Chemiluminescence immune analytic reagent kit for detecting thyroid peroxidase autoantibody
A chemiluminescent immunoassay and peroxidase technology, applied in the field of immunoassay, can solve the problem of low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of TPOAb chemiluminescence immunoassay assay kit of the present invention
[0021] 1. Preparation of TPOAb calibrator
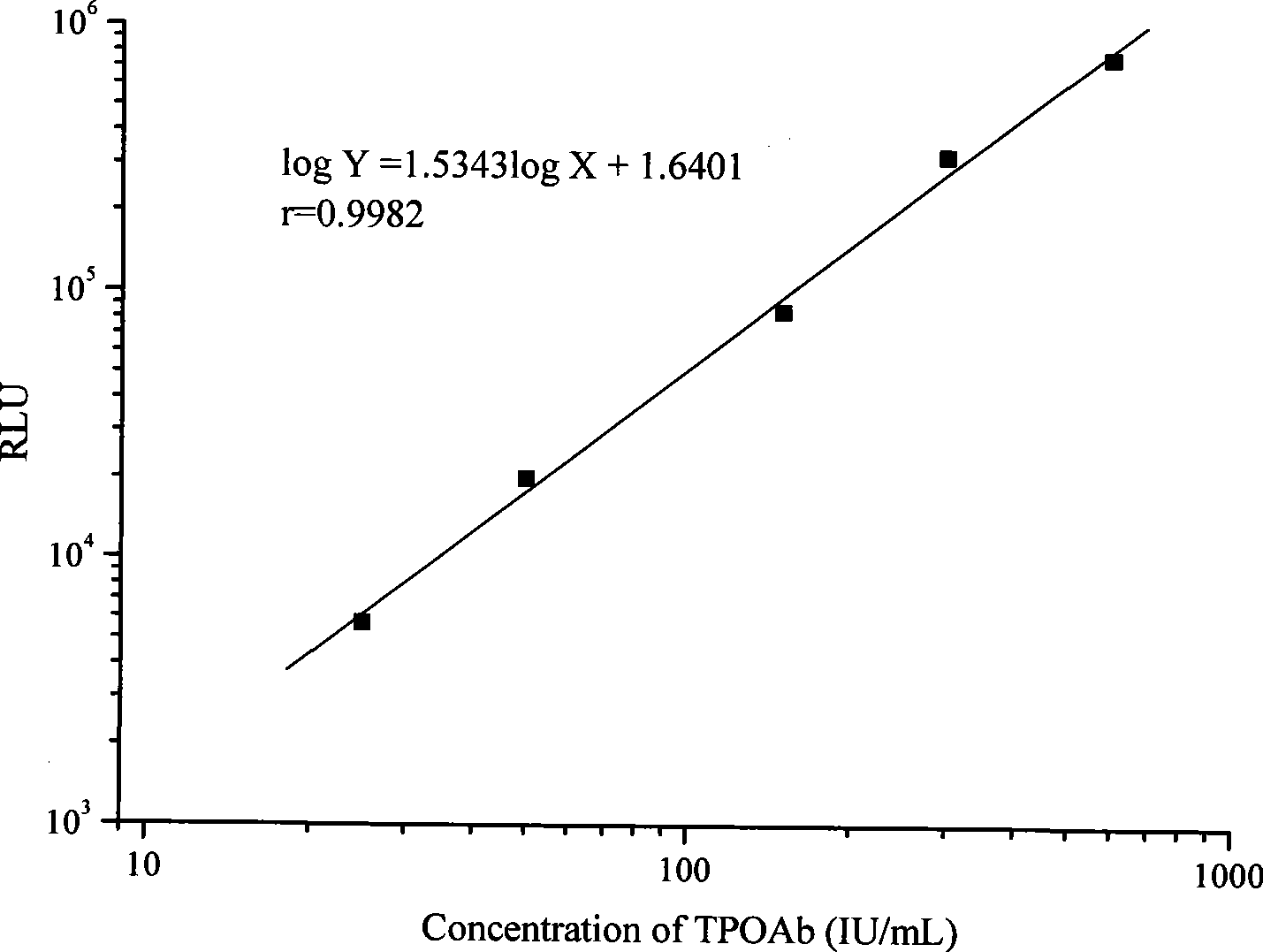
[0022] Pure TPOAb was diluted into calibrator with horse serum, the concentrations were 0, 25, 50, 150, 300, 600 IU / mL.
[0023] 2. Preparation of TPO antigen-coated microwell plates
[0024] a) coated
[0025] Mix 0.05M PBS buffer solution with pH7.2 and appropriate concentration of thyroid peroxidase antigen to make coating solution, and load it on a luminescent microwell plate;
[0026] b) washing the above-mentioned microwell plate with a washing solution; and
[0027] c) closed
[0028] Prepare hydrolyzed gelatin blocking solution, based on 1000mL of the blocking solution contains: 1.28mMNaH 2 PO 4 2H 2 O, 8.10 mM Na 2 HPO 4 12H 2 O, 1.0% hydrolyzed gelatin, 0.1% Proclin300, the pH is 7.2-7.4, and then the obtained blocking solution is loaded on the above-mentioned washed microporous plate for sealing for 2 hours, t...
Embodiment 2
[0062] Embodiment 2 The usage method of kit of the present invention
[0063] 1. Sample pretreatment
[0064] The fasting morning serum samples were taken from people, centrifuged at 3000rpm for 5min, and the upper serum was taken for analysis.
[0065] 2. Detection method
[0066] Follow the steps below to use this kit:
[0067] 1) Preparation of washing buffer: dilute the washing buffer provided in the kit with distilled water 25 times;
[0068] 2) Take the calibrator, coated microwell plate, biotin marker and enzyme marker out of the refrigerator at 4°C, place it for 20min, and equilibrate to room temperature;
[0069] 3) Add 0, 25, 50, 150, 300, 600 IU / mL of 0, 25, 50, 150, 300, 600 IU / mL to the reaction wells respectively, and add 1 blank well for each test, and then add Biotin labeling solution 100μL, react at 37℃ for 30min;
[0070] 4) Discard the solution in the well, wash 5 times with the diluted washing buffer, and pat dry on absorbent paper;
[0071] 5) Add 10...
Embodiment 3
[0077] Embodiment 3 The methodological test of the kit of the present invention
[0078] The test kit prepared in Example 1 is tested according to the conventional manufacturing and testing procedures in the art. The present invention has carried out specific experimental operations on the precision, accuracy, sensitivity, specificity and stability of the test kit. The experimental results are shown in Table 1. The results showed that the sensitivity, precision, accuracy, specificity and stability of the "Thyroid Peroxidase Autoantibody Chemiluminescent Immunoassay Assay Kit" were fully qualified.
[0079] Table 1 Test results of technical indicators of the kit
[0080] Test items Inspection standards test result accuracy The average recovery rate is 90.0~110.0% 94.2~108.6% specificity Cross-reaction rate with its analogues ≤0.1% <0.07%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com