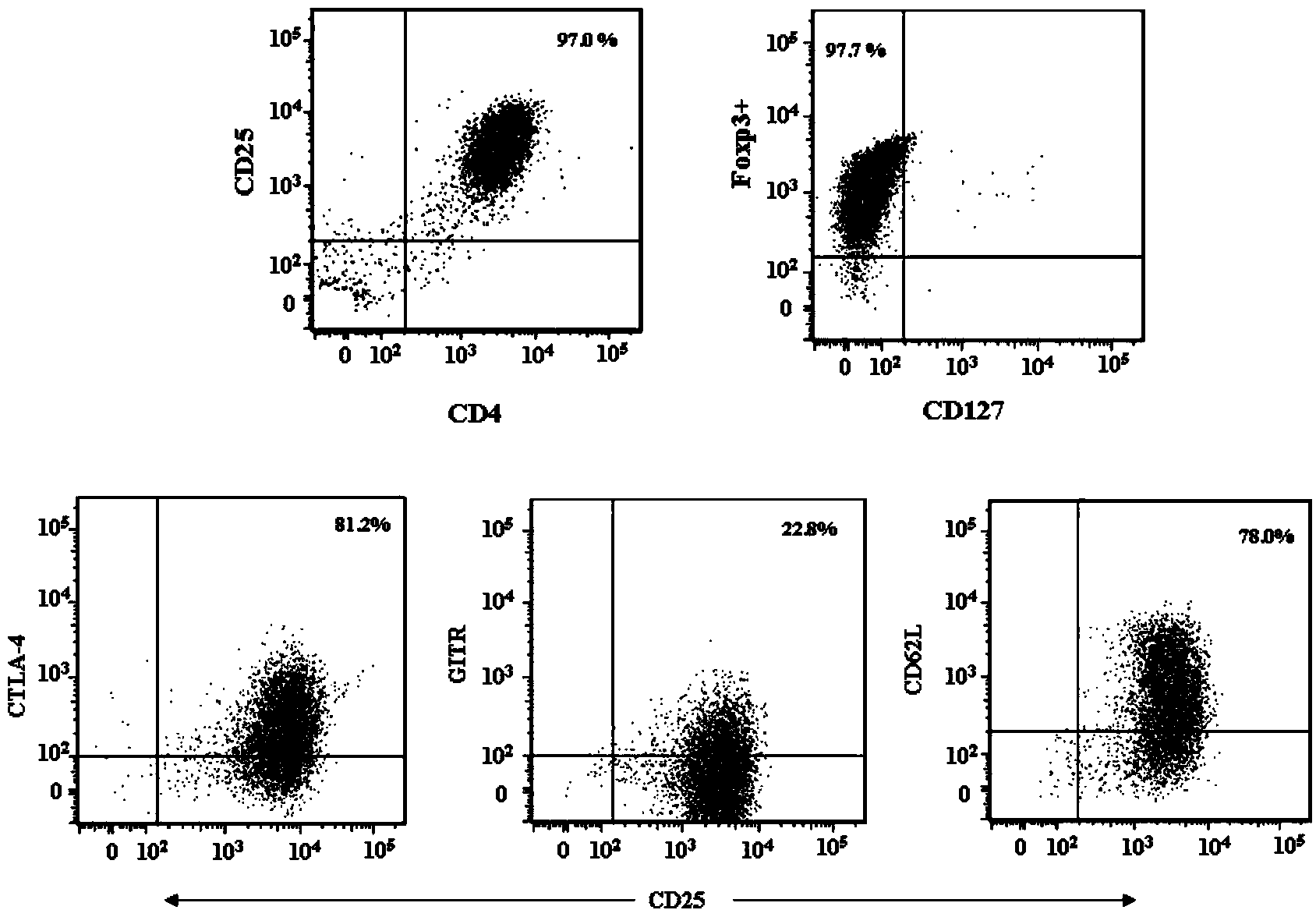
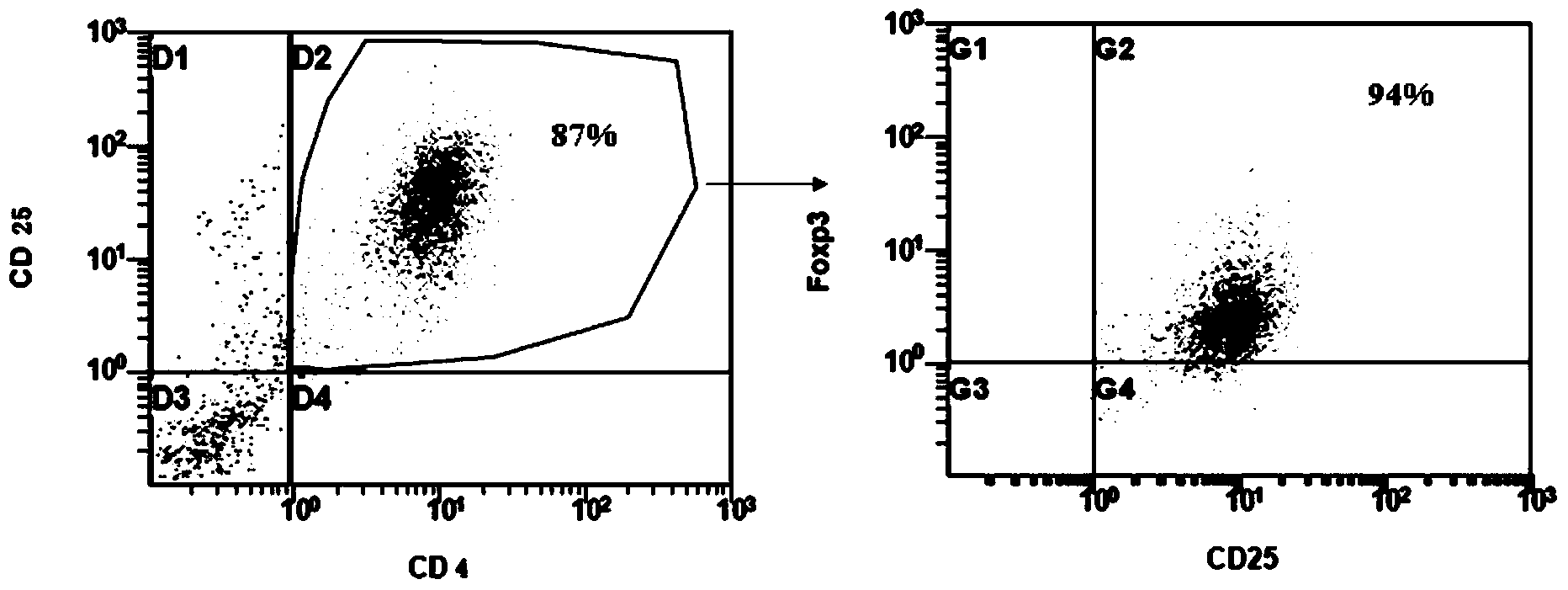
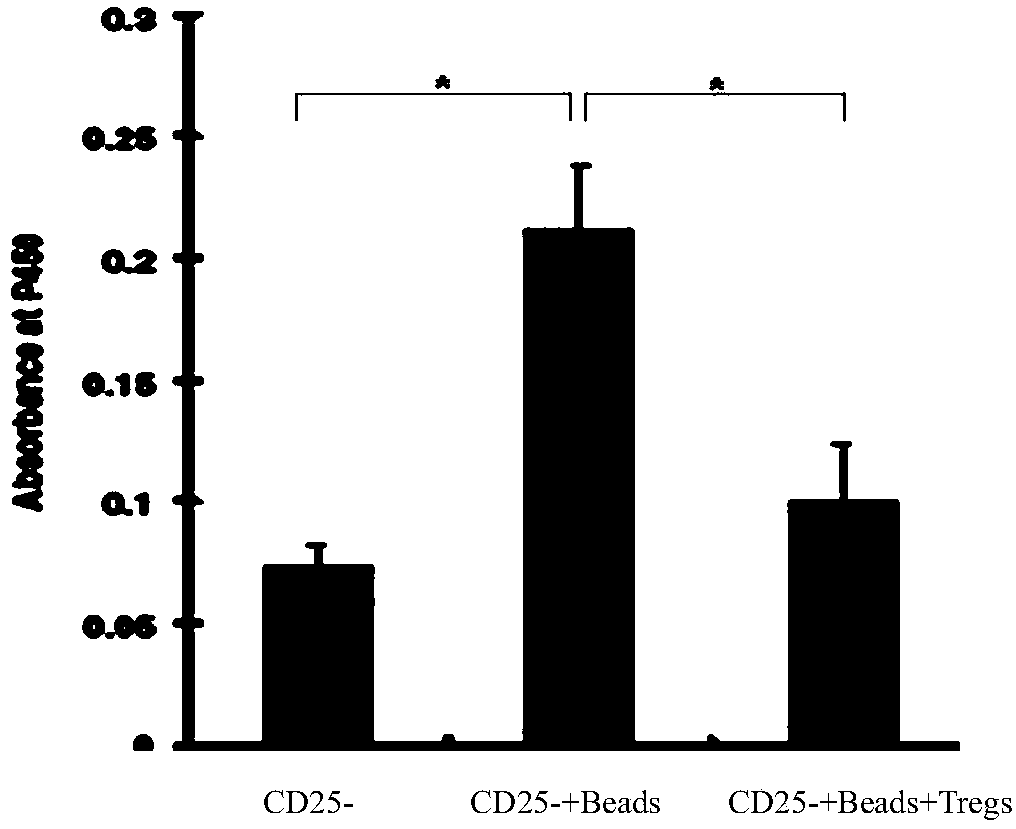
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
192 results about "Transforming growth factor beta" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transforming growth factor beta (TGF-β) is a multifunctional cytokine belonging to the transforming growth factor superfamily that includes three different mammalian isoforms (TGF-β 1 to 3, HGNC symbols TGFB1, TGFB2, TGFB3) and many other signaling proteins. TGFB proteins are produced by all white blood cell lineages.
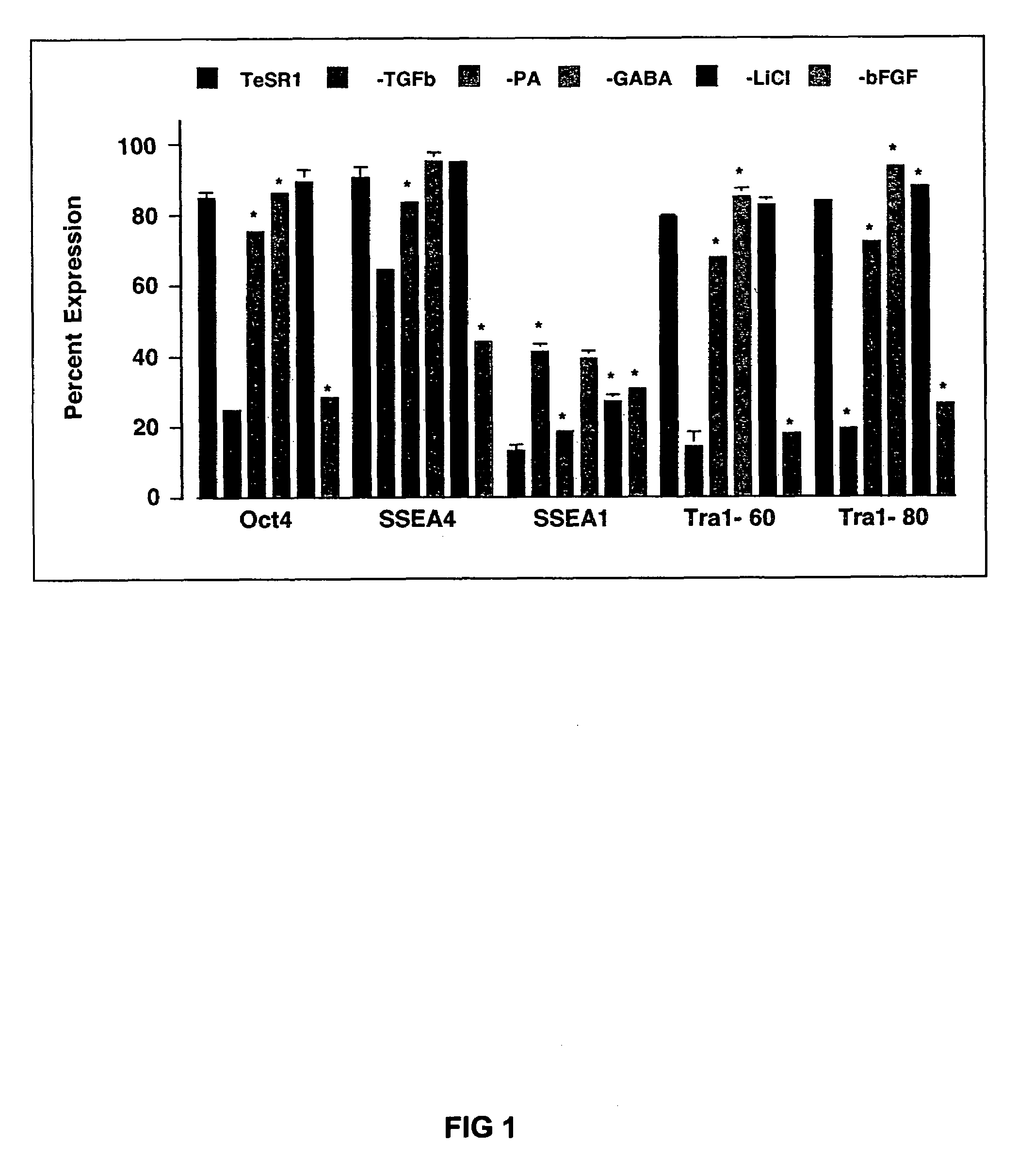
Medium containing pipecholic acid and gamma amino butyric acid and culture of embryonic stem cells
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor, gamma amino butyric acid, pipecholic acid, lithium and transforming growth factor beta are added to the medium in which the stem cells are cultured, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Transforming growth factor beta (TGF-β) blocking agent-treated stem cell composition and method
InactiveUS6841542B2Inhibition effectProlong survival timeBiocidePeptide/protein ingredientsBETA BLOCKING AGENTSExtended time
The invention relates to TGF-β blocking agent-treated HSC compositions and methods comprising the same. The TGF-β blocking agent-treated stem cells are viable for an extended time in culture without replication or differentiation and upon transfer to appropriate conditions are capable of long term hematopoietic reconstitution.
Owner:SAREPTA THERAPEUTICS INC
Compositions and Methods for Targeted Immunomodulatory Antibodies and Fusion Proteins
ActiveUS20130039911A1Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antobodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antobodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immuno-suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β)) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By conteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Antibodies to TGF-beta
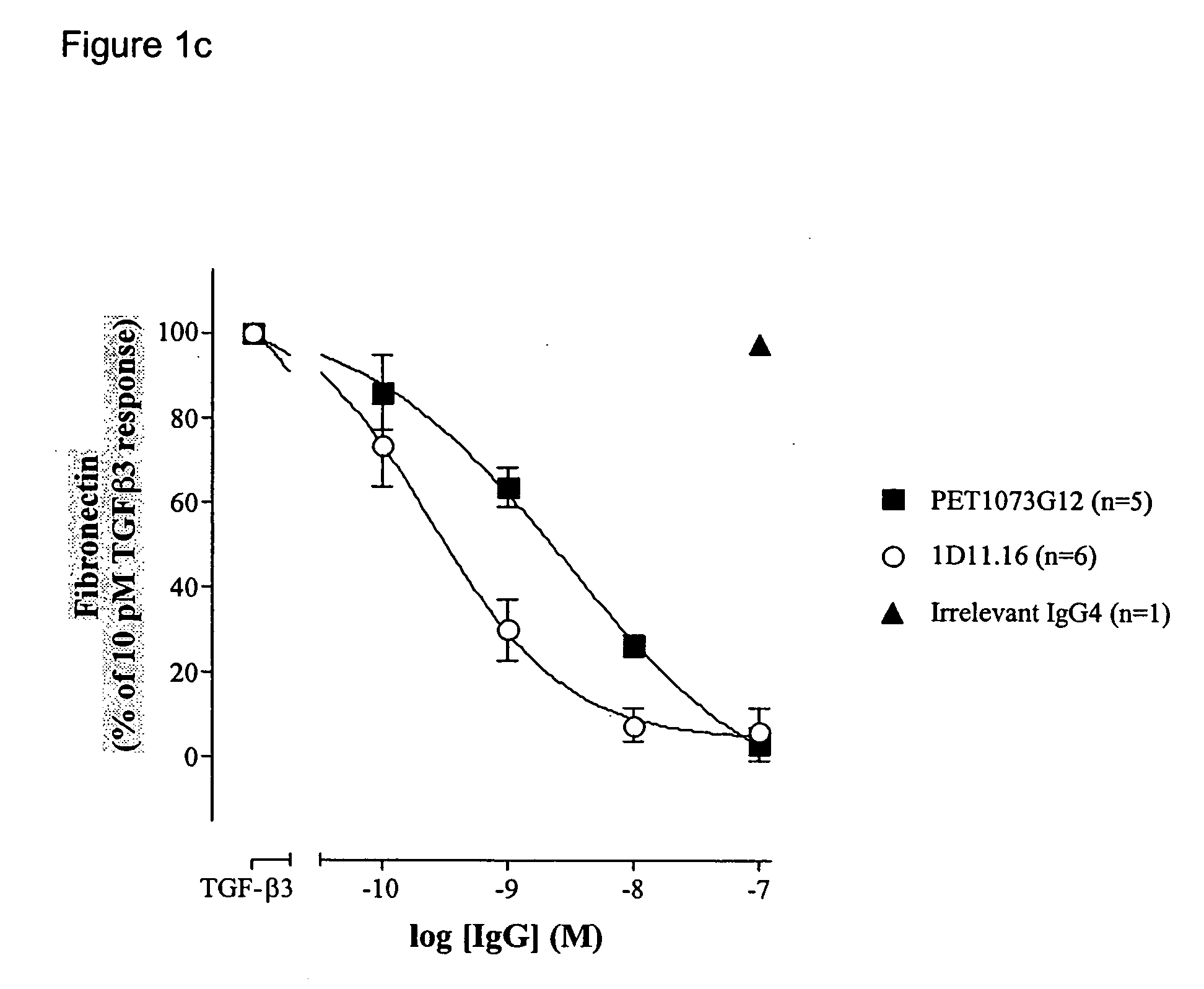
ActiveUS20060251658A1Improve effectivenessEasy to detectAnimal cellsSugar derivativesAntibody fragmentsTransforming growth factor beta
The present invention relates to antibody molecules, in particular antibody molecules that bind Transforming Growth Factor beta (TGFβ), and uses thereof. More particularly, the invention relates to antibody molecules that bind and preferably neutralise TGFβ1, TGFβ2 and TGFβ3, so-called “pan-specific” antibody molecules, and uses of such antibody molecules. Preferred embodiments within the present invention are antibody molecules, whether whole antibody (e.g. IgG, such as IgG1 or IgG4) or antibody fragments (e.g. scFv, Fab, dAb).
Owner:GENZYME CORP +1
Compositions and methods for targeted immunomodulatory antibodies and fusion proteins
ActiveUS8993524B2Function increaseOrganic active ingredientsPeptide/protein ingredientsTumor targetAntitumor immunity
The present invention is based on the seminal discovery that targeted immunomodulatory antibodies and fusion proteins can counter act or reverse immune tolerance of cancer cells. Cancer cells are able to escape elimination by chemotherapeutic agents or tumor-targeted antibodies via specific immunosuppressive mechanisms in the tumor microenvironment and such ability of cancer cells is recognized as immune tolerance. Such immune suppressive mechanisms include immunosuppressive cytokines (for example, Transforming growth factor beta (TGF-β) and regulatory T cells and / or immunosuppressive myeloid dendritic cells (DCs). By counteracting tumor-induced immune tolerance, the present invention provides effective compositions and methods for cancer treatment, optional in combination with another existing cancer treatment. The present invention provides strategies to counteract tumor-induced immune tolerance and enhance the antitumor efficacy of chemotherapy by activating and leveraging T cell-mediated adaptive antitumor immunity against resistant or disseminated cancer cells.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Antagonizing TGF-beta activity with various ectodomains of TGF-beta receptors used in combination or as fusion proteins
InactiveUS20070244042A1Peptide/protein ingredientsFusions with soluble cell surface receptorGreek letter betaAnticarcinogen
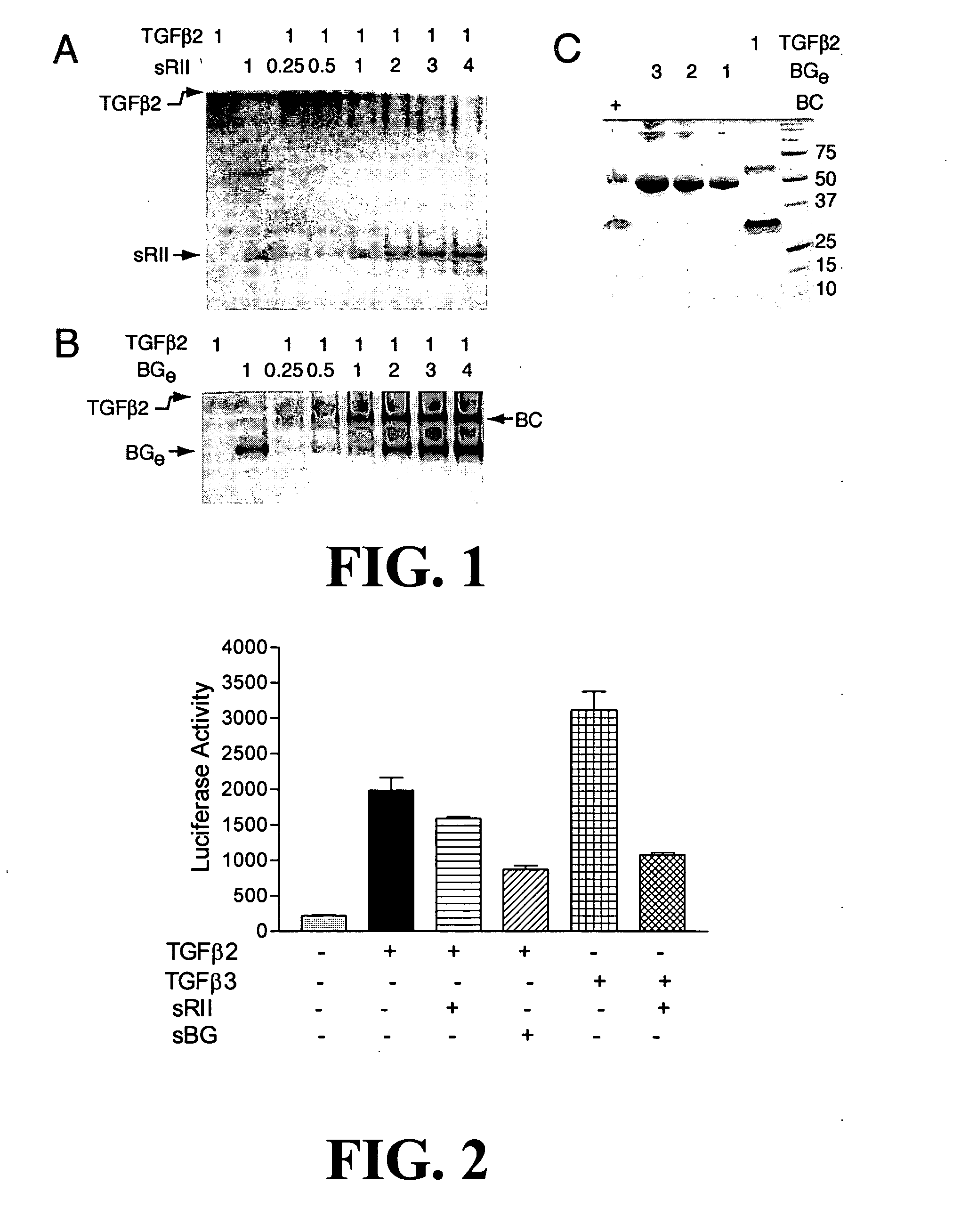
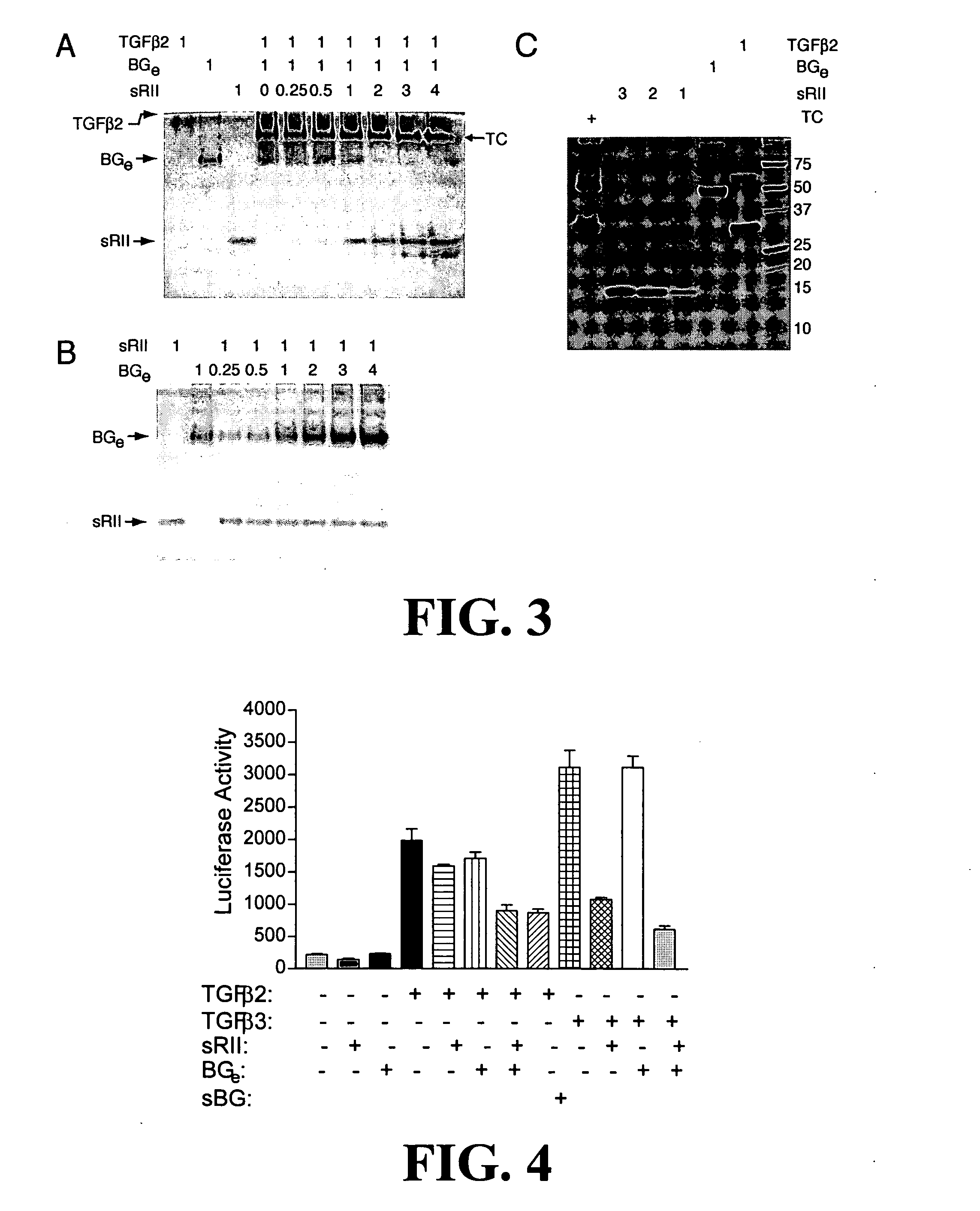
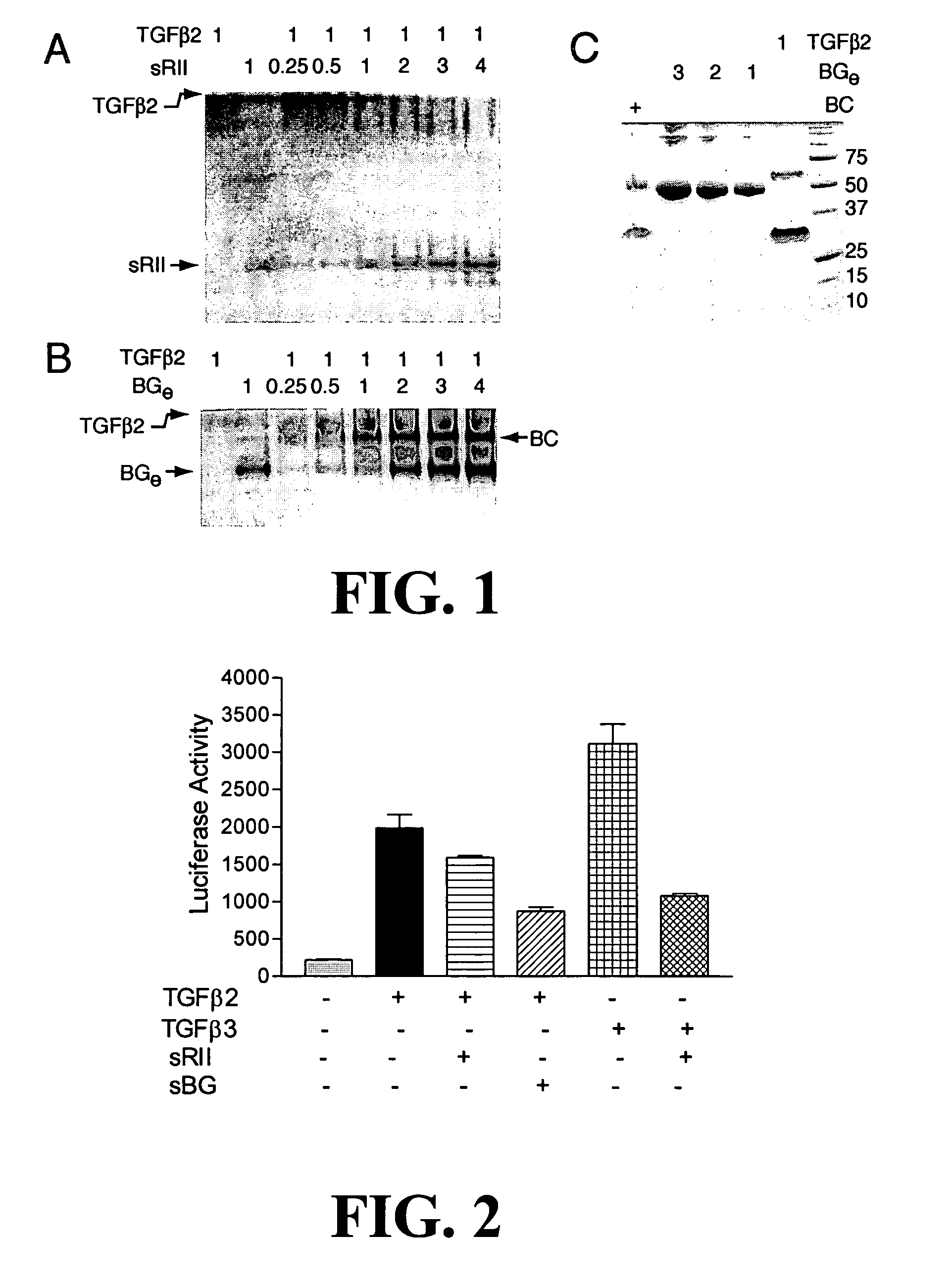
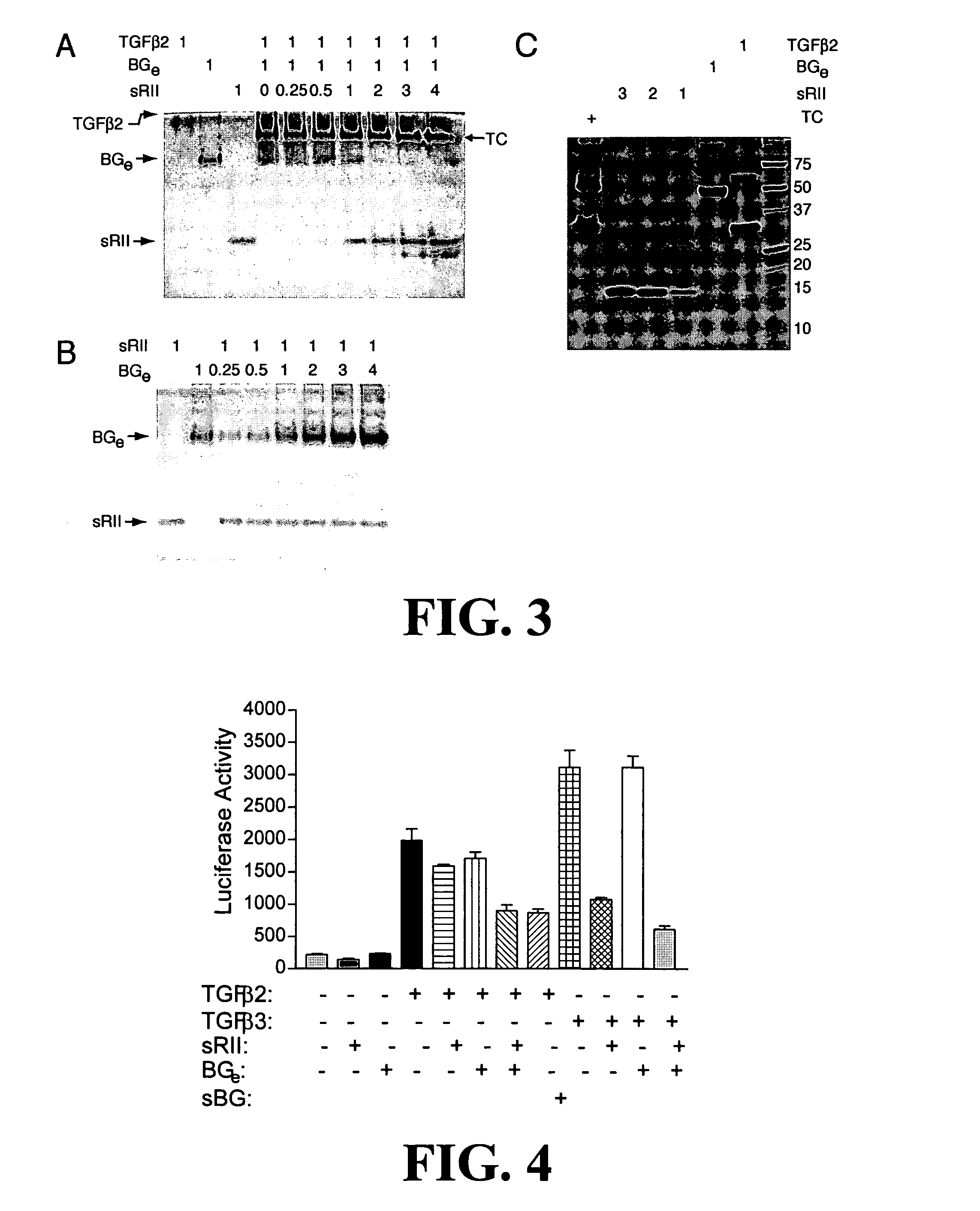
Anti-cancer agents and / or transforming growth factor beta (TGF-beta or TGFβ) antagonists are disclosed, where the agents and / or antagonists include a therapeutically effective amount of a combination of therapeutically active portions of sRII and therapeutically active portions of sRIII or a fusion polypeptide or protein comprising therapeutically active portions of sRII and therapeutically active portions of sRIII. Methods for preventing, treating and / or ameliorating the symptoms of cancer are also disclosed based on administering an effective amount of a composition of this invention.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
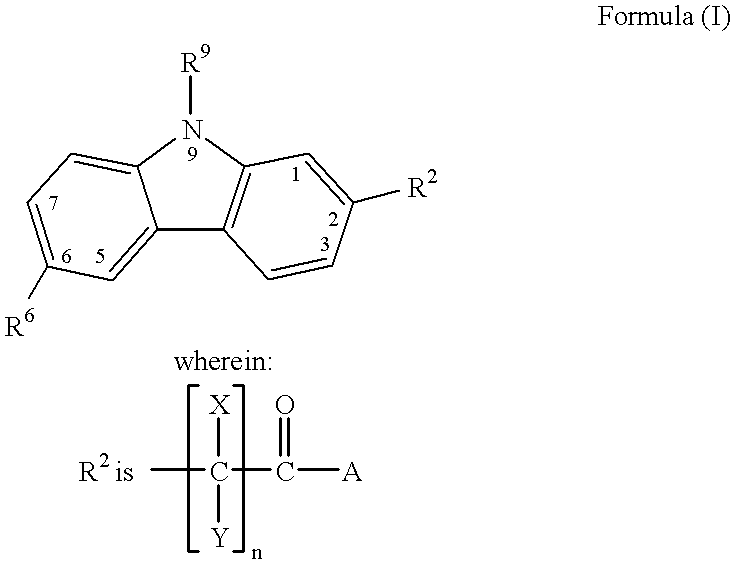
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
Human antibodies specific for TGFbeta2
InactiveUS7368111B2Peptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsDiseaseEpitope
Specific binding members comprising human antibody antigen binding domains specific for human transforming growth factor beta (TGFβ) bind specifically isoforms TGFβ2 and TGFβ1 or both, preferentially compared with TGFβ3. Specific binding members may be isolated and utilized in the treatment of disease, particularly fibrotic disease and also immune / inflammatory diseases. Therapeutic utility is demonstrated using in vitro and in vivo models. Full sequence and binding information is provided, including epitope sequence information for particularly advantageous specific binding member which binds the active form of TGFβ2, neutralizing its activity, but does not bind the latent member.
Owner:MEDIMMUNE LTD
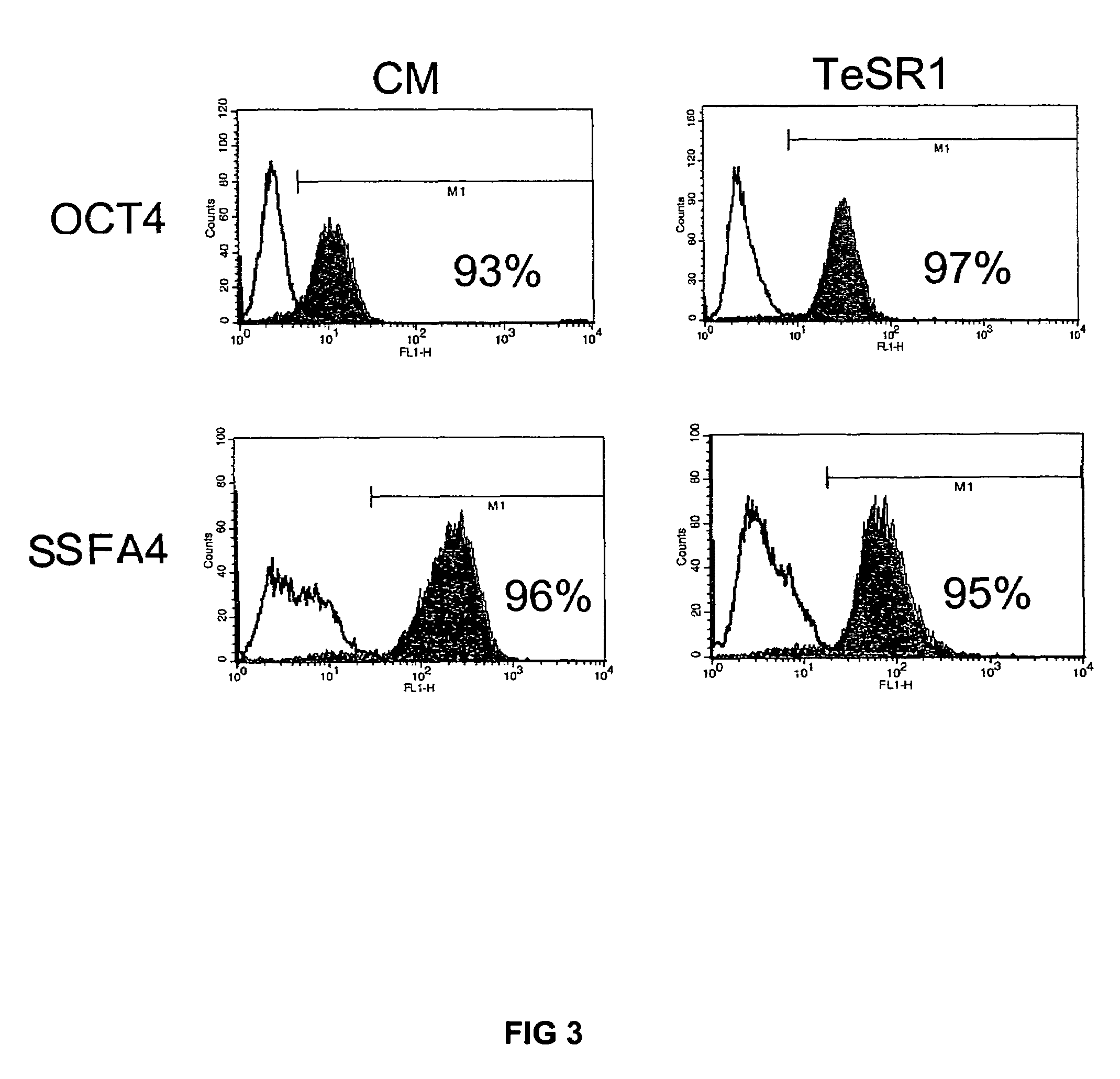
Medium and culture of embryonic stem cells
ActiveUS20060084168A1Promotes robust growthCulture processArtificial cell constructsButyric acidFibroblast growth factor
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor, gamma amino butyric acid, pipecholic acid, lithium and transforming growth factor beta are added to the medium in which the stem cells are cultured, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Cartilage and Bone Repair Composition
InactiveUS20080031915A1Promote bone formationPromote cartilage formationBiocidePeptide/protein ingredientsCollagen iWhole body
The invention relates to a cartilage and bone repair composition comprising a group of human mesenchymal stem cells that are differentiated to the chondro-osteogenic lineage, by means of the amplification thereof with human serum and a transforming growth factor-beta 1 with a molecular domain for binding to collagen I (TGF-β1-DUC) obtained in eukaryotic expression systems, and a biocompatible material which is absorbed by the cells thus treated. The inventive composition can be employed using implants in the area to be repaired or it can be employed directly by injecting the cells in suspension either at the site of the injury or into the systemic circulation for the widespread distribution thereof.
Owner:UNIV DE MALAGA
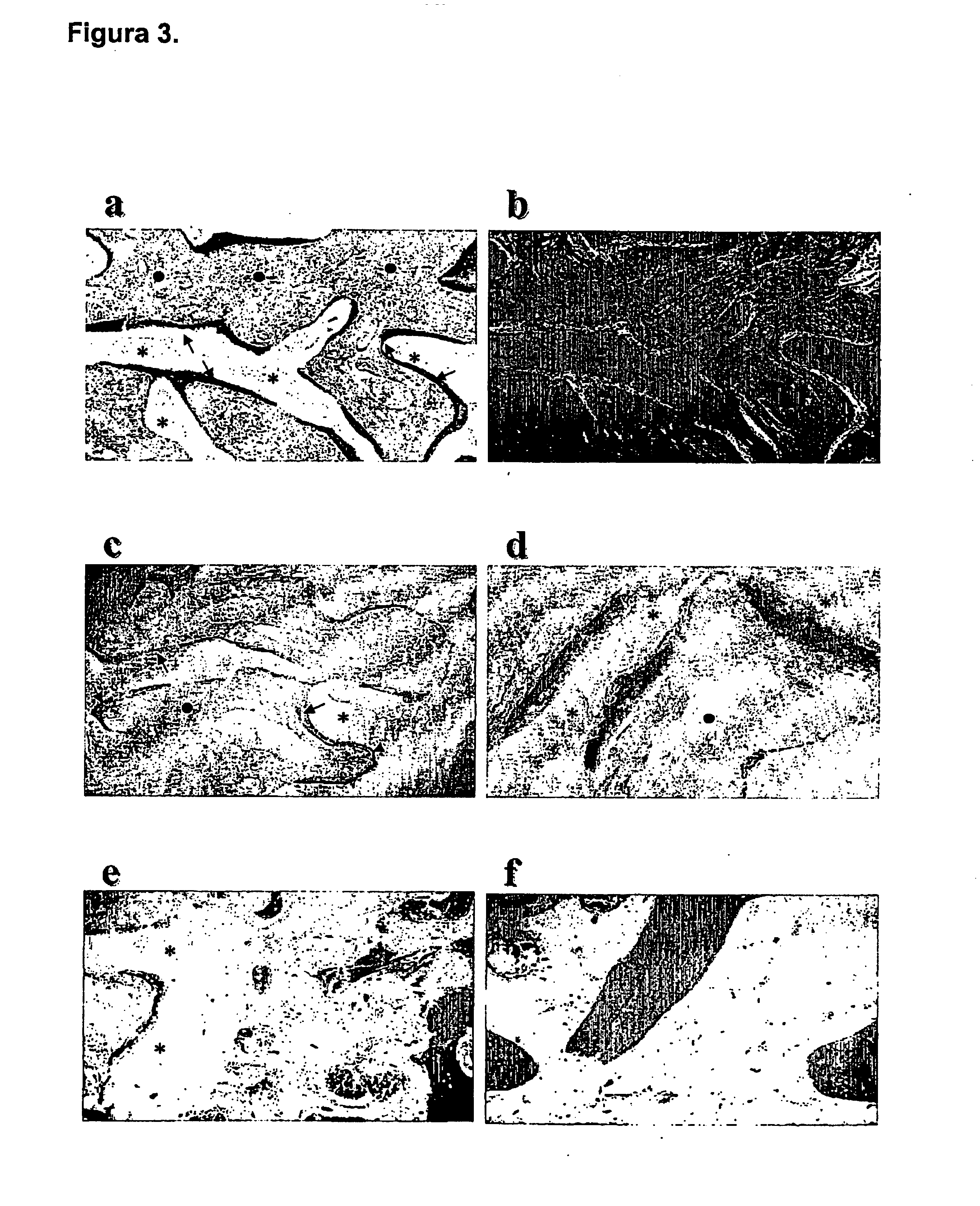
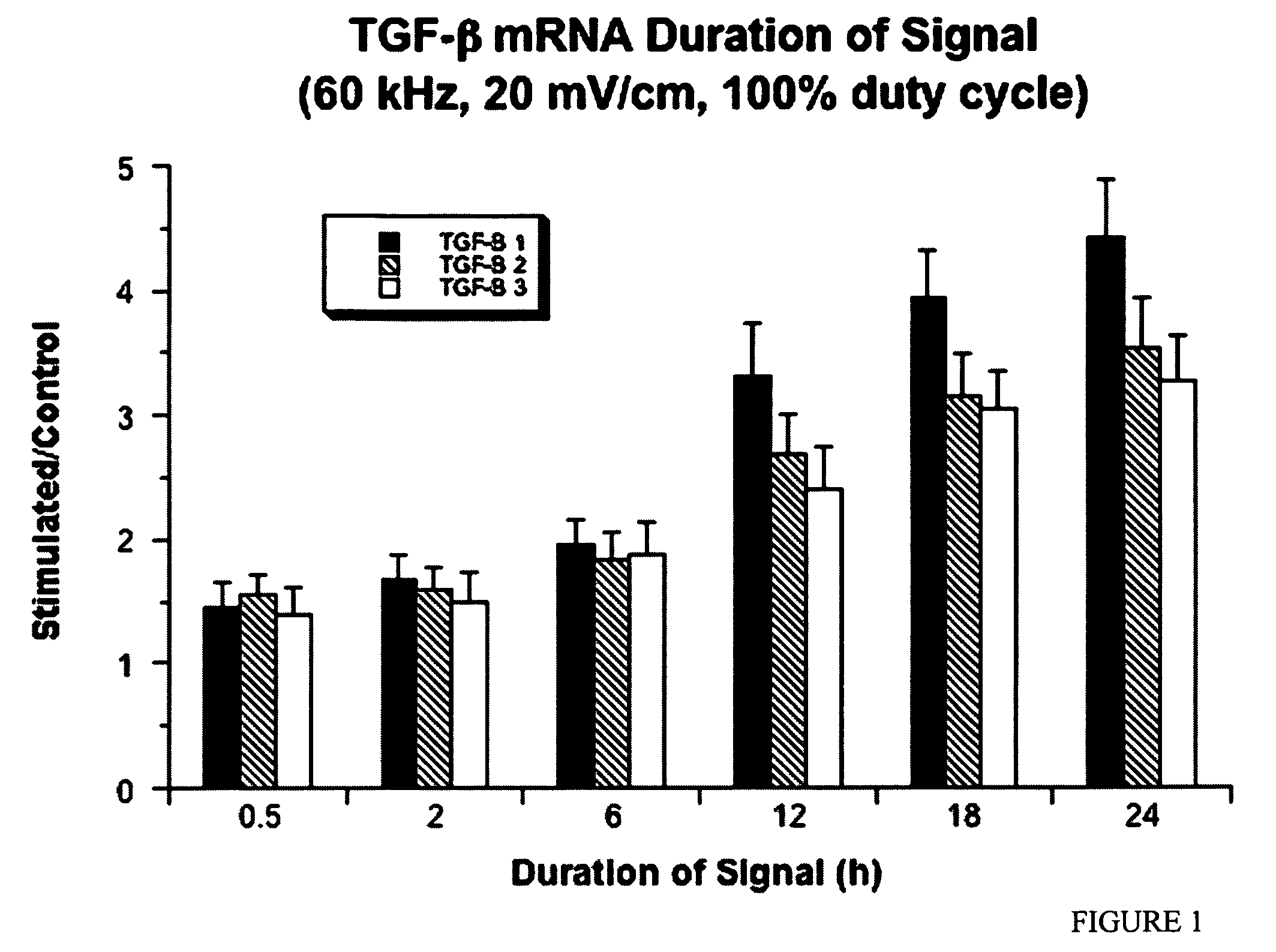
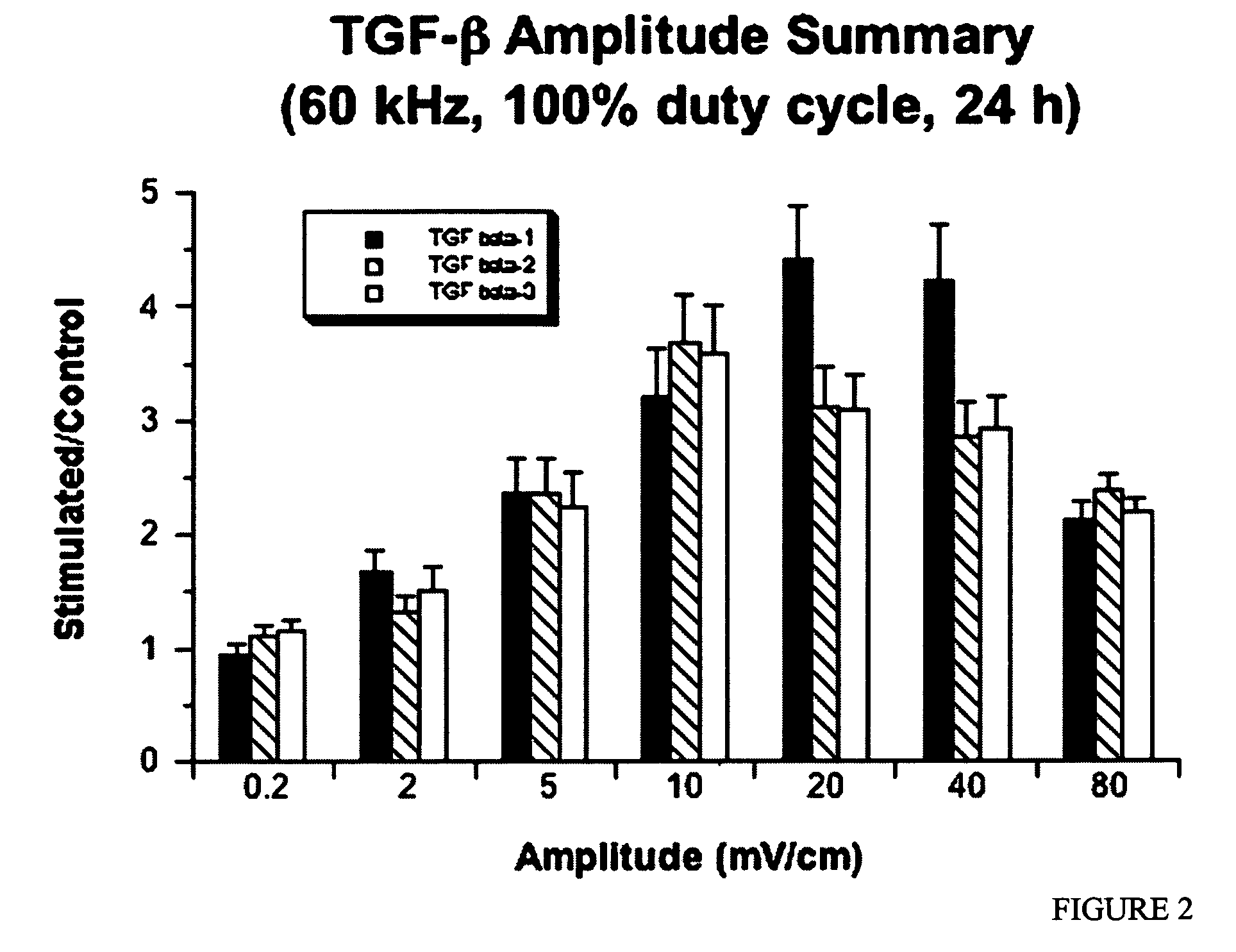
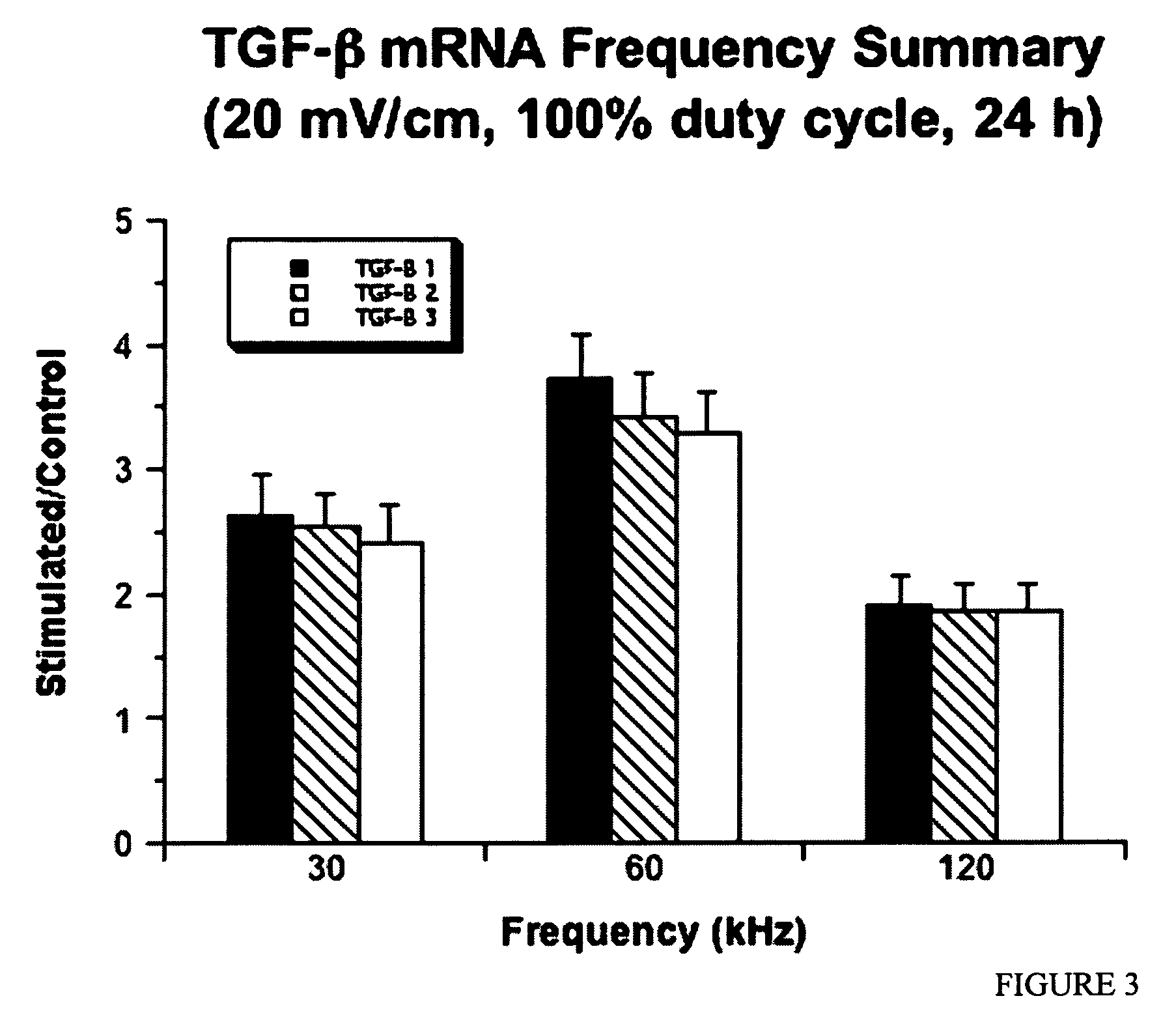
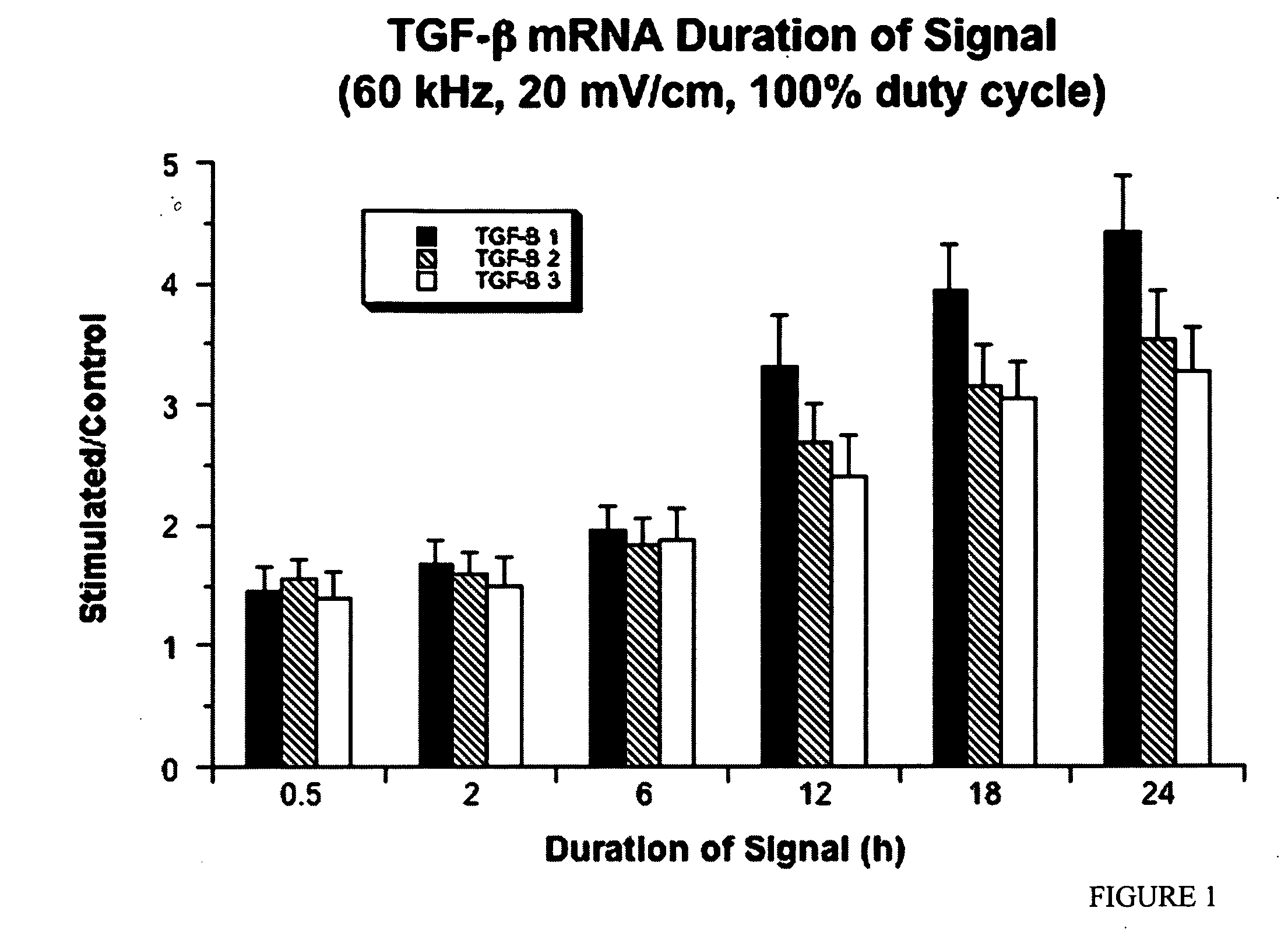
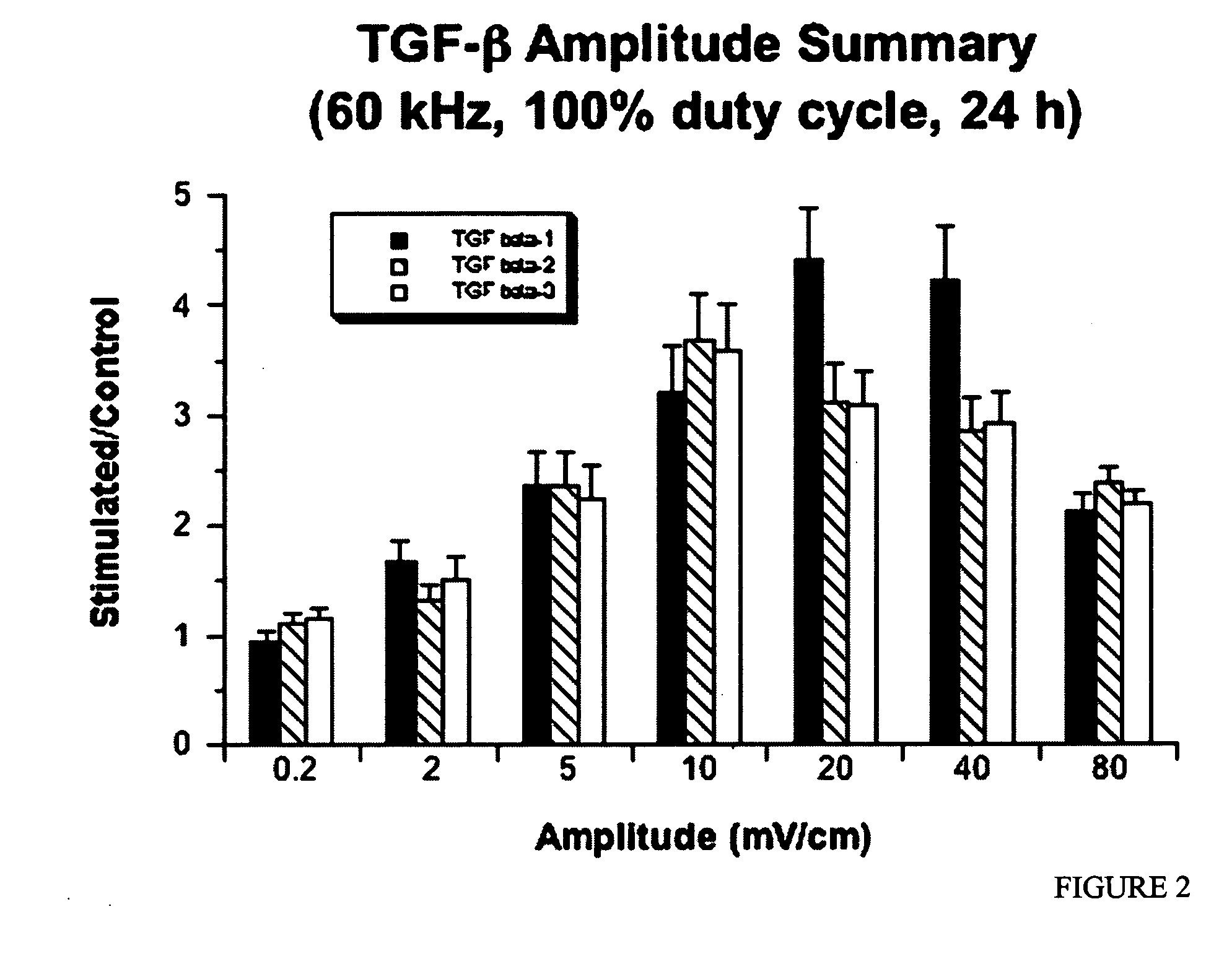
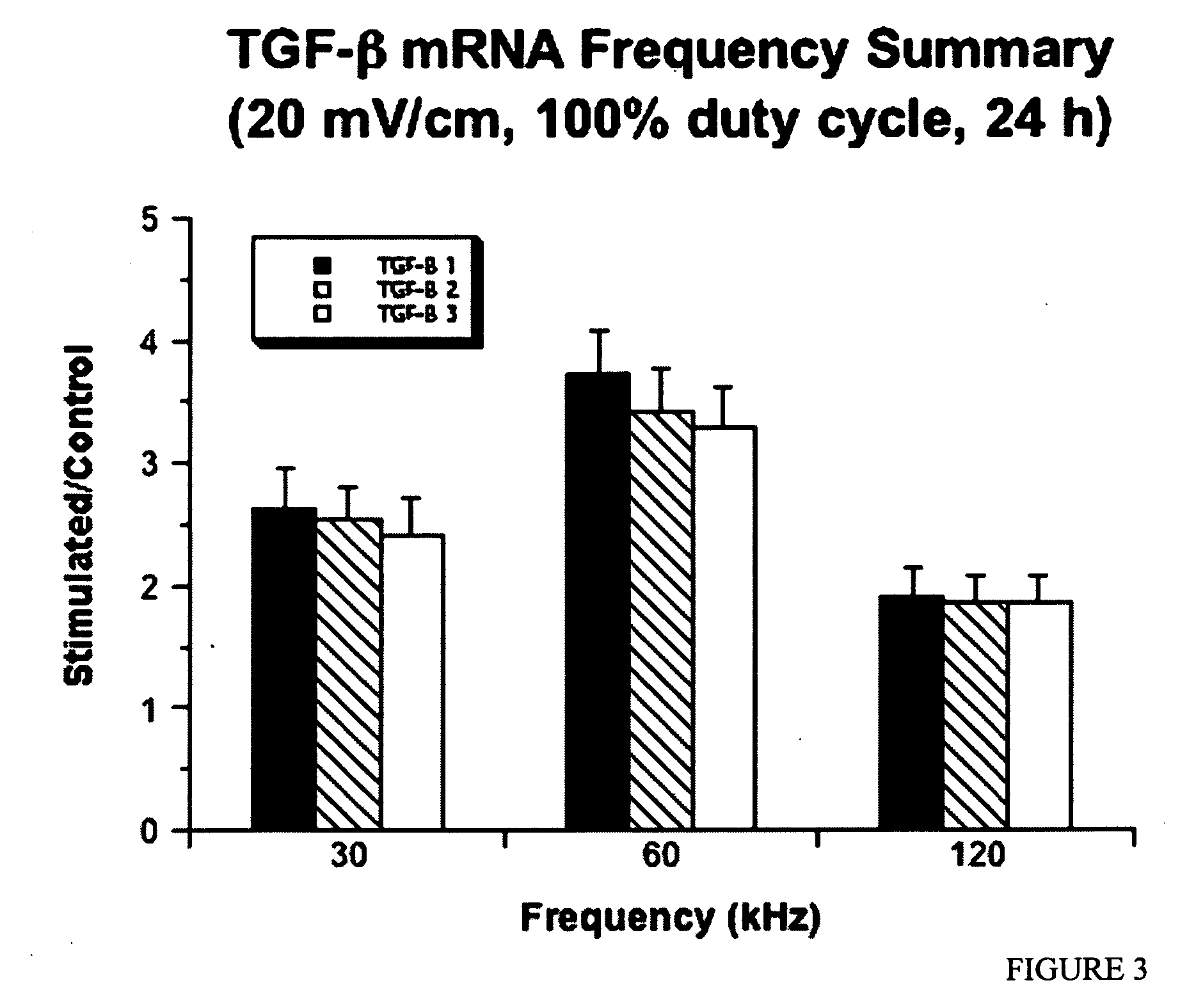
Regulation of transforming growth factor-beta (TGF-beta) gene expression in living cells via the application of specific and selective electric and electromagnetic fields
InactiveUS7465546B2ElectrotherapyMicrobiological testing/measurementElectromagnetic fieldTarget tissue
Methods and devices are described for the regulation of Transforming Growth actor (TGF)-β1, β2, and / or β3 protein gene expression in bone cells and other tissues via the capacitive coupling or inductive coupling of specific and selective electric fields to the bone cells or other tissues, where the specific and selective electric fields are generated by application of specific and selective electric and electromagnetic signals to electrodes or one or more coils or other field generating device disposed with respect to the bone cells or other tissues so as to facilitate the treatment of diseased or injured bone and other tissues. By gene expression is meant the up-regulation or down-regulation of the process whereby specific portions (genes) of the human genome (DNA) are transcribed into mRNA and subsequently translated into protein. Methods and devices are provided for the targeted treatment of injured or diseased bone and other tissue that include generating specific and selective electric and electromagnetic signals that generate fields in the target tissue optimized for increase of TGF-β1, β2, and / or β3 protein gene expression and exposing bone and other tissue to the fields generated by specific and selective signals so as to regulate TGF-β1, β2, and / or β3 protein gene expression in such tissue. The resulting methods and devices are useful for the targeted treatment of bone fractures, fractures at risk, delayed unions, nonunion of fractures, bone defects, spine fusions, osteonecrosis or avascular necrosis, as an adjunct to other therapies in the treatment of one or all of the above, in the treatment of osteoporosis, and in other conditions in which TGF-β1, β2, and / or β3 protein may be implicated.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Artificial crystalline len with transforming growth factor resistant beta2 antibody membrane on surface and manufacturing method thereof
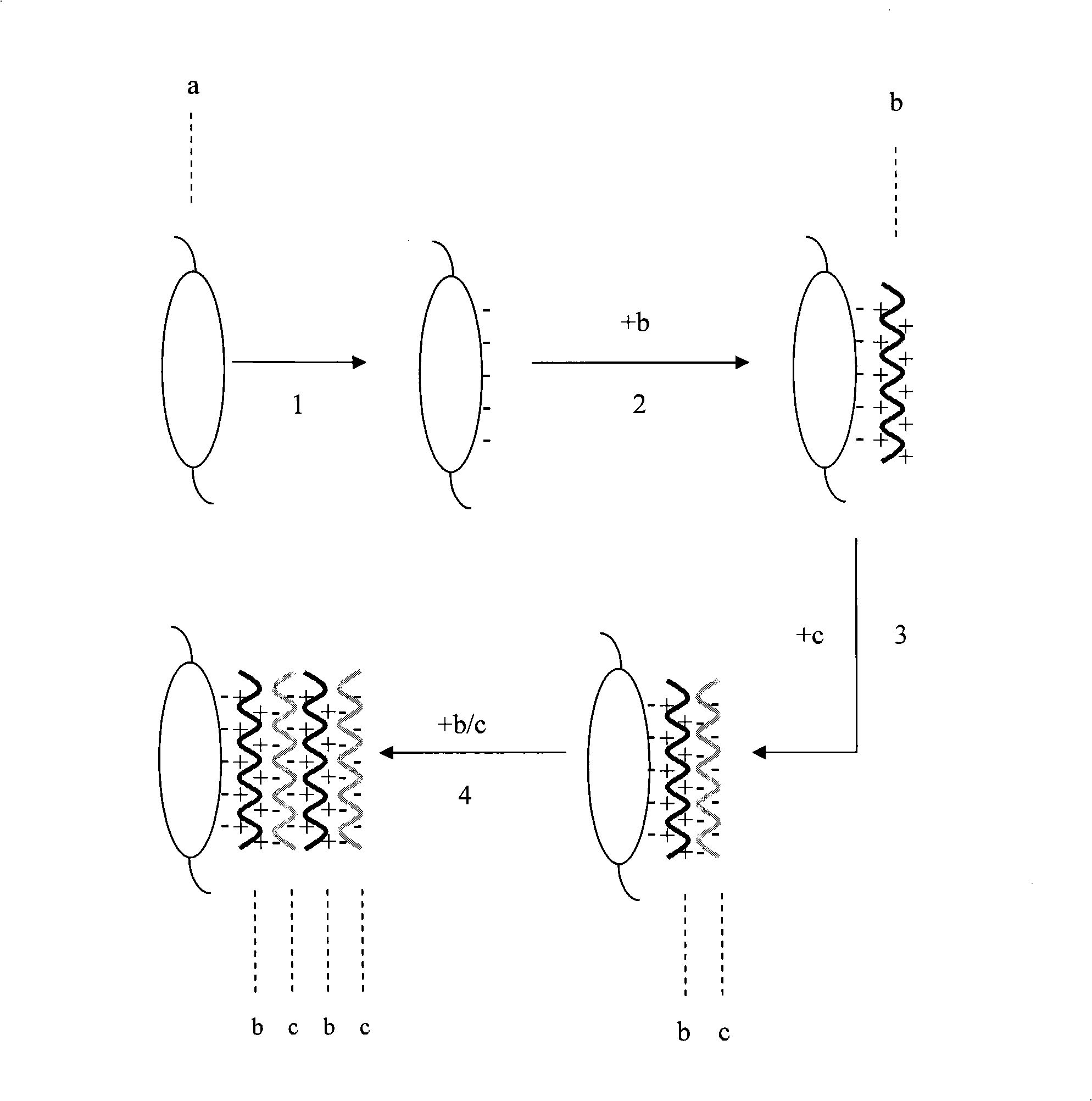
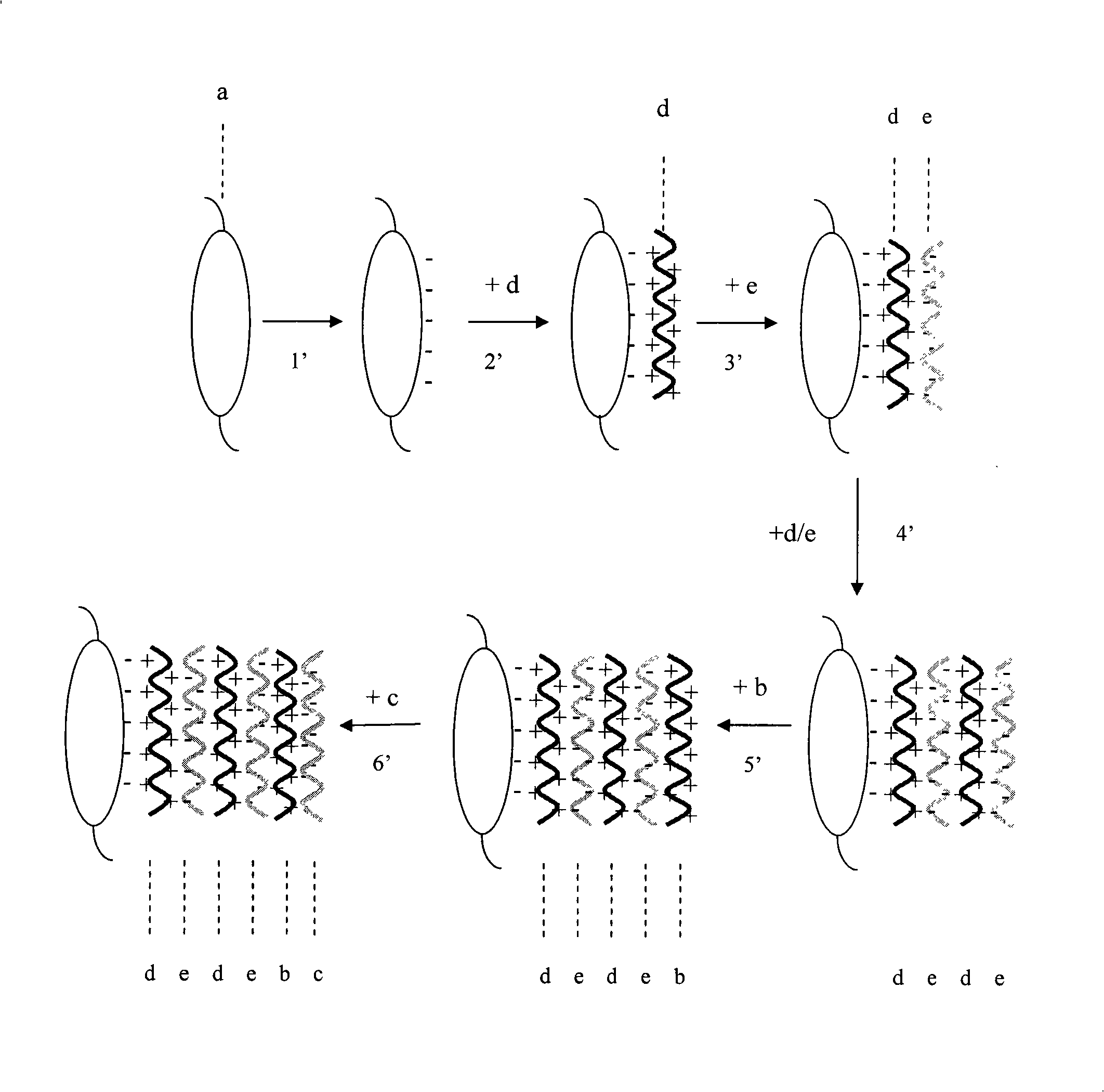
The invention provides an artificial lentis, which contains anti-transforming growth factor beta 2 antibody membrane on the surface and can inhibit intercurrent post-cataract after cataract surgery, and also provides a the production method thereof. The production method includes the steps that: the artificial lentis is charged with positive electricity or negative electricity after the artificial lentis is cleaned, dried, and pretreated on the surface; the artificial lentis is soaked in a polyelectrolyte solution the charge of which is opposite to the surface charge of the artificial lentis for adsorbing, and rinsing the artificial lentis by deionized water, and drying the artificial lentis by nitrogen gas; the artificial lentis is soaked in a phosphate buffering solution of anti-transforming growth factor beta 2 antibody, the pH value of which is 4-10, and the carried charge of which is opposite to that of the polyelectrolyte, for adsorption; finally, the artificial lentis is rinsed by phosphate buffering solution, and the artificial lentis is dried by nitrogen gas; the alternating assembly steps are repeated. The artificial lentis of the invention can inhibit the transformation and differentiation as well as cyst membrane shrinkage of the lentis epithelial cells in a target way, and then interdicts the occurrence of the post-cataract, and has excellent biocompatibility. The production method of the invention is scientific and simple, and can ensure the activity under a dry state and the safety and reliability during medical transplantation of the anti-transforming growth factor beta 2.
Owner:SECOND AFFILIATED HOSPITAL ZHEJIANG UNIV COLLEGE OF MEDICINE
Methods and compositions for expansion of stem cells and other cells
Presented herein are methods of generating a multipotent or immature cell from a mature somatic cell, involving contacting a mature somatic cell with one or more small molecule compounds selected from: a histone deacetylase (HDAC) inhibitor; a glycogen synthase kinase 3 (GSK-3) inhibitor; one or more transforming growth factor-beta receptor (TGF-βR) inhibitors; one or more lysine-specific demethylase 1 (LSD1) inhibitors; a cAMP agonist; a histone lysine methyltransferase (EZH2) inhibitor; and a histone methyltransferase (HMTase) G9a inhibitor; valproic acid. Also provided are methods of generating a multipotent or immature cell from a somatic cell, by driving expression of OCT4, or an OCT4 functional homolog or derivative, under the control of a high expressing promoter. Presented herein are also methods of stem cell expansion, stem cell regeneration and differentiation, which comprise contacting stem cells with one or more small chemical compounds.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Regulation of transforming growth factor-beta (TGF-beta) gene expression in living cells via the application of specific and selective electric and electromagnetic fields
InactiveUS20060235473A1ElectrotherapyMicrobiological testing/measurementHuman DNA sequencingElectromagnetic field
Methods and devices are described for the regulation of Transforming Growth actor (TGF)-β1, β2, and / or β3 protein gene expression in bone cells and other tissues via the capacitive coupling or inductive coupling of specific and selective electric fields to the bone cells or other tissues, where the specific and selective electric fields are generated by application of specific and selective electric and electromagnetic signals to electrodes or one or more coils or other field generating device disposed with respect to the bone cells or other tissues so as to facilitate the treatment of diseased or injured bone and other tissues. By gene expression is meant the up-regulation or down-regulation of the process whereby specific portions (genes) of the human genome (DNA) are transcribed into mRNA and subsequently translated into protein. Methods and devices are provided for the targeted treatment of injured or diseased bone and other tissue that include generating specific and selective electric and electromagnetic signals that generate fields in the target tissue optimized for increase of TGF-β1, β2, and / or β3 protein gene expression and exposing bone and other tissue to the fields generated by specific and selective signals so as to regulate TGF-β1, β2, and / or β3 protein gene expression in such tissue. The resulting methods and devices are useful for the targeted treatment of bone fractures, fractures at risk, delayed unions, nonunion of fractures, bone defects, spine fusions, osteonecrosis or avascular necrosis, as an adjunct to other therapies in the treatment of one or all of the above, in the treatment of osteoporosis, and in other conditions in which TGF-β1, β2, and / or β3 protein may be implicated
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Antagonizing TGF-beta activity with various ectodomains TGF-beta receptors used in combination or as fusion proteins
InactiveUS7795389B2Peptide/protein ingredientsFusions with soluble cell surface receptorAnti-Carcinogenic AgentsPharmacology
Anti-cancer agents and / or transforming growth factor beta (TGF-beta or TGFβ) antagonists are disclosed, where the agents and / or antagonists include a therapeutically effective amount of a combination of therapeutically active portions of sRII and therapeutically active portions of sRIII or a fusion polypeptide or protein comprising therapeutically active portions of sRII and therapeutically active portions of sRIII. Methods for preventing, treating and / or ameliorating the symptoms of cancer are also disclosed based on administering an effective amount of a composition of this invention.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
TGF-β1 specific antibodies and methods and uses thereof
ActiveUS9518112B2Less responseLess toxicityImmunoglobulins against growth factorsAntibody ingredientsDiseaseAnticarcinogen
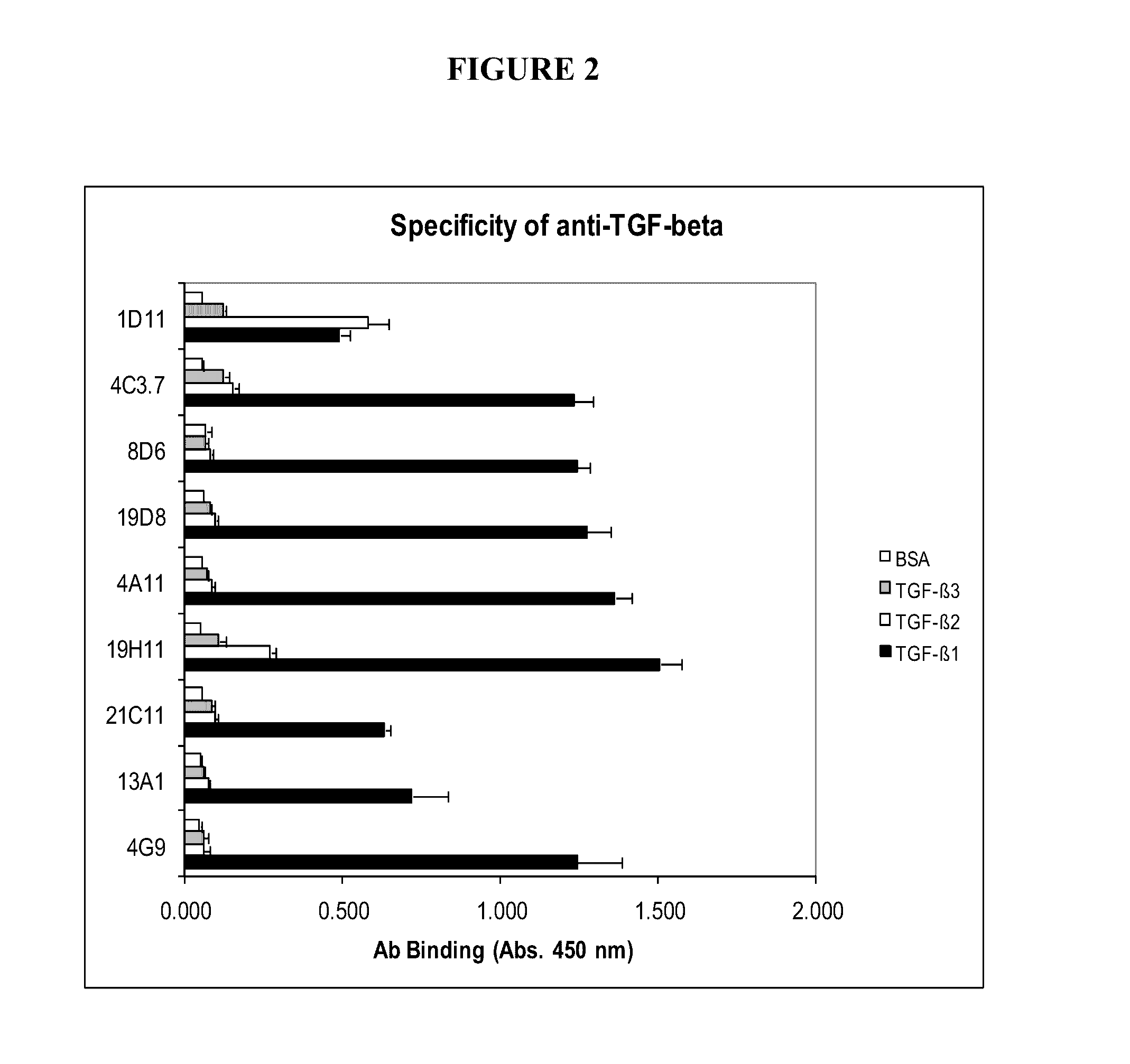
Specific binding members, particularly antibodies and fragments thereof, which bind to transforming growth factor beta 1 (TGF-β1) are provided, particularly recognizing human and mouse TGF-β1 and not recognizing or binding TGF-β2 or TGF-β3. Particular antibodies are provided which specifically recognize and neutralize TGF-β1. These antibodies are useful in the diagnosis and treatment of conditions associated with activated or elevated TGF-β1, including cancer, and for modulating immune cells and immune response, including immune response to cancer or cancer antigens. The anti-TGF-β1 antibodies, variable regions or CDR domain sequences thereof, and fragments thereof may also be used in therapy in combination with chemotherapeutics, immune modulators, or anti-cancer agents and / or with other antibodies or fragments thereof. Antibodies of this type are exemplified by the novel antibodies hereof, including antibody 13A1, whose sequences are provided herein.
Owner:LUDWIG INST FOR CANCER RES LTD
Inhibiting transforming growth factor beta to prevent accumulation of extracellular matrix
InactiveUS20040028682A1Connective tissue peptidesTetrapeptide ingredientsRESPIRATORY DISTRESS SYNDROME ADULTCell-Extracellular Matrix
The present invention provides a method for treating or arresting the progress of pathologies characterized by an accumulation of extracellular matrix components by providing an agent to suppress the activity of transforming growth factor beta (TGF-beta) a peptide growth factor which is anabolic and leads to fibrosis and angiogenesis. In one embodiment, such agent is anti-TGF-beta antibody. Pathologies which can be so treated include, but are not limited to, glomerulonephritis, adult respiratory distress syndrome and cirrhosis of the liver. The invention further provides a method for the diagnosis of pathologies, or incipient pathologies, which are characterized by the accumulation of extracellular matrix components in tissues by determining the levels of TGF-beta in the tissues, a high level being indicative of such pathologies.
Owner:BORDER WAYNE A +1
Therapeutic compositions and methods using transforming growth factor-beta mimics
The invention provides methods, compositions, and kits for treating a variety of conditions using substances that display one or more activities of transforming growth factor-β (TGF-β mimics). Conditions include skin conditions, wounds, and the need for soft tissue augmentation. Compositions that may be used to treat such conditions contain a TGF-β mimic. Kits that may be used to treat such conditions contain a TGF-β mimic.
Owner:BHATNAGAR RAJENDRA
Transforming growth factor beta crystals
InactiveUS6294656B1Improve concentrationPrevent penetrationPeptide/protein ingredientsMicroorganism based processesOxidizing agentCrystallization
The invention concerns TGF-beta in a crystalline form which shows no adsorption or less adsorption to the walls of vials than the soluted TGF-beta and which is more stable towards oxidative agents than the soluted form. TGF-beta crystals of the invention can be used for structure determination and for drug design and for the production of a slow release pharmaceutical preparation.
Owner:NOVARTIS AG
Human skin epidermal cell culture medium and application thereof
InactiveCN105950542AShorten the timeMeet the needs of clinical treatmentCulture processEpidermal cells/skin cellsSerum free mediaCell culture media
The invention provides a human skin epidermal cell culture medium and application thereof. The culture medium is prepared by the following steps: mixing a K-SFM (keratinocyte serum-free medium), a DMEM (dulbecco's modified eagle medium) and a F12 culture medium according to the ratio of 2:1:1, and adding a BPE (bovine pituitary extract), an EGF (epidermal growth factor), an SCGF (stem cell growth factor), an FGF (fibroblast growth factor), a TGF-beta (transforming growth factor-beta), a VEGF (vascular endothelial growth factor), CaCl2, glutamine and a double-antibody. The culture medium is free of serum, and can be used for human skin epidermal cell primary culture and subculture. The human skin epidermal cell culture medium can obtain abundant epidermal cells by in-vitro culture by using only a small amount of patient skin tissues. Compared with the traditional epidermal cell culture medium, the human skin epidermal cell culture medium provided by the invention greatly shortens the time required by amplifying the same cell count, and satisfies the demands for clinical therapy.
Owner:JINAN PANSHENG BIOTECH
Adult regulatory T cell in-vitro amplification culture medium and application method thereof
ActiveCN104278012AHigh amplification rateReduce oxidative damageBlood/immune system cellsDiseaseImmunologic disorders
The invention provides an adult regulatory T cell in-vitro amplification culture medium and an application method thereof. The adult regulatory T cell in-vitro amplification culture medium comprises transform growth factor-beta (1-4 ng / ml), bone morphogenesis protein 4 (8-12 ng / ml), recombinant human interleukin-2 (300-500U / ml), rapamycin (80-100nM / ml), al-trans vitamin A acid (1-4 uM / mL), 4-hydroxyethylpiperazinoethylsulfonic acid (20-30mM / ml), L-glutamine (2mM / ml), 2-mercaptoethanol (40-50uM / ml), 5% human AB type serum, CD3CD28 magnetic bead, penicillin (50U / ml) and streptomycin (50ug / ml). When being used for performing amplification culture and induced differentiation on regulatory T cells, the culture medium can shorten the amplification and differentiation time of the regulatory T cells, and can obtain the high-purity Foxp3CD4+CD25+CD127-regulatory T cells. Therefore, the regulatory T cells can be used in the aspect of clinical treatment of anti-transplantation immunity rejection, autoimmune disease, allergic disease and the like to perform a novel clinical cell therapy.
Owner:HUNAN XENO LIFE SCI
Separation and cultivation method of human adipose stem cells
ActiveCN108220230ANormal growthNormal metabolismCell dissociation methodsCulture processSerum free mediaParenchyma
The invention discloses a separation and cultivation method of human adipose stem cells. The method comprises the following steps: first, digesting a fat suction matter by using a Liberase digestive enzyme solution, neutralizing digestive enzyme after digestion is ended, centrifuging, filtering, and further removing parenchyma cells by using a lymphocyte separation solution so as to obtain the human adipose stem cells; second, cultivating the human adipose stem cells by using a serum-free medium, wherein following components are added in the serum-free medium: a recombinant human epidermal growth factor, a recombinant human basic fibroblast growth factor, a recombinant human transforming growth factor-beta, a recombinant human platelet-derived growth factor-BB, a recombinant human stem cell factor, reduced glutathione, coenzyme A, biotin, MEM vitamin solution, MEM amino acid solution, MEM non-essential amino acid solution, a GlutaMAX additive, insulin-transferrin-selenium solution andgentamicin. The method can remarkably increase the yield of adipose stem cells and improve the activity of the adipose stem cells, so as to obtain more adipose stem cells with proliferation ability.
Owner:SHANGHAI LIFE SCI & TECH CO LTD
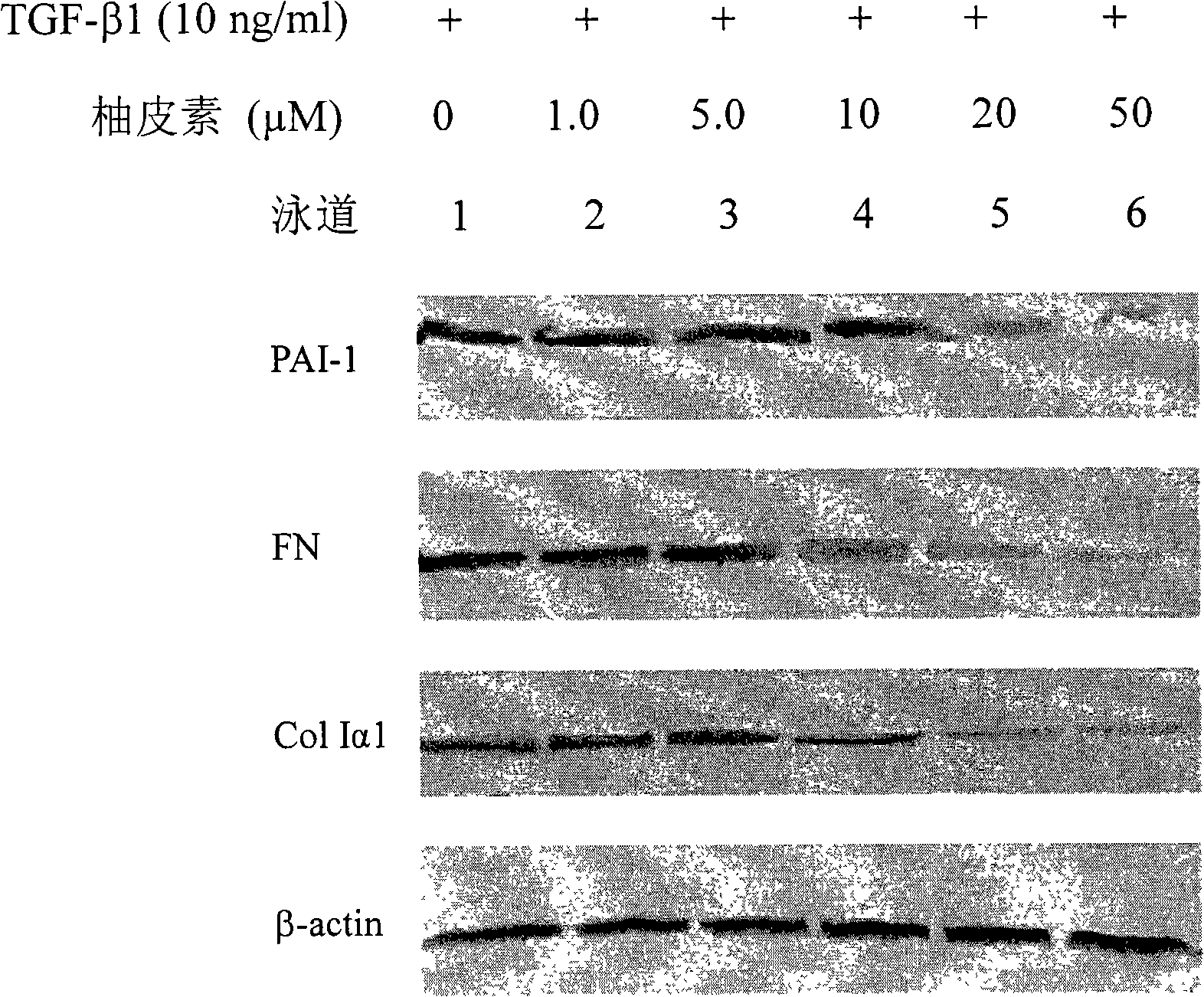
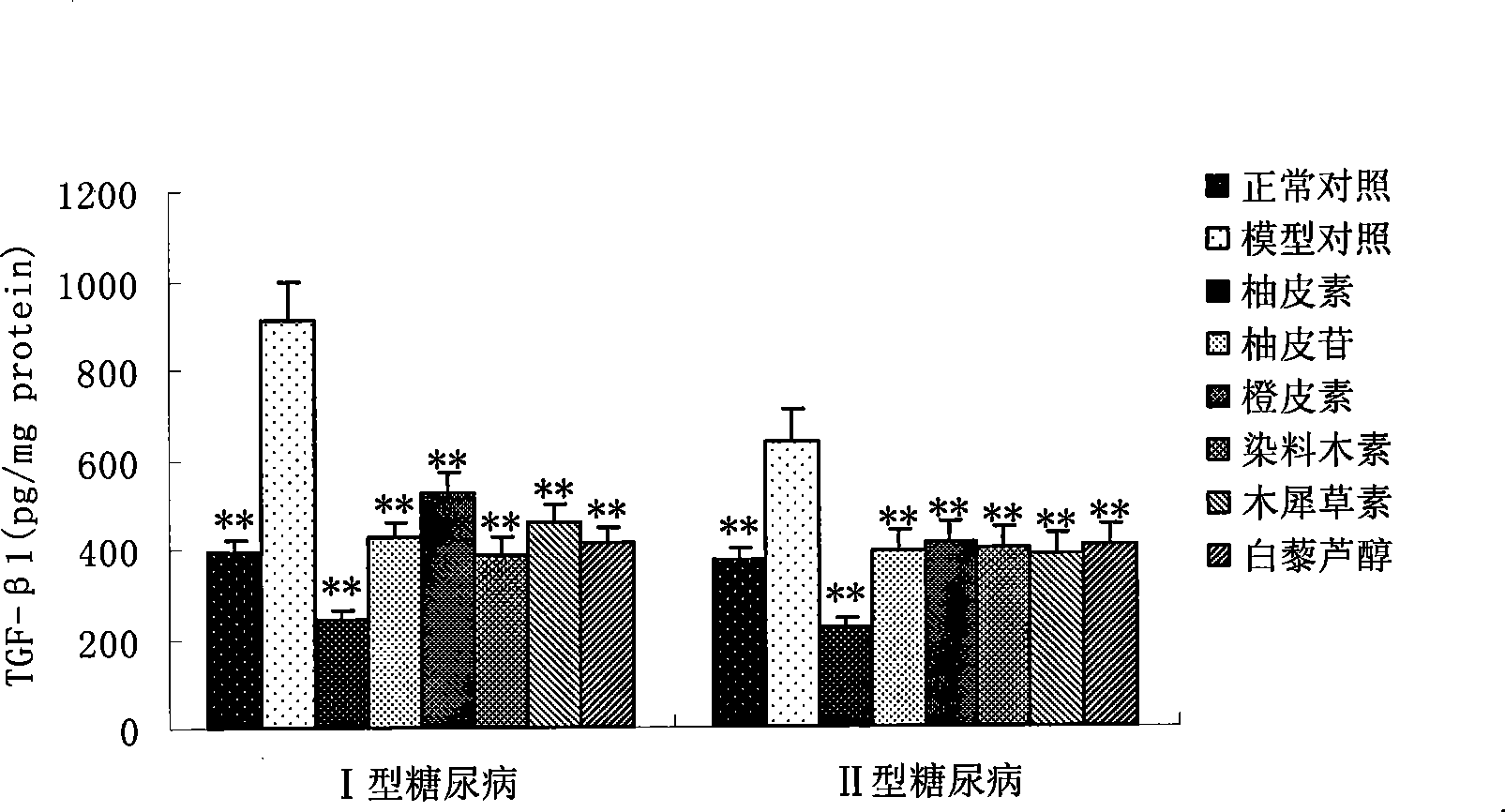
Applications of naringenin and naringin as signal pathway inhibitor of transforming growth factor-beta 1
ActiveCN101322700AReduce liver fibrosisReduce pulmonary fibrosisOrganic active ingredientsDigestive systemNaringinFibrosis
The invention discloses the application of naringenin and aurantiin as the inhibitor of the signal passage of transforming growth factor-Beta1, in particular the application to the treatment or prevention of fibrosis and tumors.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES +1
High-efficiency non-integrated human iPSC induction platform
ActiveCN104673741AImprove induction efficiencyEasy to separateVertebrate cellsArtificial cell constructsAdenosineSomatic cell
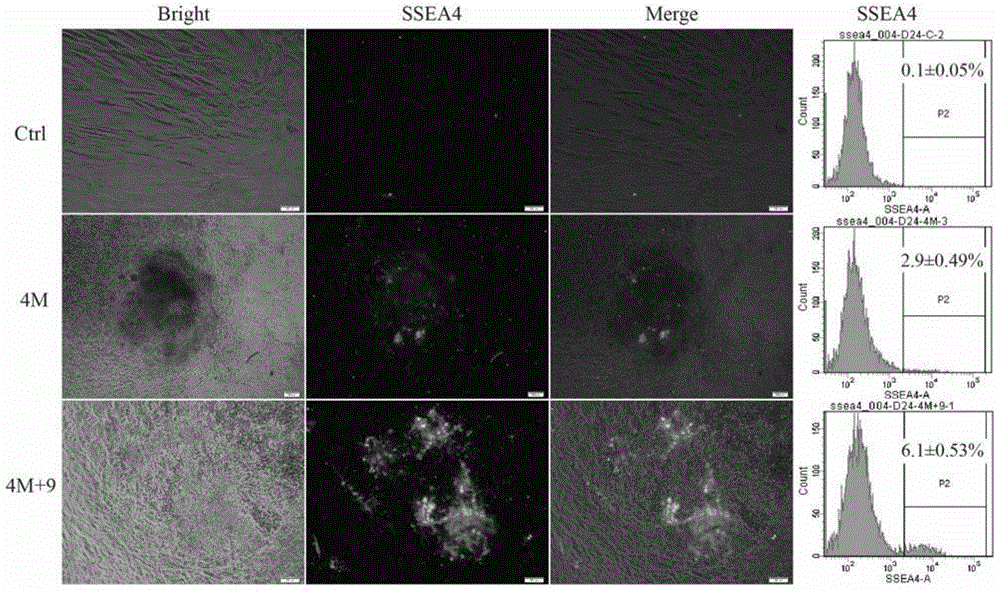
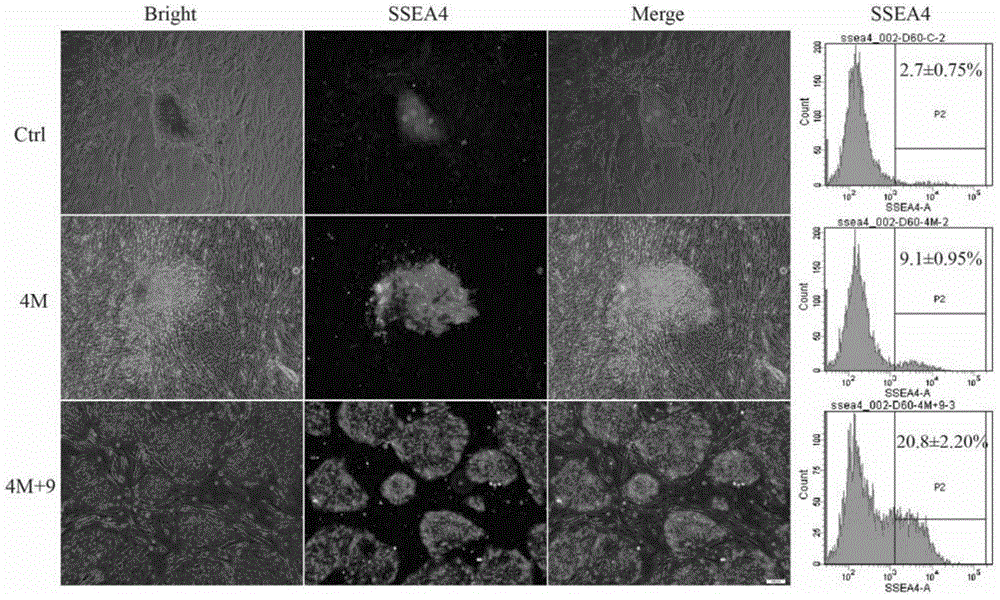
The invention discloses a high-efficiency non-integrated human iPSC induction platform which comprises a composition, wherein the composition is used for inducing a human cell into iPSC, and the composition comprises a previous inducer and a later inducer; the previous inducer comprises the following active components: a transforming growth factor beta inhibitor, a glycogen synthetase kinase 3 inhibitor, a cAMP agonist, an S-adenosyl homocysteine hydrolase inhibitor and a p21 activated kinase inhibitor; and the later inducer comprises the following active components: the glycogen synthetase kinase 3 inhibitor, a selective ATP noncompetitive MEK inhibitor and the S-adenosyl homocysteine hydrolase inhibitor. The high-efficiency non-integrated human iPSC induction platform disclosed by the invention achieves the previous induction efficiency of the iPSC of 6.4% under the action of a previous inducer composition, can achieve the induction of a full culture of 20.8% through later induction culture or be used for totally further inducing a previous inductor clone into a human iPSC clone approaching to ESC and is far higher than the prior art in induction efficiency; in addition, the obtained iPSC is high in maturity, free of being inserted with an exogenous gene, is more approaching to the ESC in cell morphology and property and has the advantages of good stability and great high application value.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
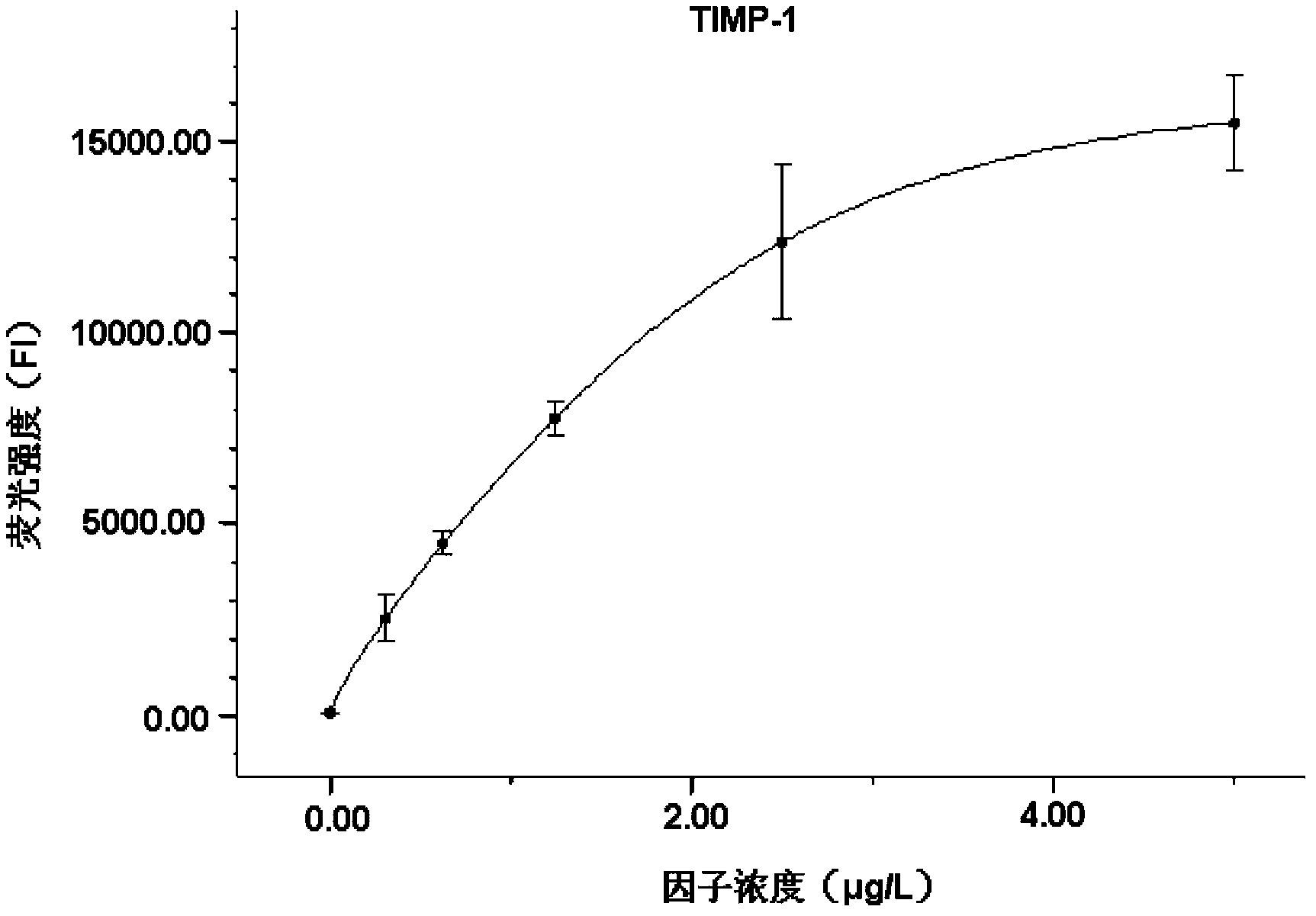
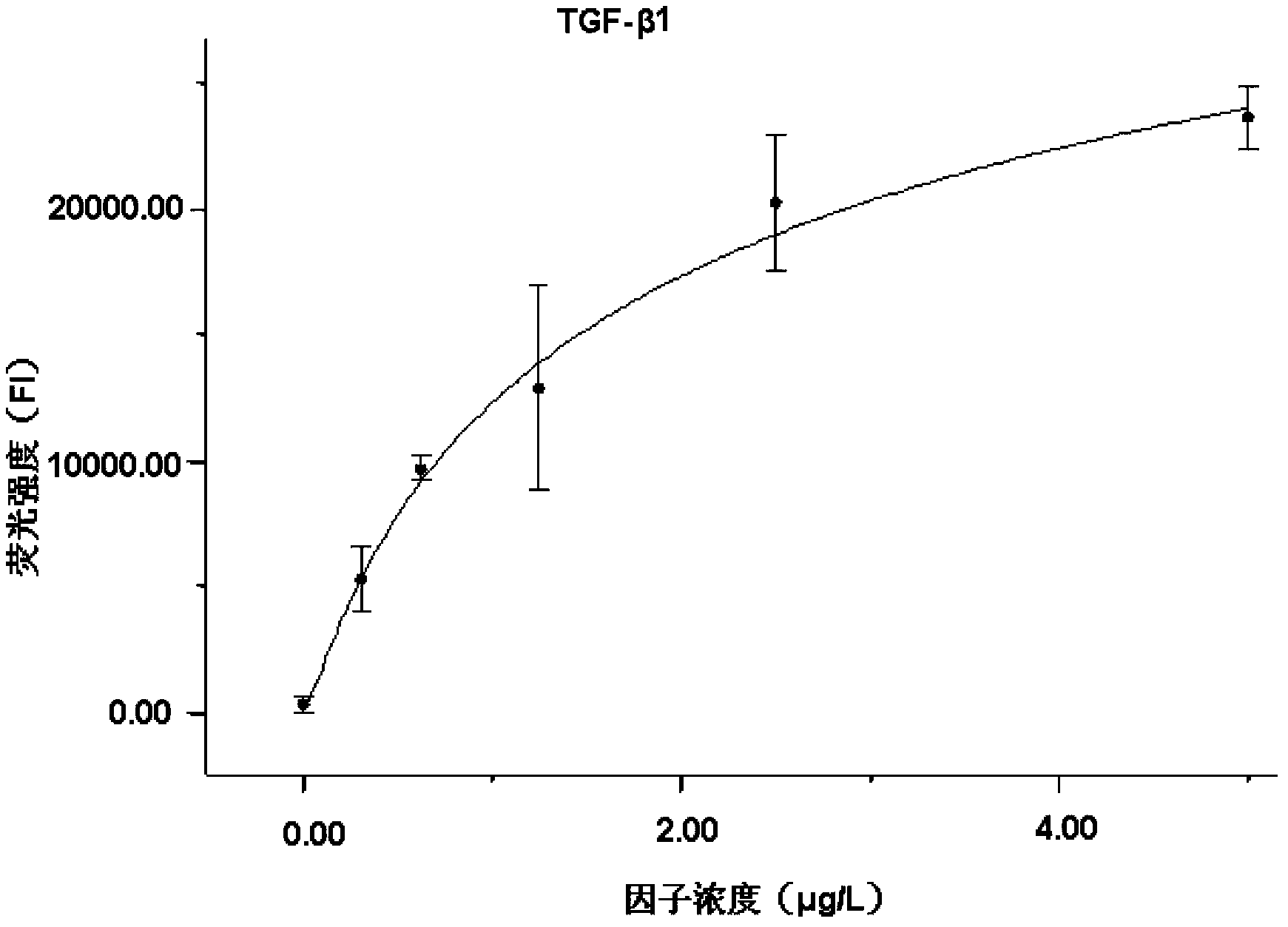
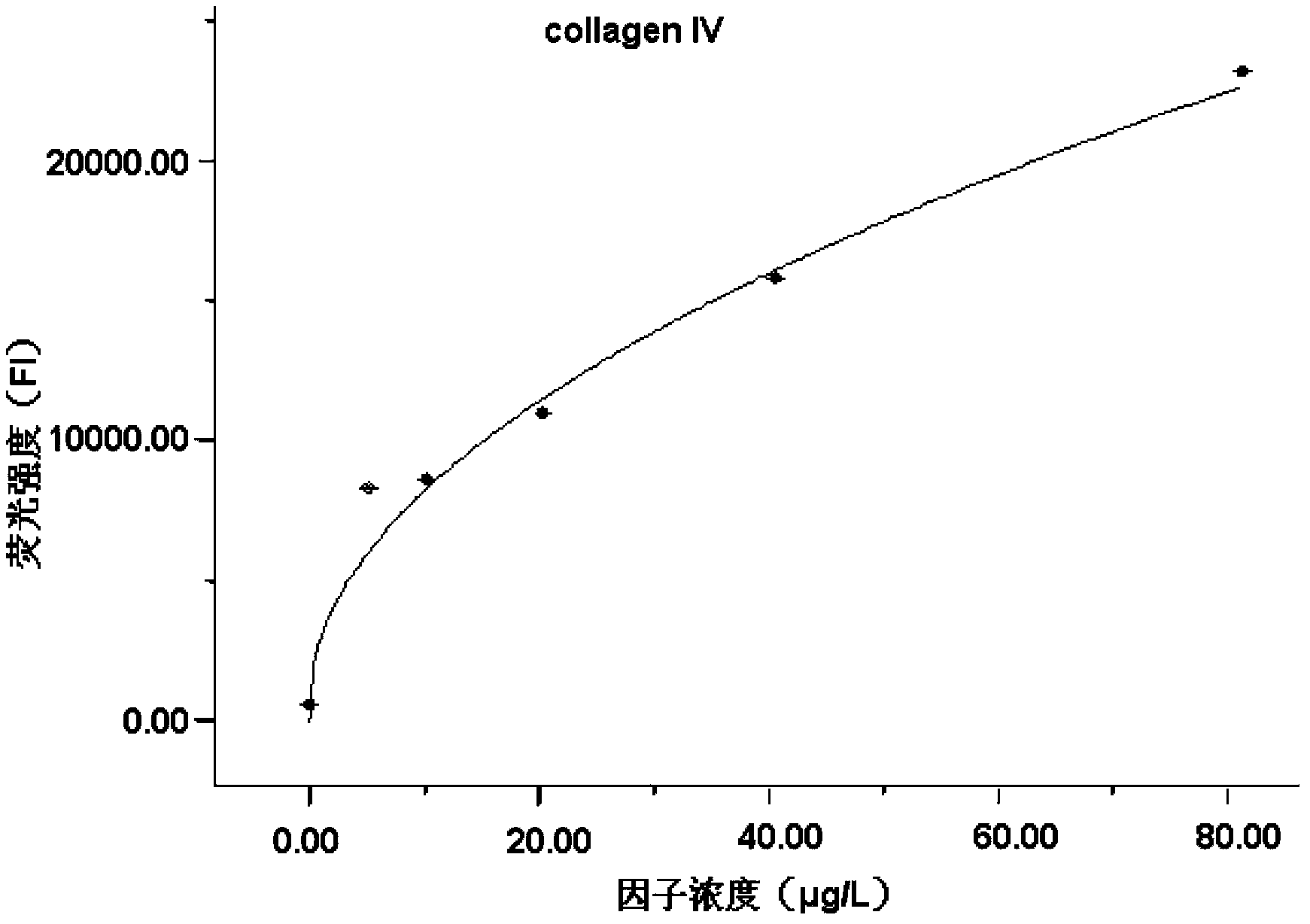
Liquid protein chip kit for detecting liver fibrosis degree
InactiveCN103399158AShorten detection timeSerum sample volume is smallBiological testingFluorescence/phosphorescenceTumor necrosis factor alphaPlatlet-Derived Growth Factor
The invention provides a liquid protein chip kit for detecting a liver fibrosis degree. In the liquid protein chip kit, coupling antibody microspheres comprise 1, fluorescent microspheres coupling with procollagen peptide III and / or a metalloprotease tissue inhibitor factor-1 capture antibody, 2, one or more of fluorescent microspheres coupling with collagen peptide IV, fibronectin, a transforming growth factor-beta 1, a platelet-derived growth factor, a tumor necrosis factor alpha capture antibody, and an angiotensin II capture antibody, and 3, fluorescent microspheres coupling with a serum hyaluronic acid capture antibody and / or a laminin capture antibody. The liquid protein chip kit can realize combined detection of a plurality of liver fibrosis-related markers for reflection of different liver fibrosis stages, can shorten detection time to more than ten minutes from a few hours, can finish detection of a plurality of markers only by 1 microliter of a serum sample, can be operated simply and has a low diagnosis cost.
Owner:SHANGHAI TONGJI HOSPITAL
Low-density-tolerant feeder-layer-free human pluripotency stem cell culture medium
ActiveCN106032527AProliferate fastLow costArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsMagnesium phosphateReprogramming
The present invention discloses a feeder-layer-free human pluripotency stem cell culture medium, which contains L-ascorbate-2 magnesium phosphate, sodium selenite, recombinant human insulin, human apotransferrin, basic fibroblast growth factor, transforming growth factor beta 1, heparin lithium and / or heparin sodium, a DMEM / F-12 base culture medium and an osmotic pressure regulator. According to the present invention, with the culture medium, when the hiPSC culture is performed in low density and normal density, the proliferation rate is high, and the cell morphology and the pluripotency are well maintained; the use of the expensive heparitin sulfate is not required so as to substantially reduce the cost; and almost all of the hiPSCs maintaining cultures at the current stage can be met, and various small molecule compounds are added to perform induction reprogramming culture (removal of TGFB1 and DM), such the culture medium is suitable for extensive researches of various basic scientific researches and clinical scientific research experiments.
Owner:FUTURE HOMO SAPIENS INST OF REGENERATIVE MEDICINE CO LTD (FHSR)
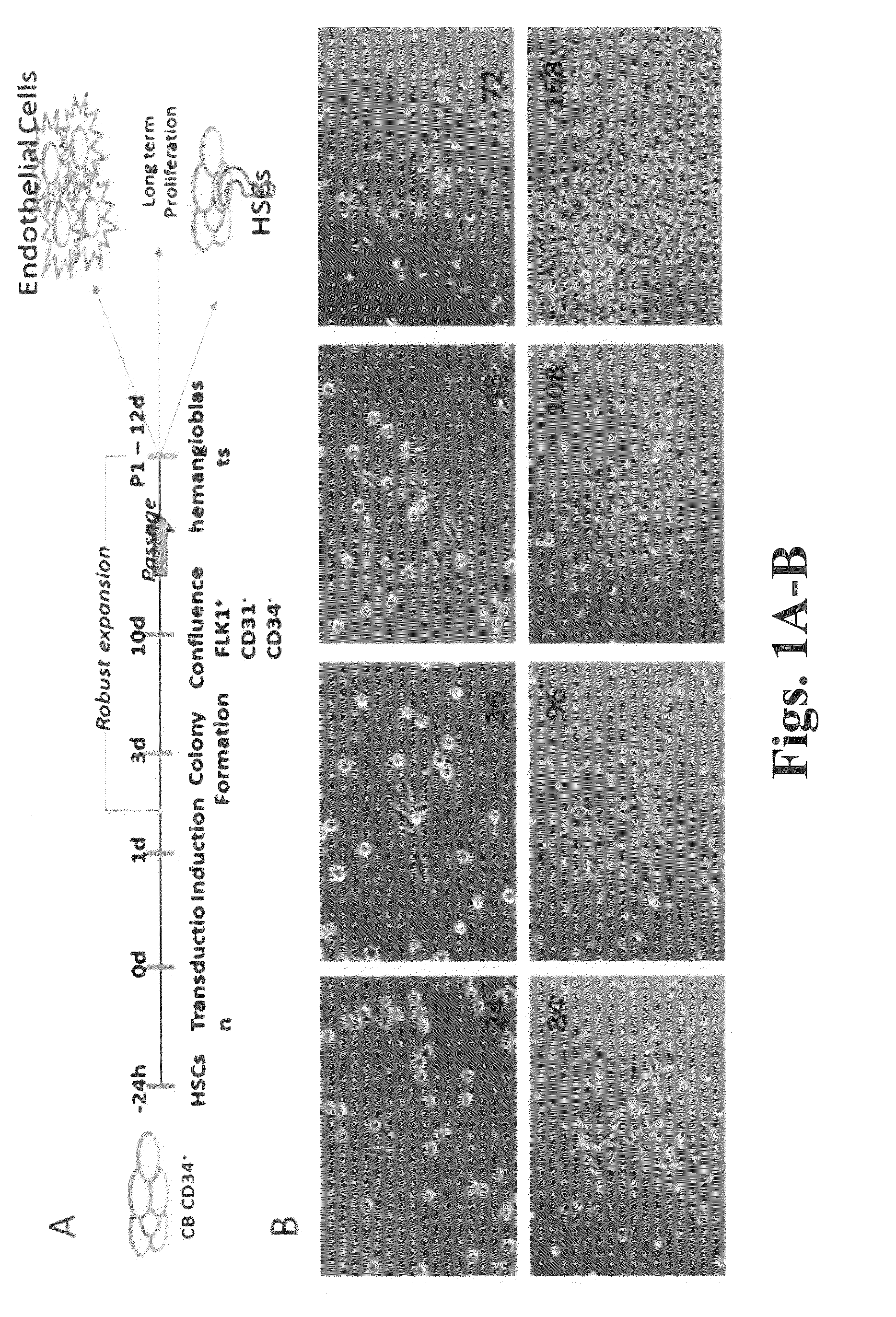
Culture method of vascular endothelial progenitor cells
ActiveCN105062957AExcellent self-renewal abilityAvoid uncertaintyVertebrate cellsArtificial cell constructsProgenitorSodium bicarbonate
The invention discloses a culture method of vascular endothelial progenitor cells, which comprises the following steps: 1) inducing differentiation of multipotent stem cells in a culture medium A to form mesendoderm precursor cells; and 2) inducing differentiation of the mesendoderm precursor cells in a culture medium B to form the vascular endothelial progenitor cells. The culture medium A contains a DMEM (dulbecco's modified eagle medium) culture medium, an F12 culture medium, sodium selenate, sodium bicarbonate, vitamin C, insulin, activin A, a bone formation protein 4 and a glycogen synthetase kinase-3 inhibitor. The culture medium B contains a DMEM culture medium, an F12 culture medium, vitamin C, insulin, a vascular endothelial growth factor and a transforming growth factor beta signal pathway inhibitor. The culture media used in the culture method do not contain any animal-derived component, and can provide sufficient nutrient substances and stable living environments for the cultured cells, thereby efficiently inducing the differentiation of the human multipotent stem cells to form the vascular-grid vascular endothelial progenitor cells which are highly similar to the human cord vessel endothelial cells.
Owner:宁波医诺生物技术有限公司
Use of resveratrol or naringenin in preventing and treating diabetic nephropathy
The invention discloses the application of resveratrol and naringenin, particularly the application of the resveratrol and the naringenin in restraining the level increasing of the transforming growth factor Beta 1 in the human body and preparing medicines used for preventing or treating diabetes and nephropathy.
Owner:ZHAOMING ZEKANG (BEIJING) BIO-PHARM TECH CO LTD
Chemical reprogramming of human glial cells into neurons for brain and spinal cord repair
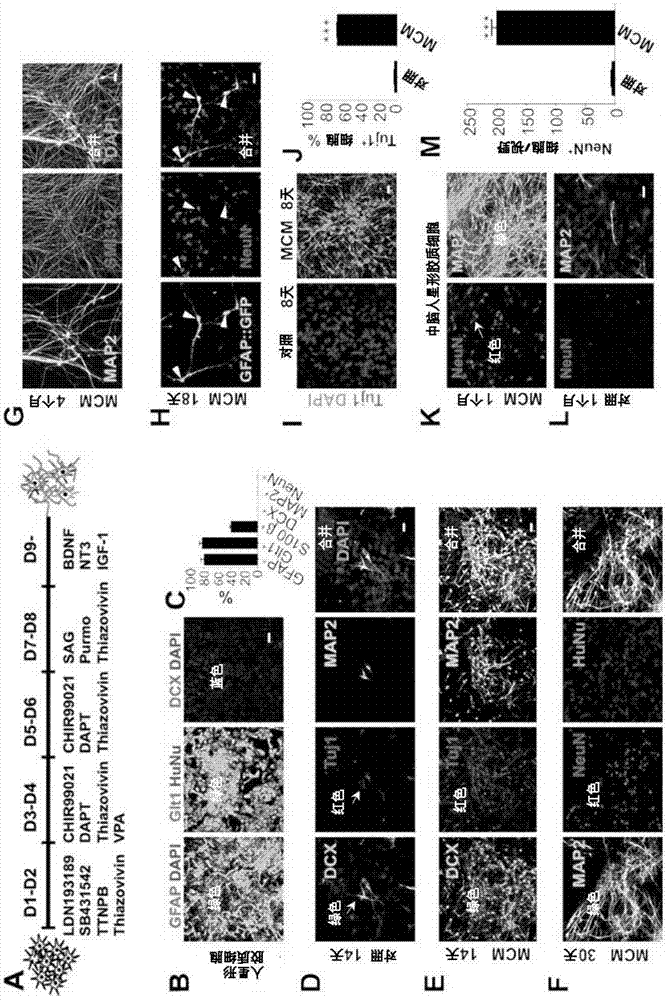
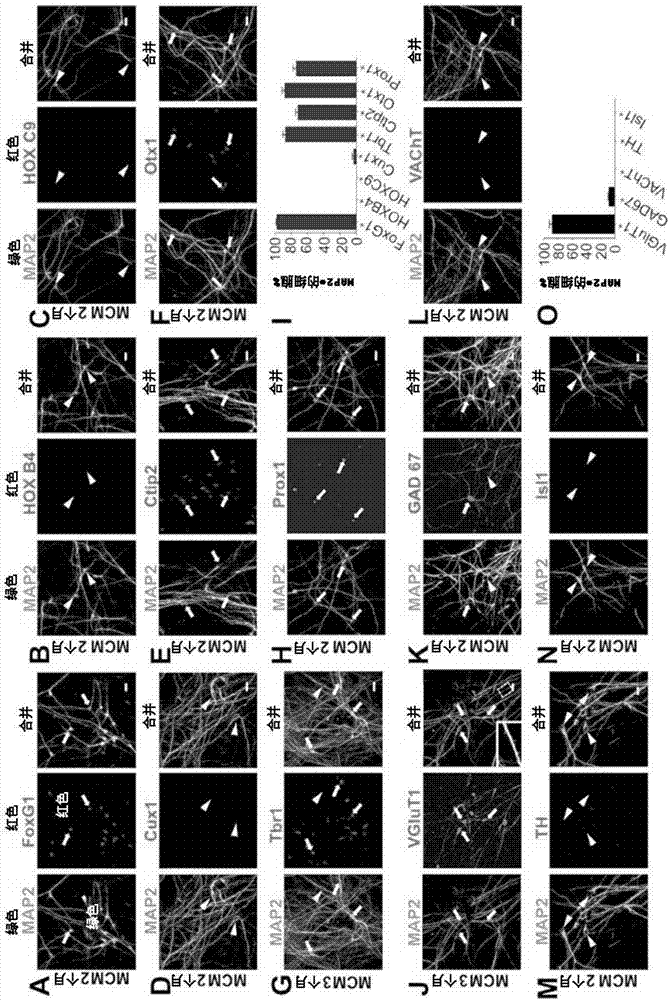
Provided are methods and compositions from reprogramming human glial cells into human neurons. The reprogramming is achieved using combinations of compounds that can modify signaling via Transforming growth factor beta (TGF-beta), Bone morphogenetic protein (BMP), glycogen synthase kinase 3 (GSK-3), and gamma-secretase / Notch pathways. The reprogramming is demonstrated using groups of three or four compounds that are chosen from the group thiazovivin, LDN193189, SB431542, TTNPB, CHIR99021, DAPT, VPA, SAG, purmorphamine. Reprogramming is demonstrated using the group of LDN193189 / CHIR99021 / DAPT, the group of B431542 / CHIR99021 / DAPT, the group of LDN193189 / DAPT / SB431542, the group of LDN 193189 / CHIR99021 / SB431542, a three drug combination of SB431542 / CHIR99021 / DAPT. Reprogramming using functional analogs of the compounds is also provided, as are pharmaceutical formulations that contain the drug combinations.
Owner:PENN STATE RES FOUND
Vascular endothelial cell culture method
ActiveCN104928230AExcellent self-renewal abilityAvoid uncertaintyArtificial cell constructsVertebrate cellsProgenitorSodium bicarbonate
The invention discloses a vascular endothelial cell culture method. The method comprises the following steps: 1) differentiating pluripotent stem cells into mesendoderm precursor cells in a culture medium A; 2) differentiating the mesendoderm precursor cells into progenitor cells of vascular endothelial cells in a culture medium B; 3) differentiating the progenitor cells of the vascular endothelial cells into the vascular endothelial cells in a culture medium C. The culture medium A contains a DMEM, an F12 culture medium, sodium selenate, sodium bicarbonate, vitamin C, insulin, an activin A , bone morphogenetic protein-4 and a glycogen synthase kinase-3 inhibitor; the culture medium B contains a DMEM, an F12 culture medium, sodium selenate, sodium bicarbonate, vitamin C, insulin, a vascular endothelial growth factor and a transforming growth factor beta signaling pathway inhibitor; the culture medium C contains a DMEM, an F12 culture medium, sodium selenate, sodium bicarbonate, vitamin C, insulin, a vascular endothelial growth factor, an epidermal growth factor and a fibroblast growth factor. According to the method, the pluripotent stem cells can be differentiated into the vascular endothelial cells.
Owner:宁波医诺生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com