Adult regulatory T cell in-vitro amplification culture medium and application method thereof
A medium and regulation technology, applied in the field of cell culture, can solve the problems of low cell proliferation ability, small number, low purity, etc., and achieve the effect of high expansion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Culture medium of the present invention and culture method test
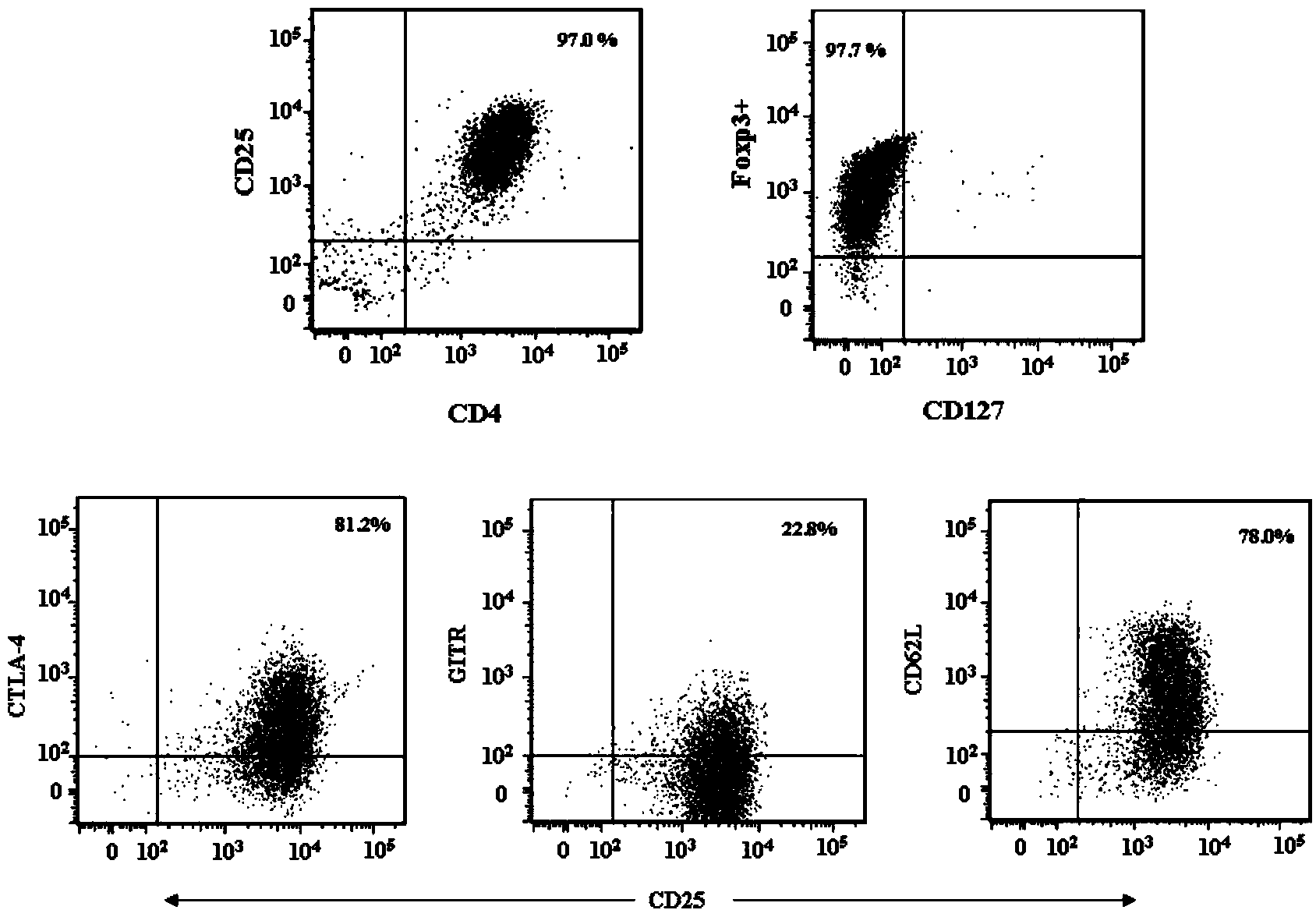
[0032] In the present invention, Treg cells are isolated from adult peripheral blood in vitro and cultured, and the initial Foxp3CD4+CD25+CD127-regulatory T cells and CD3CD28 magnetic beads are mixed in a ratio of 1:3 (CD3CD28 magnetic beads are purchased from Invitrogen Corporation ), add transforming growth factor-β (TGF-β) 1-4ng / ml (preferably 2ng / ml), bone morphogenetic protein 4 (BMP-4) 8-12ng / ml (preferably 10ng / ml) in the medium without phenol red 1640 / ml), all-trans retinoic acid (ATRA) 1-4uM / mL (preferably 2uM / mL), recombinant human interleukin-2300-500U / ml, rapamycin 80-100nM (preferably 100nM), 4-hydroxyethyl Piperazine ethanesulfonic acid 20-30mM (preferably 25mM), L-glutamine 2mM, 2-mercaptoethanol 40-50uM (preferably 50uM), human AB serum 5%, penicillin 50U / ml, streptomycin 50ug / ml.
[0033] Resuspend the isolated starting cells in the above medium and place them in a U-bott...
Embodiment 2
[0035] Example 2 Comparison of the culture effects of the culture medium of the present invention and the common regulatory T cell culture medium of the present invention without adding transforming growth factor-β, bone morphogenetic protein-4 and all-trans retinoic acid.
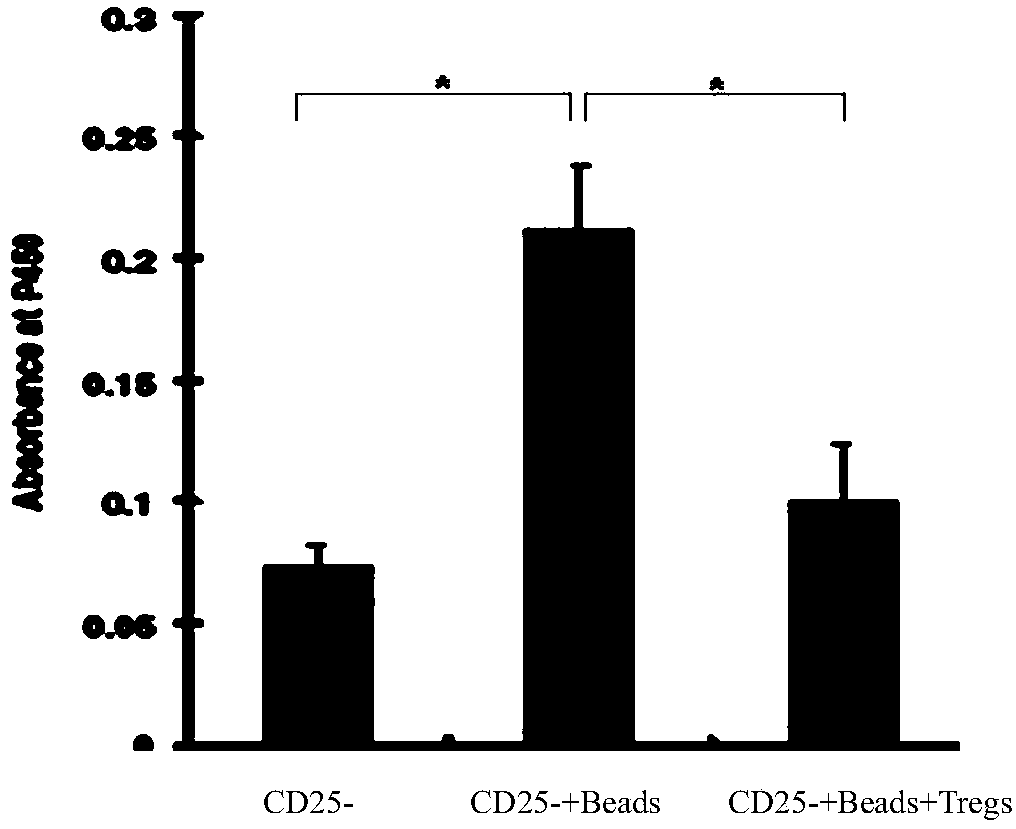
[0036] Peripheral blood was collected from adult lupus erythematosus patients who voluntarily donated blood. Systemic lupus erythematosus is a typical autoimmune disease. Although the etiology is complex, immune regulatory dysfunction plays an important role in the pathogenesis of lupus. Due to the impairment of the body's immune regulation function, normal autoimmune tolerance cannot be maintained, so many self-antigens become target antigens, autoreactive T cells and B cells increase, and excessive autoantibodies are produced, resulting in damage to organs and tissues. Compared with normal people, the ratio of regulatory T cells is lower, and the level of TGF-β is lower than normal people. Therefore, the...
Embodiment 3
[0040] Example 3 Comparison of the culture effects between the medium of the present invention and the common regulatory T cell medium of the present invention without adding bone morphogenetic protein-4 and all-trans retinoic acid.
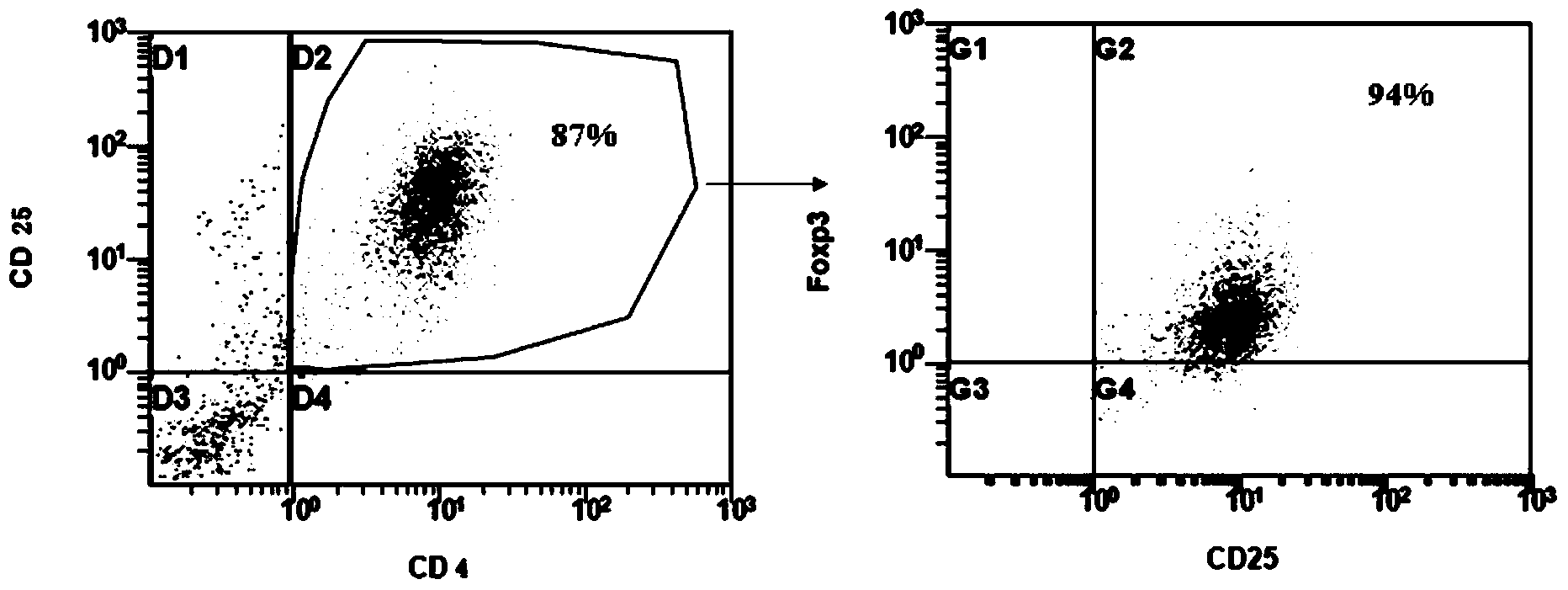
[0041] Similarly, Foxp3CD4+CD25+CD127-regulatory T cells were isolated from the peripheral blood of adult lupus erythematosus patients, and the culture method was as in Example 1. After three weeks of culture, the present invention had 20 more cells than the culture medium without BMP-4 and ATRA. times,
[0042] The purity ( Figure 4 ) compared with the purity of normal culture medium ( Figure 5 ) high, CD4+CD25+T cells 94.3% after culture in the present invention, among them the cells of Foxp3+ expression in the double-positive cells are 98.7% ( Figure 4 ), 90.8% of CD4+CD25+ T cells cultured in the medium without BMP-4 and ATRA, among which the cells expressing Foxp3+ in the double positive cells were 93.0% ( Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com