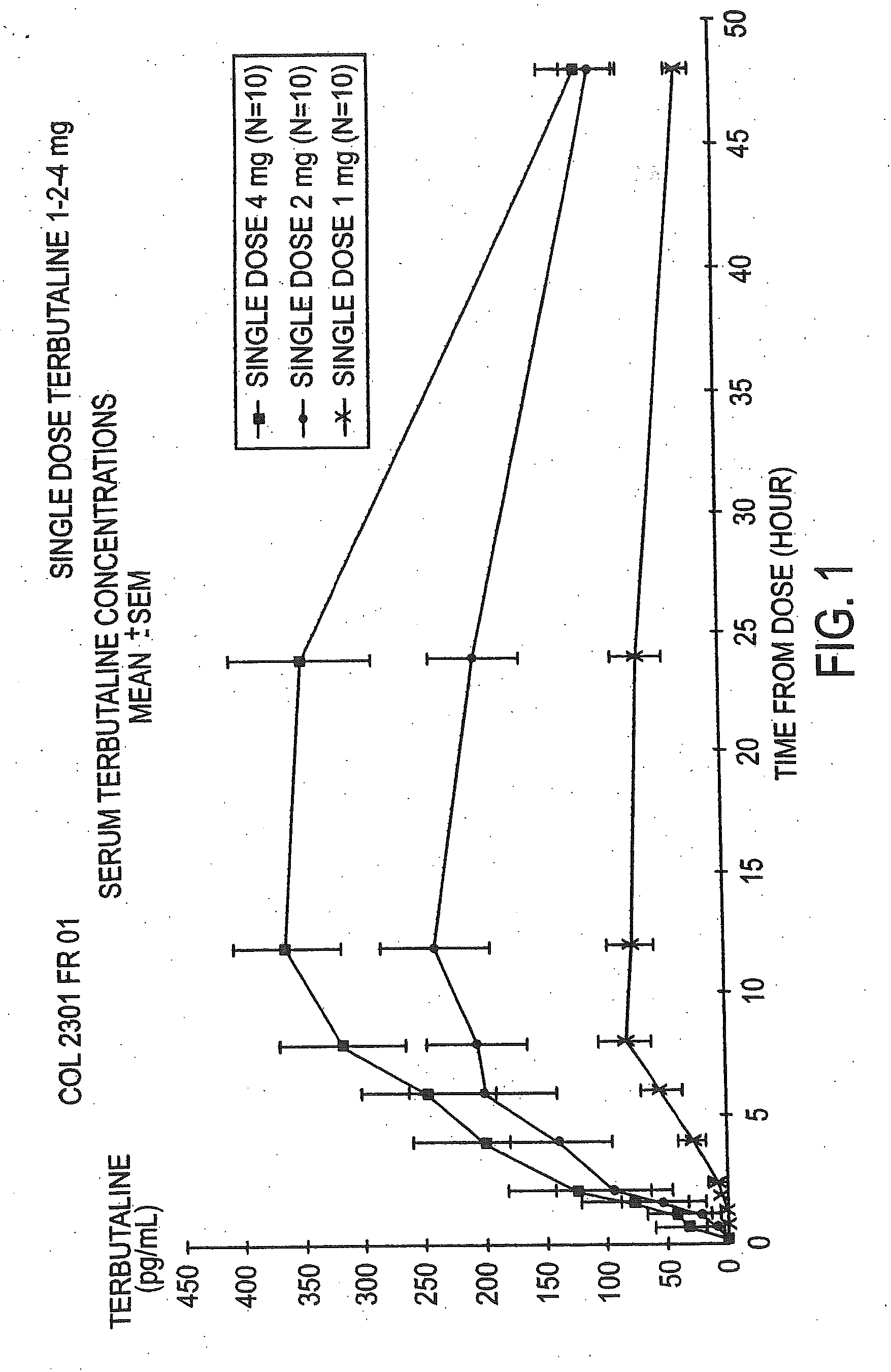
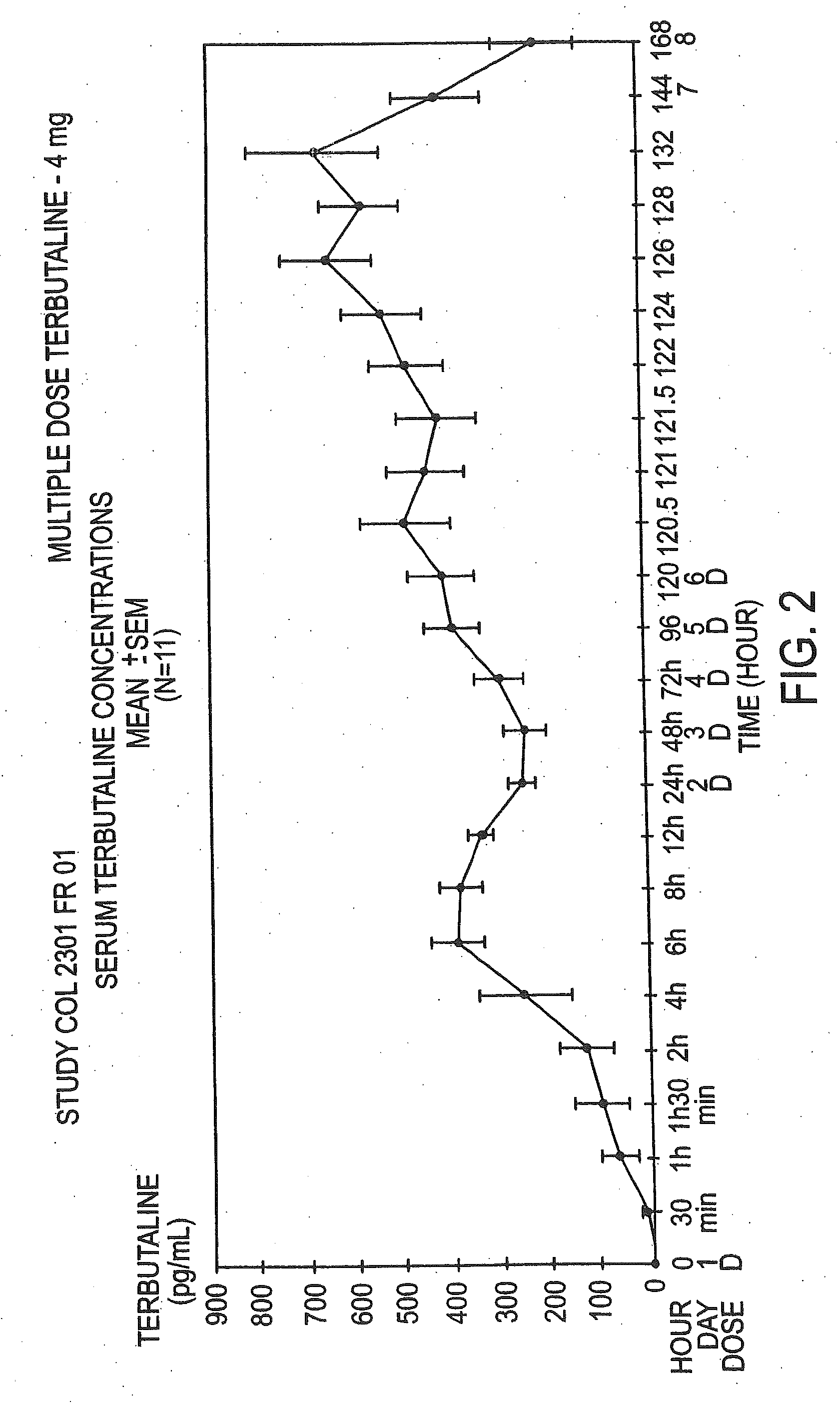
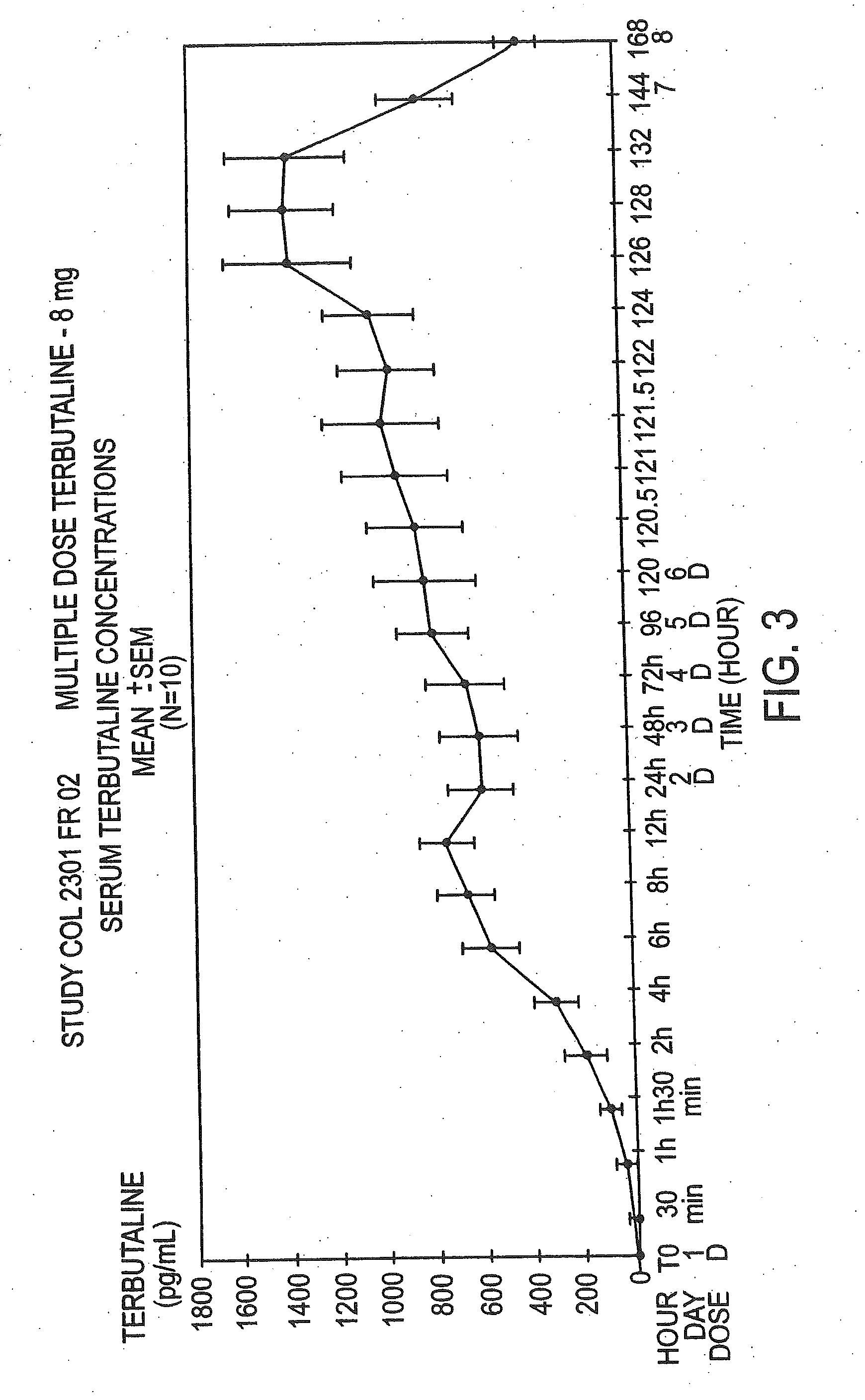
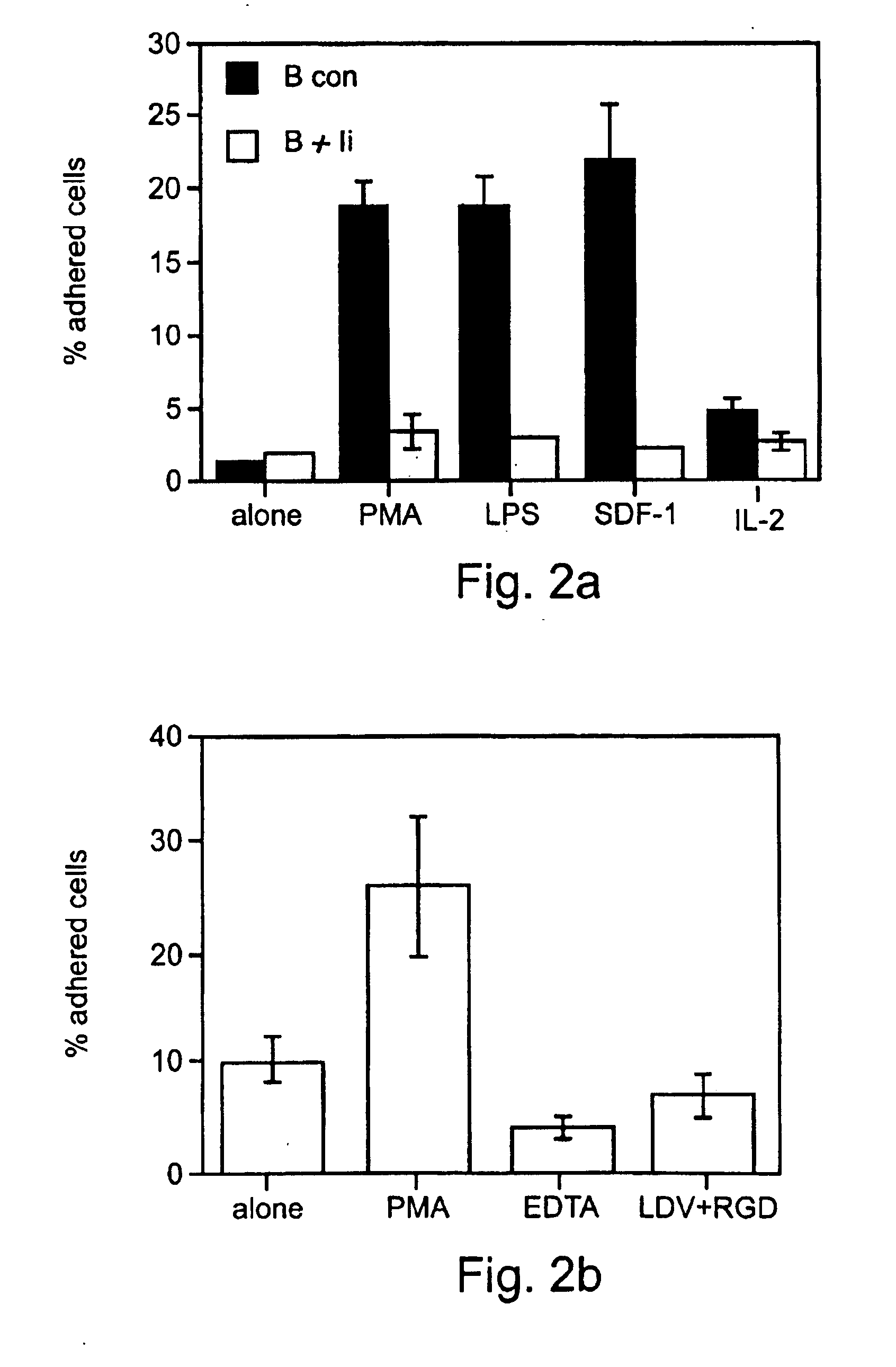
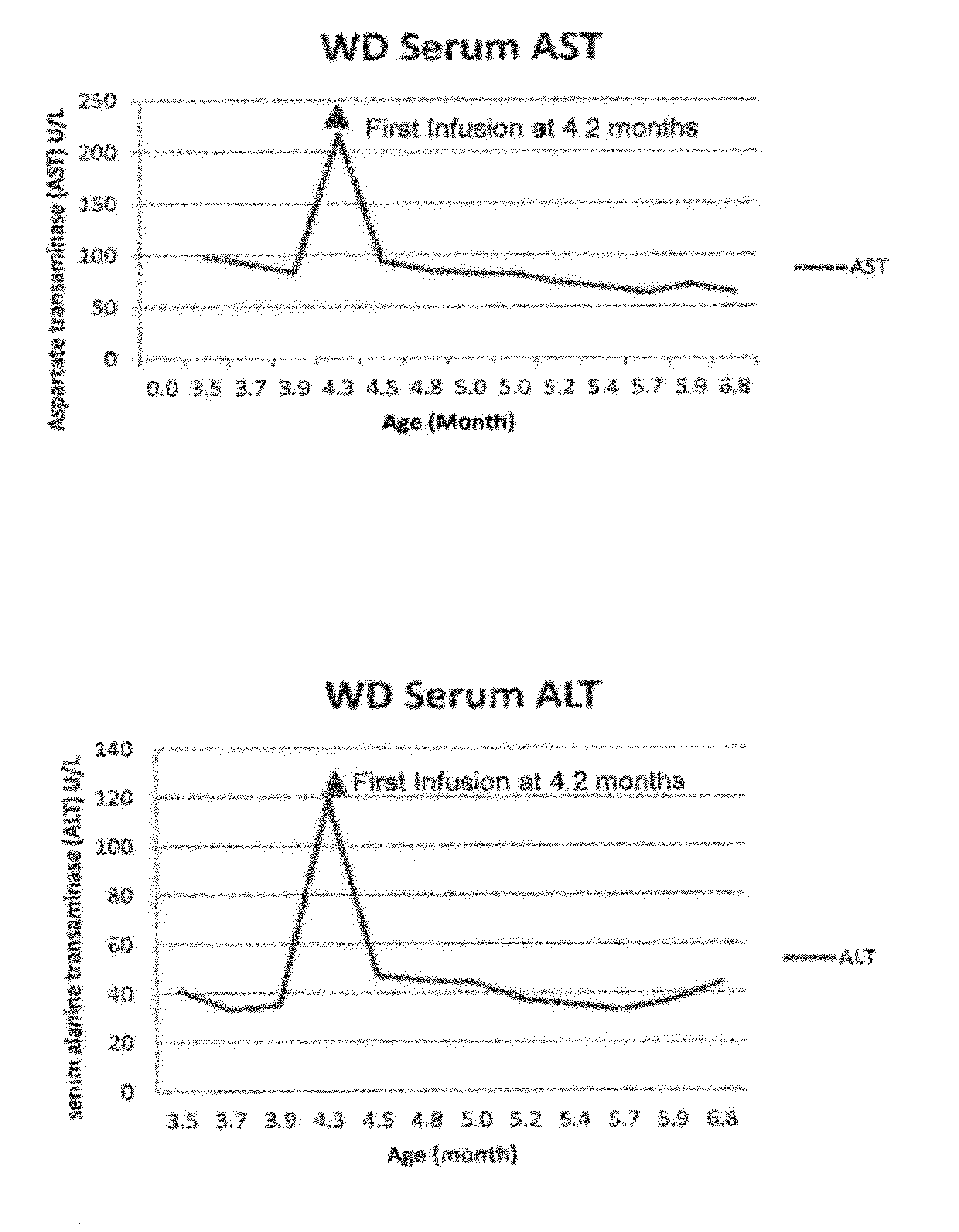
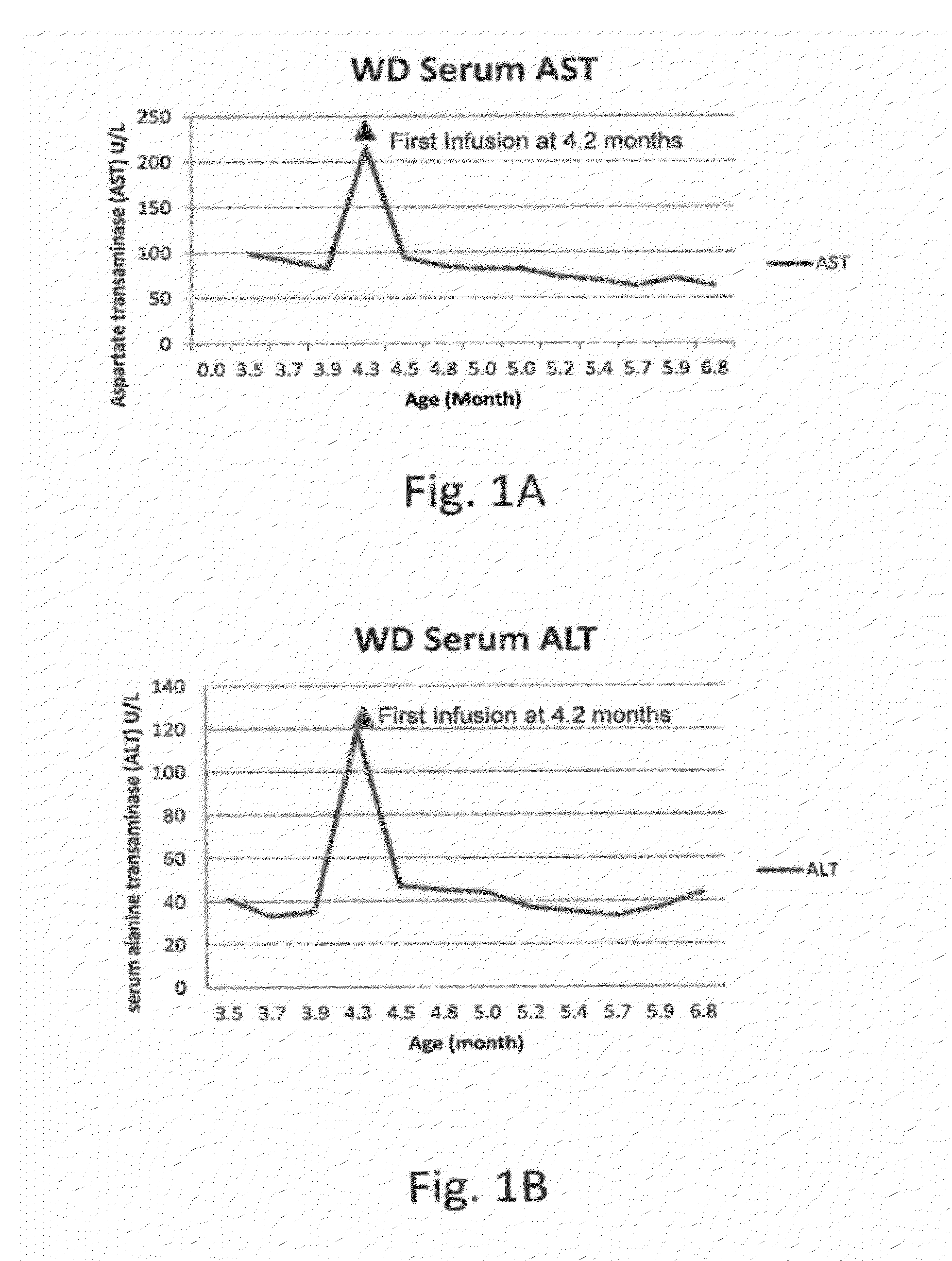
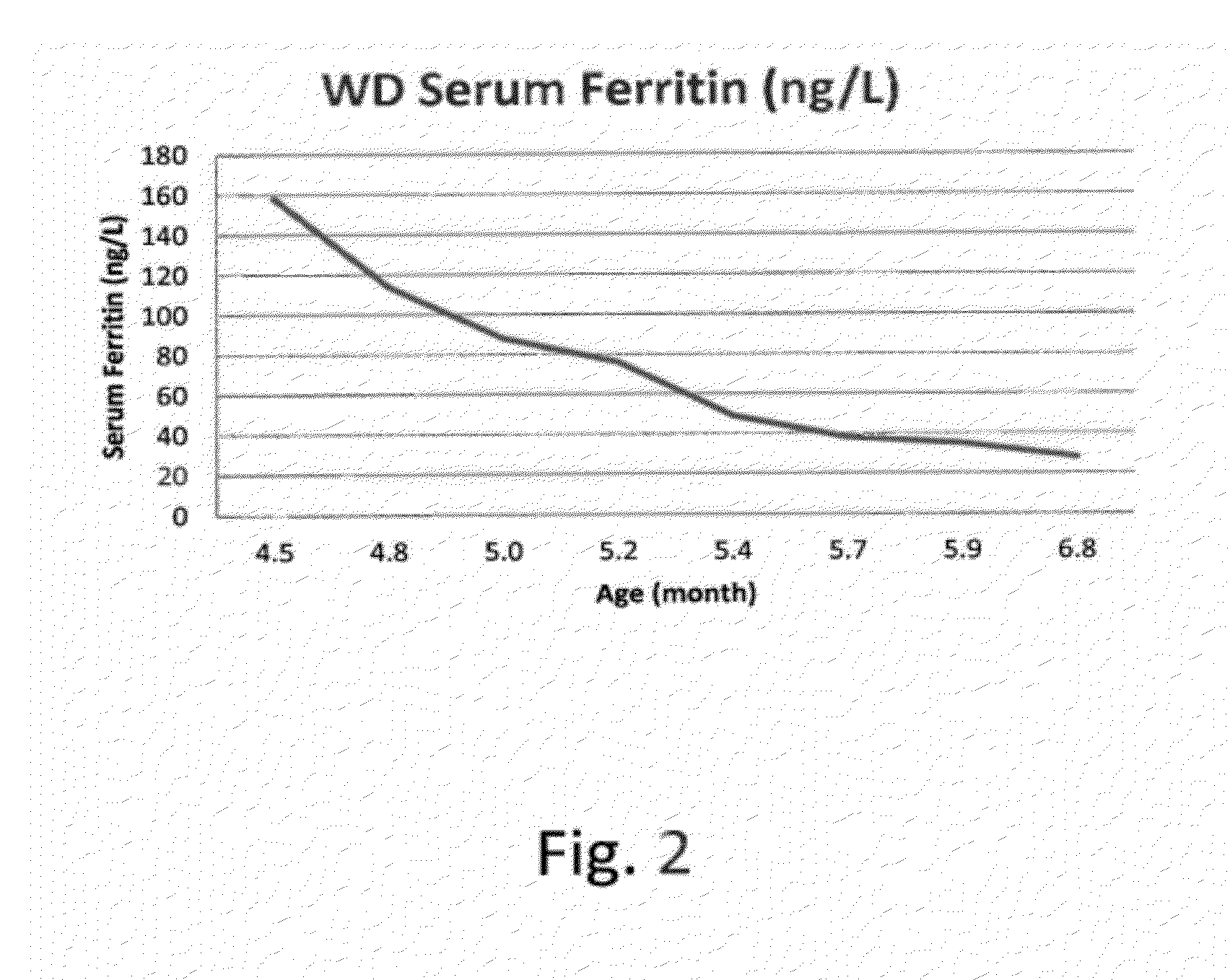
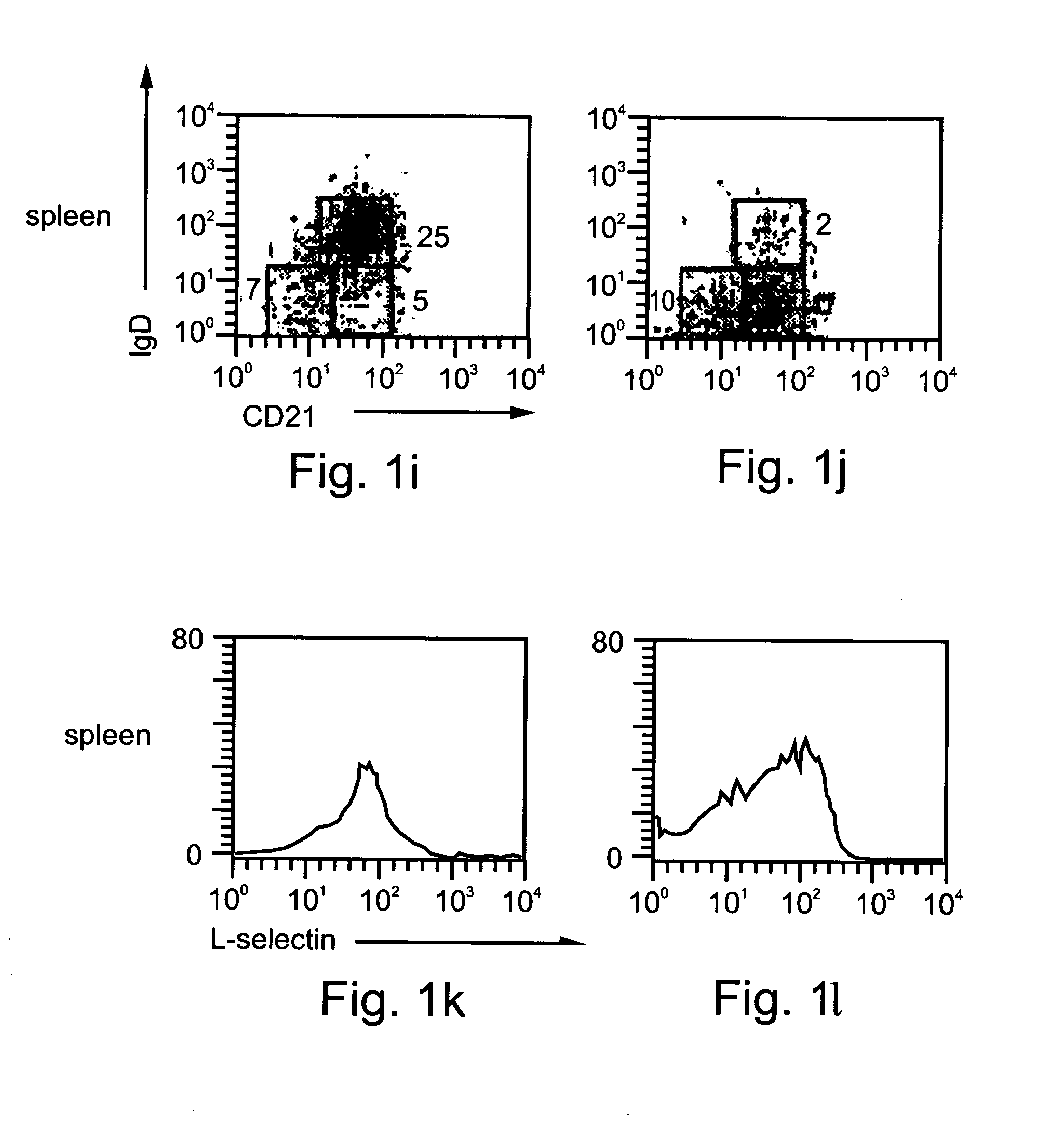
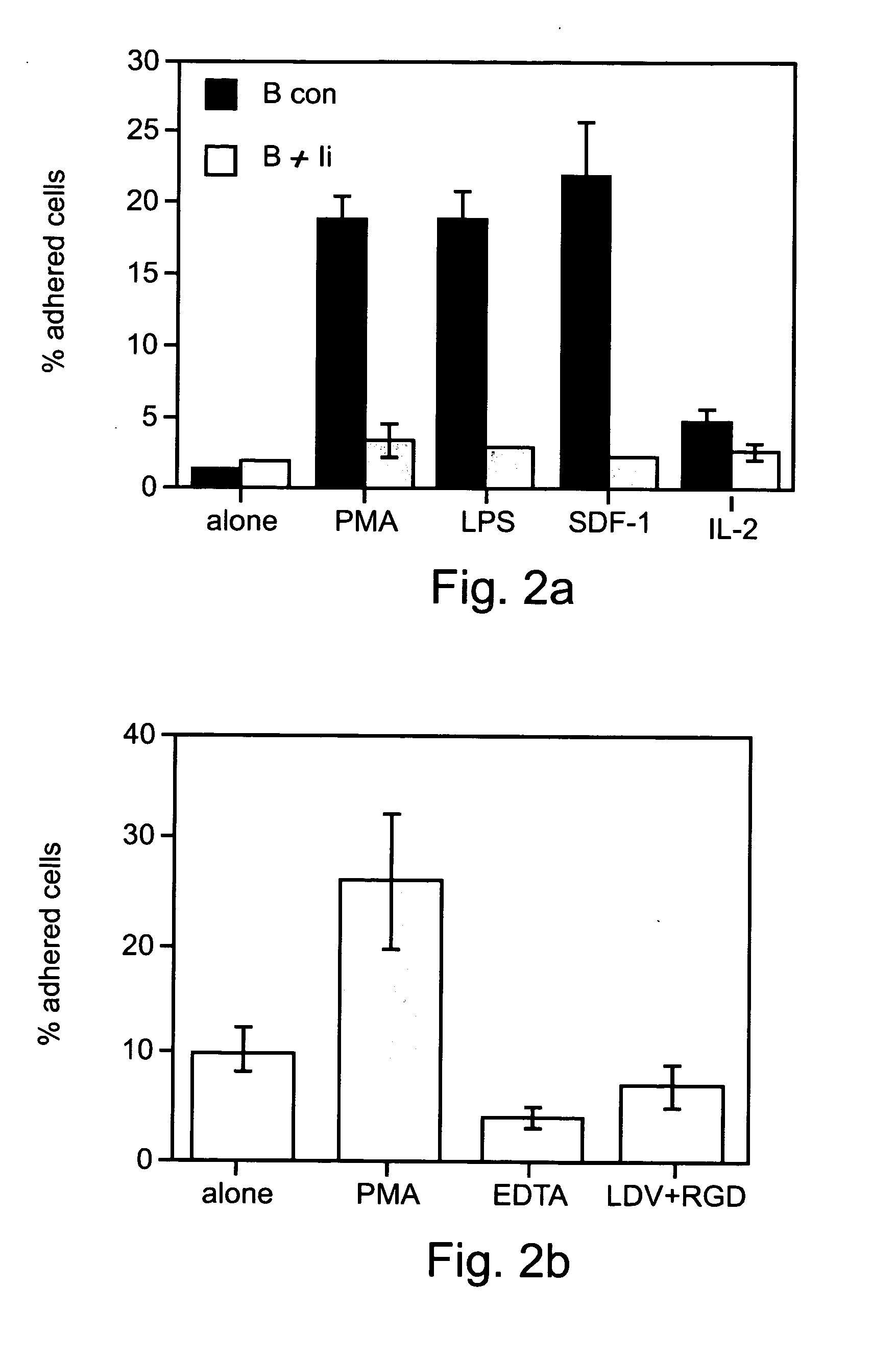
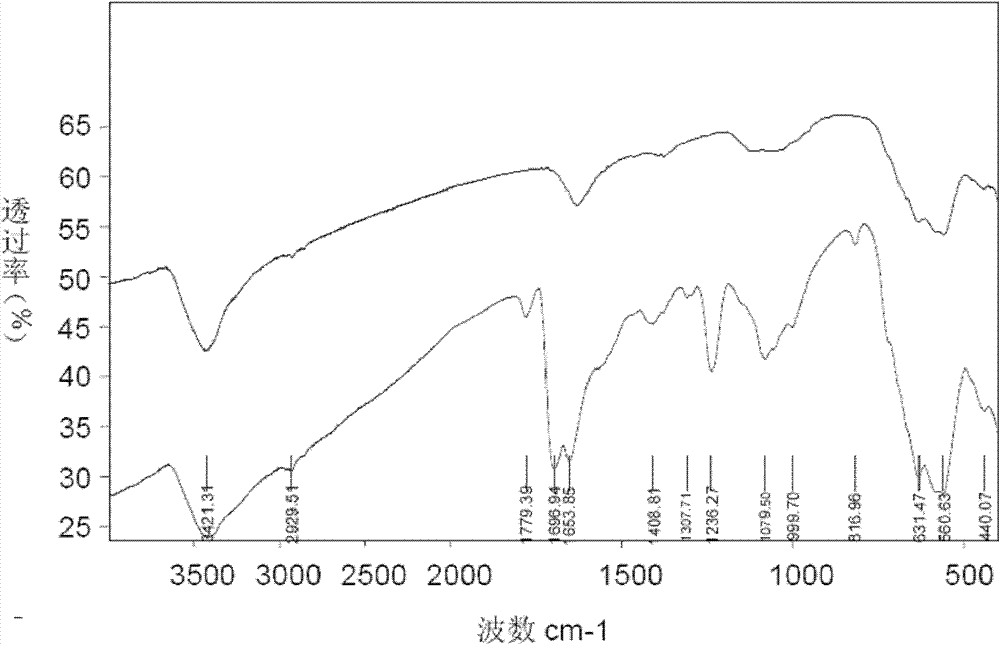
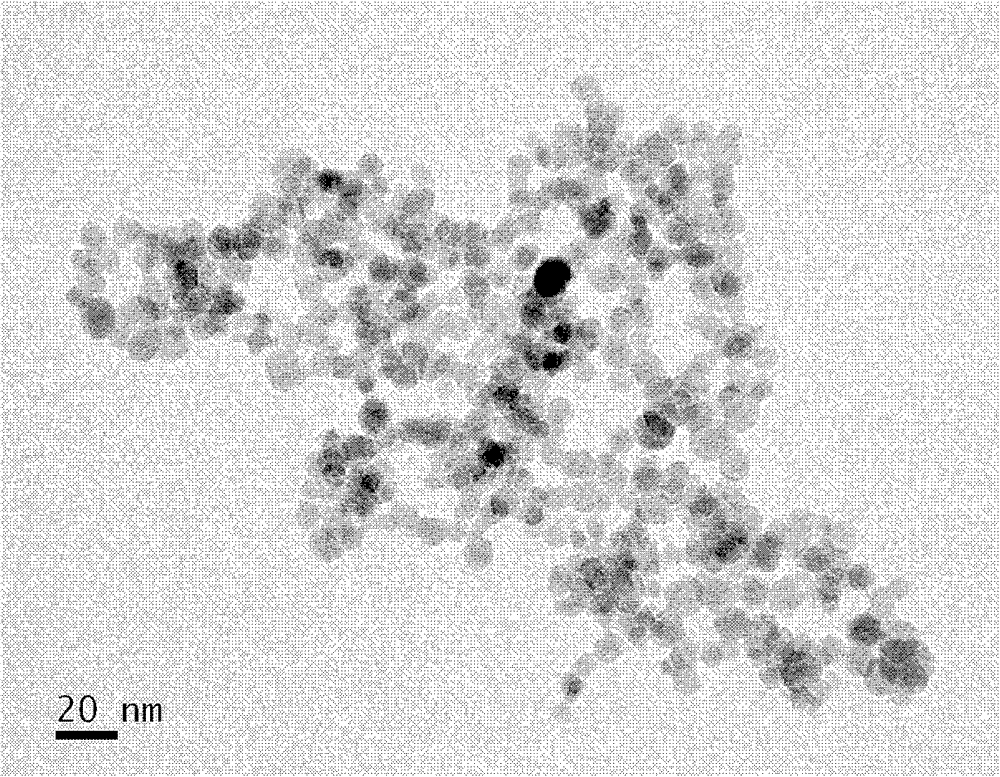
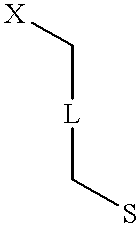Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
43 results about "Tissue concentrations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Localized vaginal delivery without detrimental blood levels
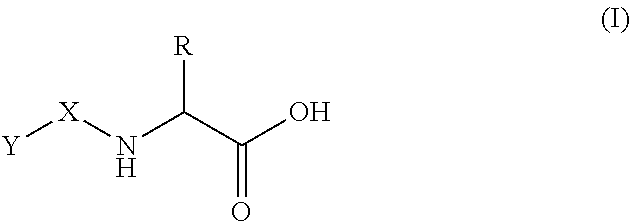
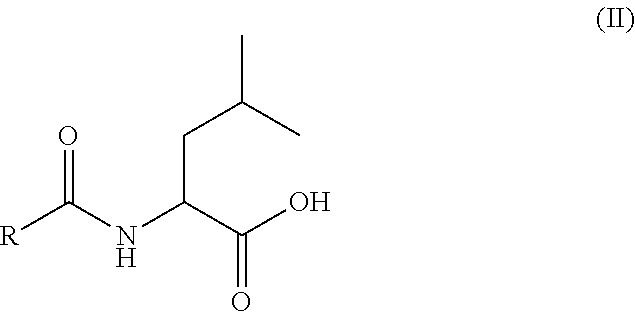
The invention relates to a pharmaceutical composition for vaginal administration of a treating agent normally associated with undesired side effects at detrimental blood levels. The composition releases the treating agent at a rate to achieve local tissue concentrations without such detrimental blood levels by using a therapeutically effective amount of the treating agent and a bioadhesive, cross-linked water swellable, but water-insoluble polycarboxylic acid polymer. Using this composition and the method of treatment provides sufficient local levels of the drug to provide therapeutic efficacy, but avoids many untoward adverse events. The invention also relates to a pharmaceutical composition for use during menses that includes a treating agent and a bioadhesive, cross-linked water swellable, but water-insoluble polycarboxylic acid polymer.
Owner:COLUMBIA LABORATORIES INC
Radiographic assessment of tissue after exposure to a compound
InactiveUS20020039401A1Facilitates efficient passageIncrease contrastComputerised tomographsTomographyConfocalClinical trial
Radiographic system and method for noninvasively assessing the response of tissue to a compound, such as a therapeutic compound. In one embodiment, a non-radioactive, radio-opaque imaging agent accumulates in tissue in proportion to the tissue concentration of a predefined cellular target. The imaging agent is administered to a live organism, and after an accumulation interval, radiographic images are acquired. The tissue being examined is transilluminated by X-ray beams with preselected different mean energy spectra, and a separate radiographic image is acquired during transillumination by each beam. An image processing system performs a weighted combination of the acquired images to produce a first image. The image processing procedure isolates the radiographic density contributed solely by differential tissue accumulation of the imaging agent. A compound is administered to the organism, and after a selected interval, a second radiographic image of the tissue is acquired. Radiographic density contributed by accumulated imaging agent in corresponding areas of tissue in the first and second images are compared. Differences in radiographic density between the images reflect changes in the concentration of the cellular target that have occurred after administration of the compound. The system and method may be used to assess therapeutic efficacy of compounds in the drug discovery process, in clinical trials, and in the evaluation of clinical treatment. In other embodiments, pharmacological and toxicological effects of a wide variety of compounds on tissue may be noninvasively assessed.
Owner:VERITAS PHARM INC
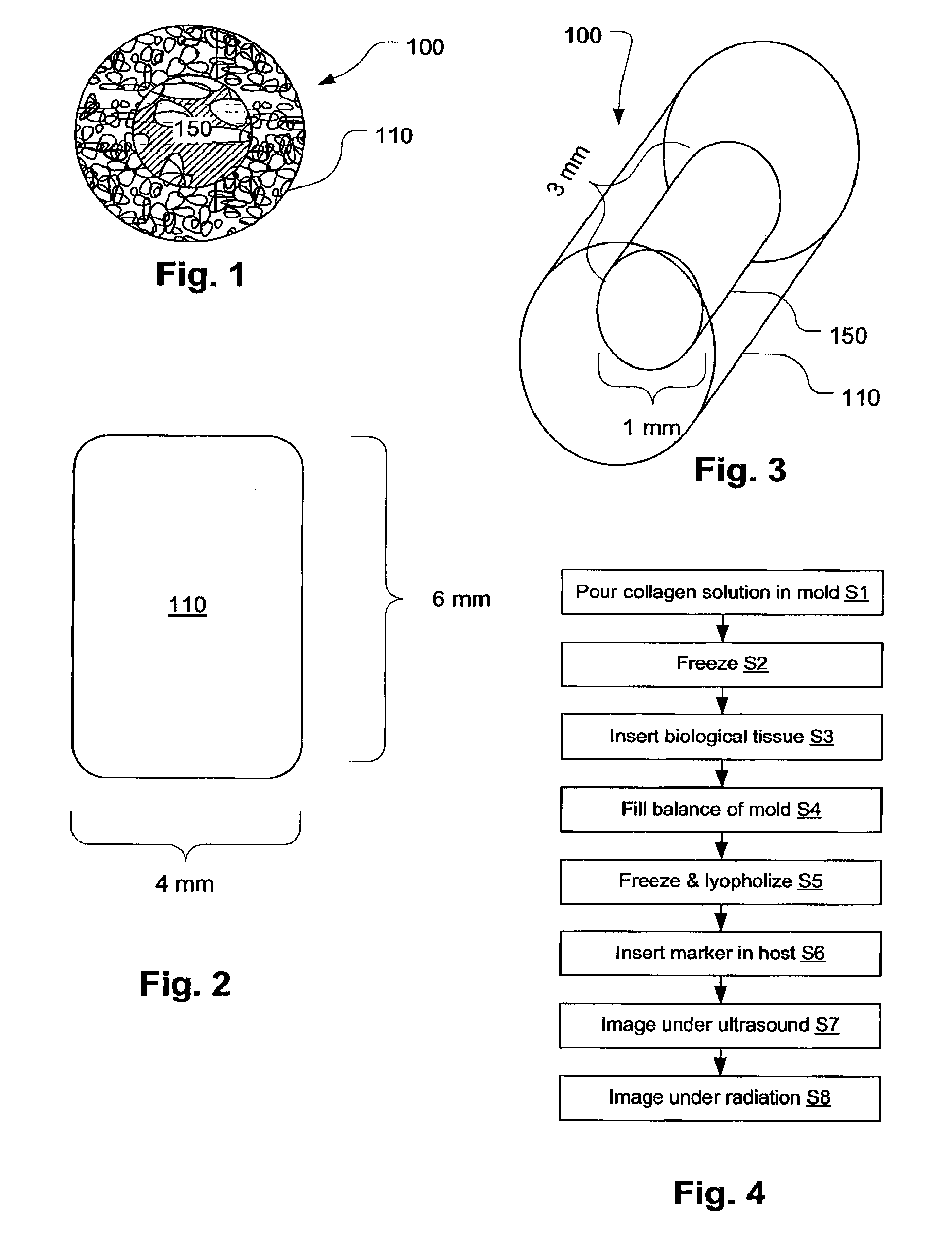
Biopsy Marker with In Situ-Generated Imaging Properties
ActiveUS20100010341A1Promote bone growthIncrease calcificationUltrasonic/sonic/infrasonic diagnosticsBiocideContrast levelCalcification
A biopsy marker having radio-opaque properties that are derived in situ, based on a natural a biological response, such as for example, calcification, accumulation or tissue-concentration of a chemical agent so as to provide an imaging contrast. A biodegradable foam such as collagen foam or gelatin foam is embedded with a biological tissue that is susceptible to the calcification. Initially the marker can be imaged using ultrasound, but over time, the embedded material calcifies causing it to become visible under radiation imaging.
Owner:CR BARD INC
Biopsy marker with in situ-generated imaging properties
ActiveUS8401622B2Promote bone growthIncrease calcificationUltrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsTissue concentrationsUltrasonic imaging
A biopsy marker having radio-opaque properties that are derived in situ, based on a natural a biological response, such as for example, calcification, accumulation or tissue-concentration of a chemical agent so as to provide an imaging contrast. A biodegradable foam such as collagen foam or gelatin foam is embedded with a biological tissue that is susceptible to the calcification. Initially the marker can be imaged using ultrasound, but over time, the embedded material calcifies causing it to become visible under radiation imaging.
Owner:CR BARD INC
Method and pharmaceutical composition for treating inflammation
A method of treating an inflammation in a subject in need thereof is disclosed. The method comprises locally or systemically administering to the subject IFN-gamma in an amount so as to achieve an IFN-gamma bulk tissue concentration at a site of inflammation of 1-8,000 units per kilogram body weight, thereby ameliorating the inflammation.
Owner:YEDA RES & DEV CO LTD
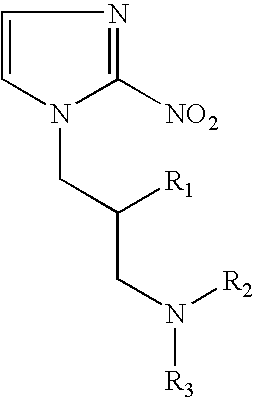
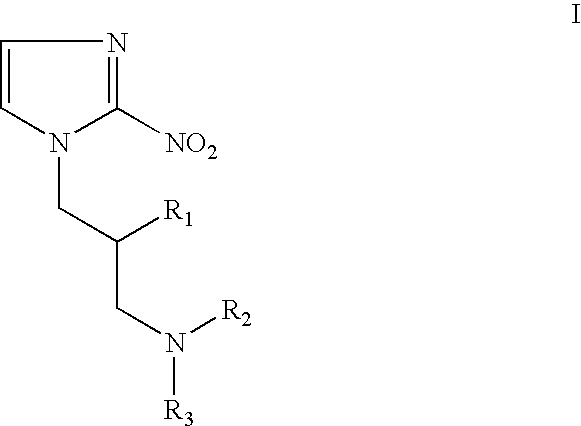
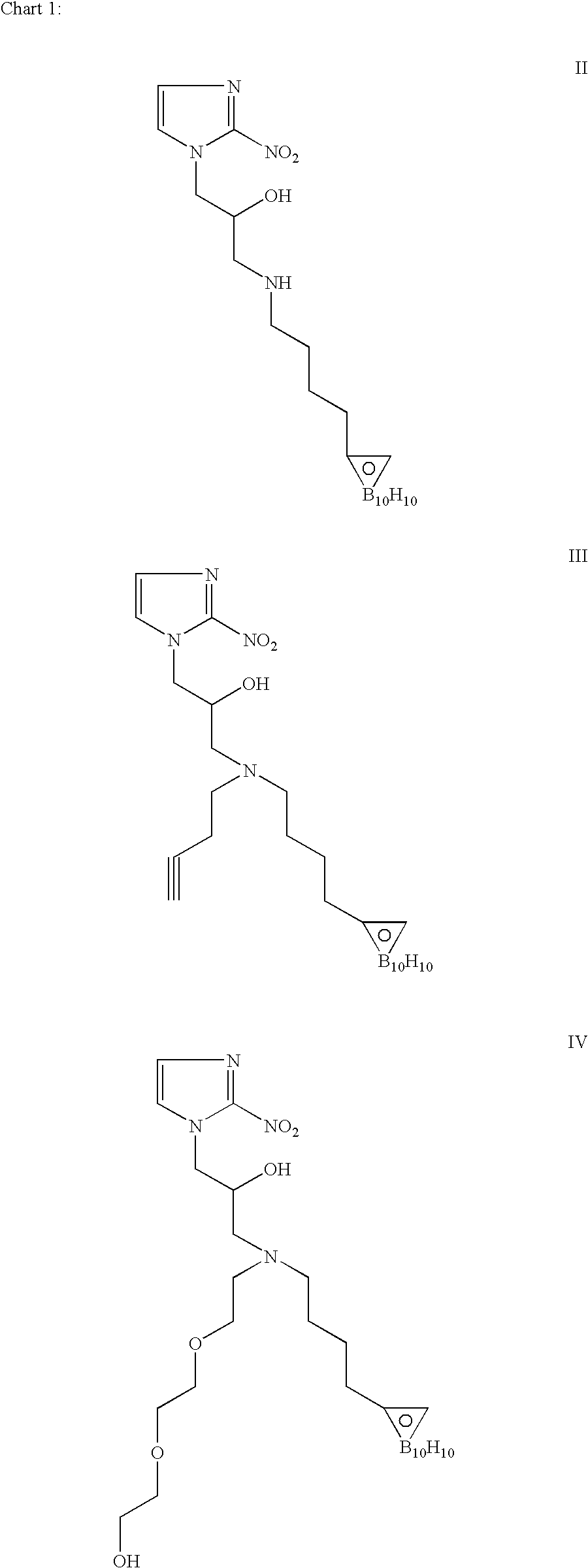
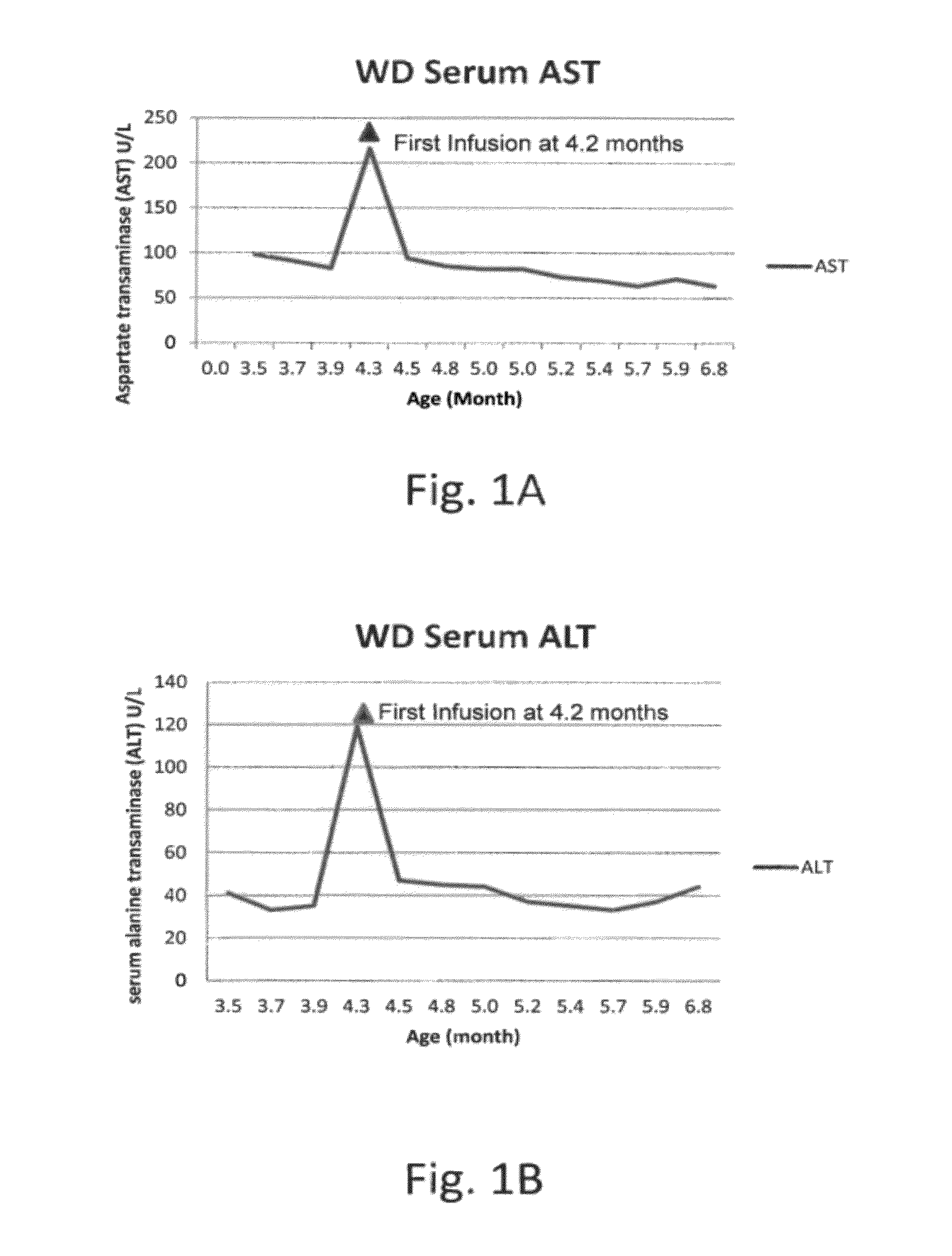
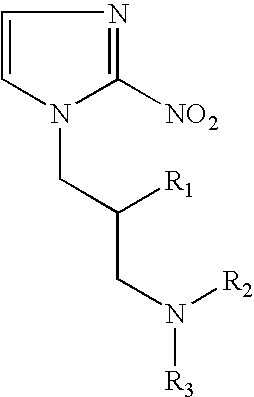
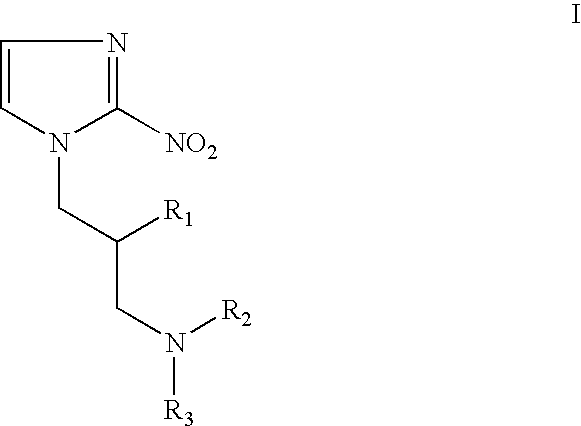
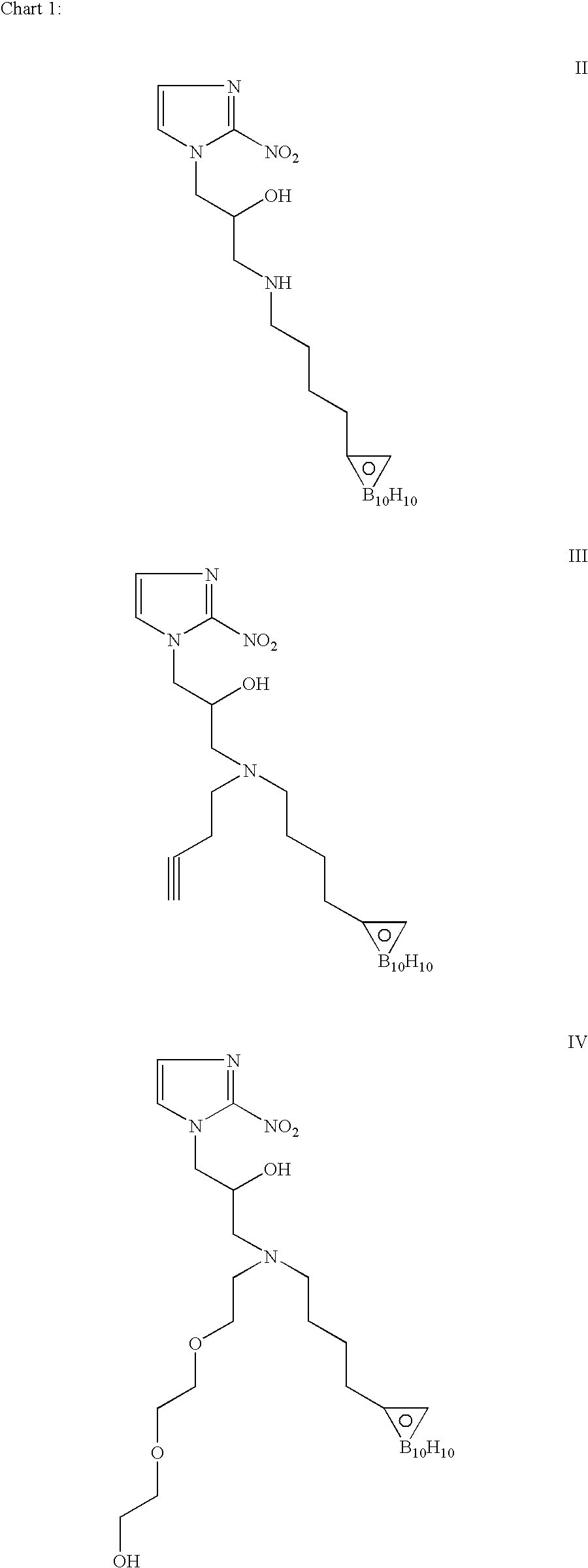
Hypoxia-selective, weakly basic 2-nitroimidazole delivery agents and methods of use thereof
InactiveUS20080102026A1Reduce deliveryImprove solubilityBiocideOrganic active ingredientsNitroimidazoleTissue concentrations
The invention features a class of 2-nitroimidazole compounds with a secondary basic nitrogen atom and a linker bearing one or more therapeutic agents, cytotoxic agents, detectable labels, or chelating groups. In particular, the invention provides 2-nitroimidazole compounds containing a cluster of boron atoms for use in boron neutron capture therapy (BNCT). The 2-nitroimidazole compounds can be used to treat hypoxic conditions, including, e.g., cancer, inflammation, and ischemia. The weakly basic 2-nitroimidazole compounds target to hypoxic tissue and provide increased tissue concentration overall.
Owner:NATURAL PHARMACIA INT
Externally applied formulation of cetirizine hydrochloride
InactiveCN1634063APharmacologically activeIncreased free drug concentrationOrganic active ingredientsAerosol deliverySkin sensitizationWhole body
Pharmacological test shows that the externally-used preparation of Cetirizine has ideal allergy resistant and anti-inflammatory action to the rat passive cutaneous anaphylaxis (Rat PCA) model and dimethylbenzene caused mouse otitis model, and also prevents the adverse effect to the central system caused by whole body administration. It is also found in the test, that the externally used preparation of Cetirizine also has very fine inhibitory action to dinitrofluorobenzene caused mouse porphyria hypersensitivity (PTH), the novel pharmacological action of the Cetirizine shows that the Cetirizine external preparation has good therapeutic action to skin inflammations which mainly include porphyria hypersensitivity.
Owner:LUNAN PHARMA GROUP CORPORATION
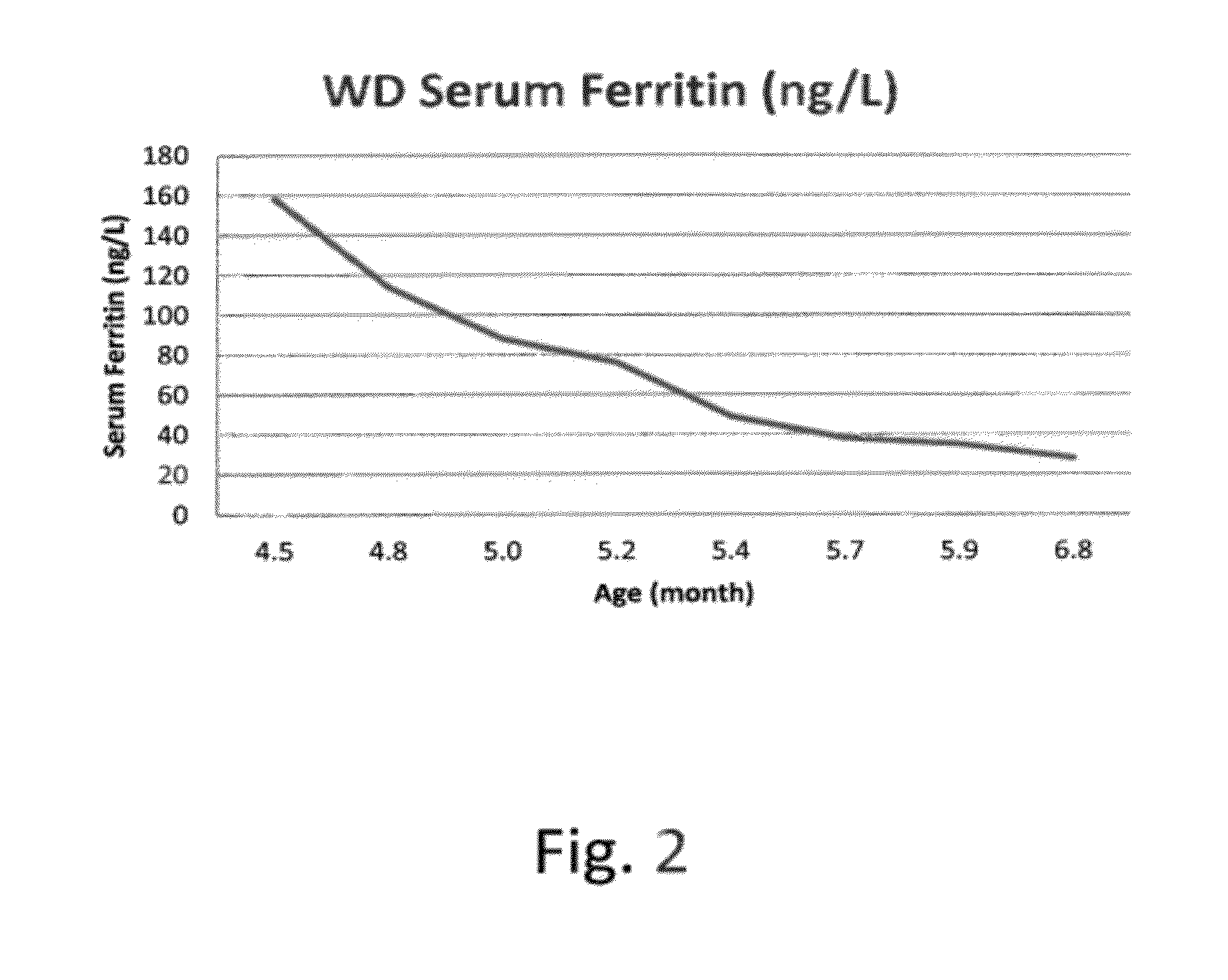
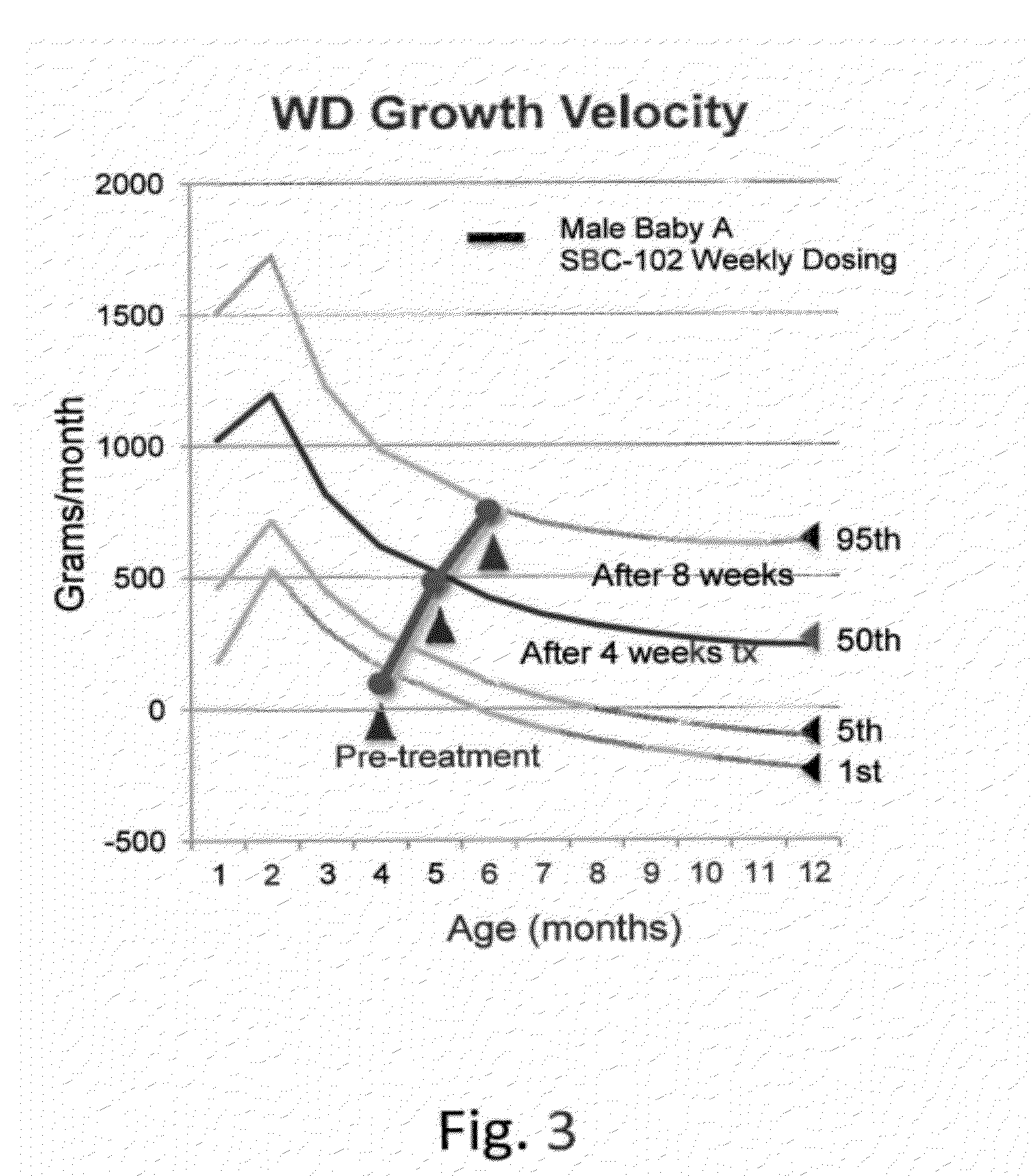
Methods for treating lysosomal acid lipase deficiency in patients
ActiveUS8663631B2Organic active ingredientsPeptide/protein ingredientsTissue concentrationsLysosomal acid lipase deficiency
The present invention provides methods of treating LAL deficiency comprising administering to a mammal a therapeutically effective amount of lysosomal acid lipase with an effective dosage frequency. Methods of improving growth and liver function, increasing LAL tissue concentration, and increasing LAL activity in a human patient suffering from LAL deficiency are also provided.
Owner:SYNAGEVA BIOPHARMA CORP
Method and pharmaceutical composition for treating inflammation
InactiveUS6911198B2Improve inflammationAvoid unwanted side-effectsPeptide/protein ingredientsAntipyreticTissue concentrationsWhole body
A method of treating an inflammation in a subject in need thereof is disclosed. The method comprises locally or systemically administering to the subject IFN-γ in an amount so as to achieve an IFN-γ bulk tissue concentration at a site of inflammation of 1-8,000 units per kilogram body weight, thereby ameliorating the inflammation.
Owner:YEDA RES & DEV CO LTD
Methods for Treating Lysosomal Acid Lipase Deficiency in Patients
ActiveUS20120064055A1Organic active ingredientsPeptide/protein ingredientsTissue concentrationsLysosomal Lipase
The present invention provides methods of treating LAL deficiency comprising administering to a mammal a therapeutically effective amount of lysosomal acid lipase with an effective dosage frequency. Methods of improving growth and liver function, increasing LAL tissue concentration, and increasing LAL activity in a human patient suffering from LAL deficiency are also provided.
Owner:SYNAGEVA BIOPHARMA CORP
Desloratadine patch and preparation method thereof
InactiveCN101933914AImprove solubilityGood curative effectOrganic active ingredientsImmunological disordersCross-linkSolubility
The invention relates to a desloratadine patch and a preparation method thereof. The desloratadine patch comprises a patch film and medicament components on the patch film, wherein the medicament components comprise main component desloratadine and auxiliary materials; and the auxiliary materials comprise a solubilizing agent, a substrate, a cross-linking agent, an excipient and an osmosis reinforcing agent. The invention overcomes the bias that in the prior art, the desloratadine is considered to be difficult to externally penetrate through the skin cuticle and not to reach effective tissue concentration in the skin tissue so as not to exert the curative effects of allergic resistance and inflammation resistance, solves the problem of poor solubility of the desloratadine, improves the permeability of the desloratadine on the skin cuticle, ensures that the desloratadine rapidly arrives at the affected part and takes the effect rapidly and solves the problems of inconspicuous effect-taking and obvious first pass effect on gastrointestinal tract of the traditional patch. The desloratadine patch has the advantages of good curative effect, rapid action, less toxic or side effect and convenient use.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +1
Hypoxia-selective, weakly basic 2-nitroimidazole delivery agents and methods of use thereof
InactiveUS7842278B2Reduce deliveryImprove solubilityBiocideOrganic active ingredientsTissue concentrationsNitroimidazole
The invention features a class of 2-nitroimidazole compounds with a secondary basic nitrogen atom and a linker bearing one or more therapeutic agents, cytotoxic agents, detectable labels, or chelating groups. In particular, the invention provides 2-nitroimidazole compounds containing a cluster of boron atoms for use in boron neutron capture therapy (BNCT). The 2-nitroimidazole compounds can be used to treat hypoxic conditions, including, e.g., cancer, inflammation, and ischemia. The weakly basic 2-nitroimidazole compounds target to hypoxic tissue and provide increased tissue concentration overall.
Owner:NATURAL PHARMACIA INT
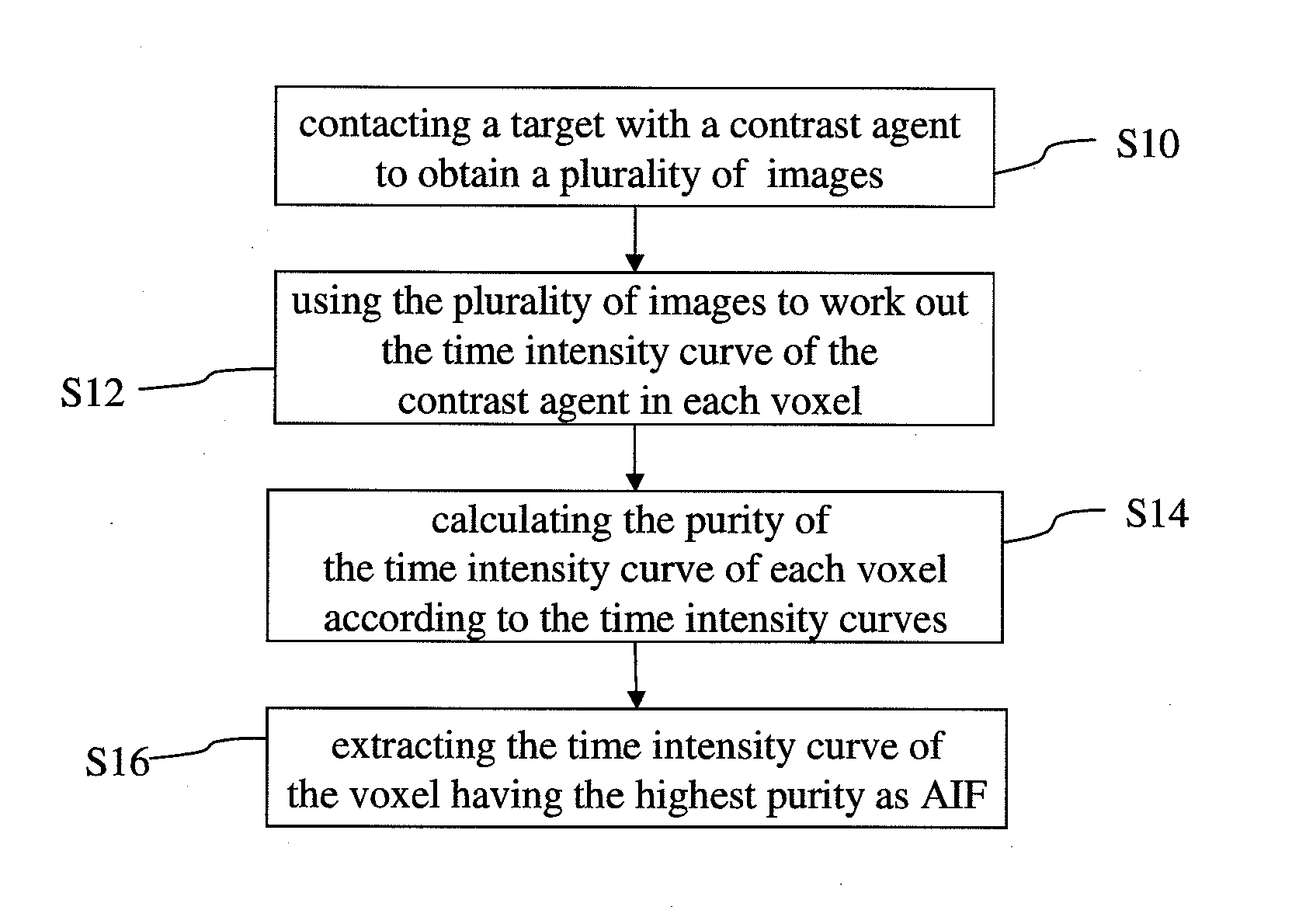
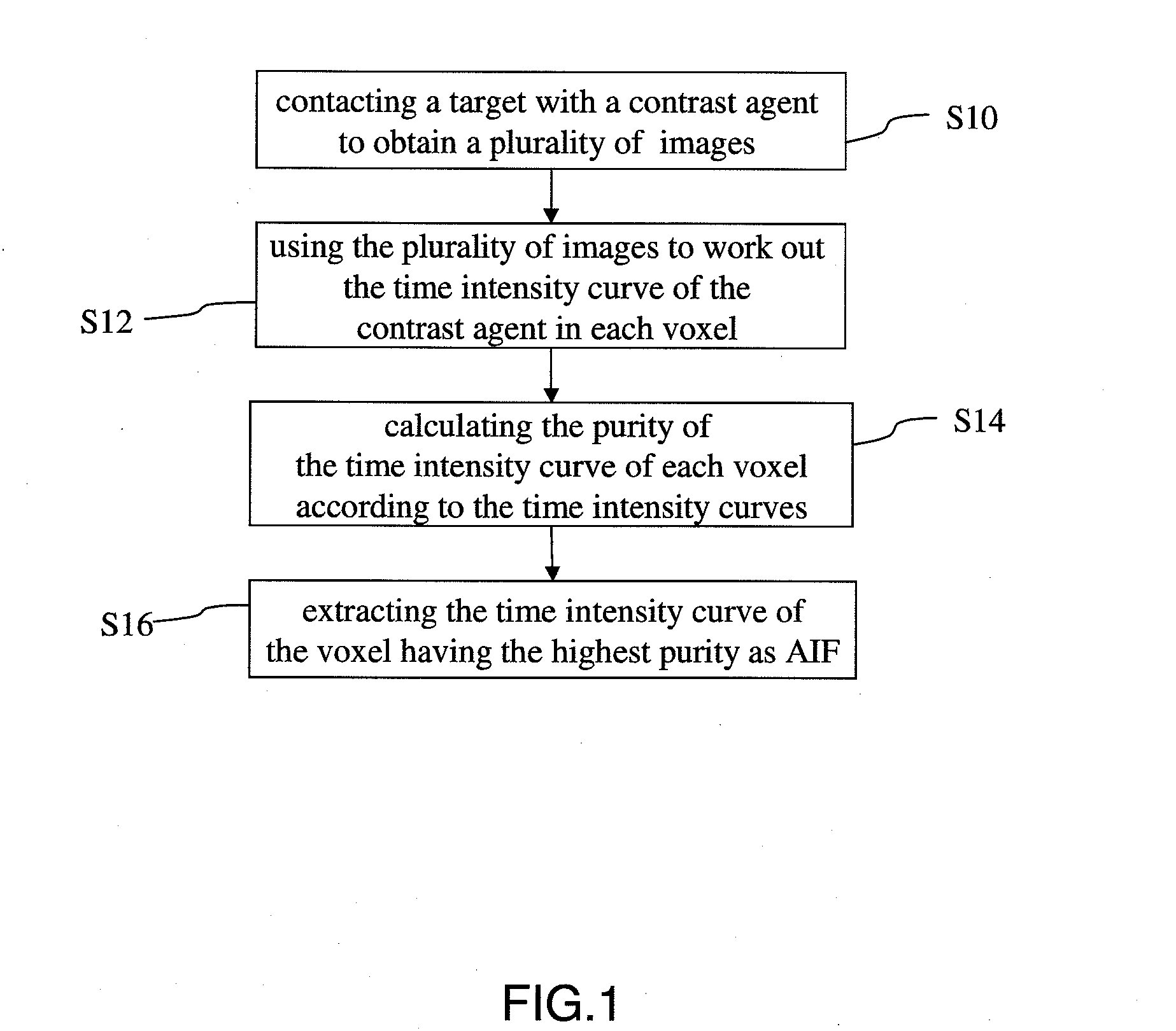
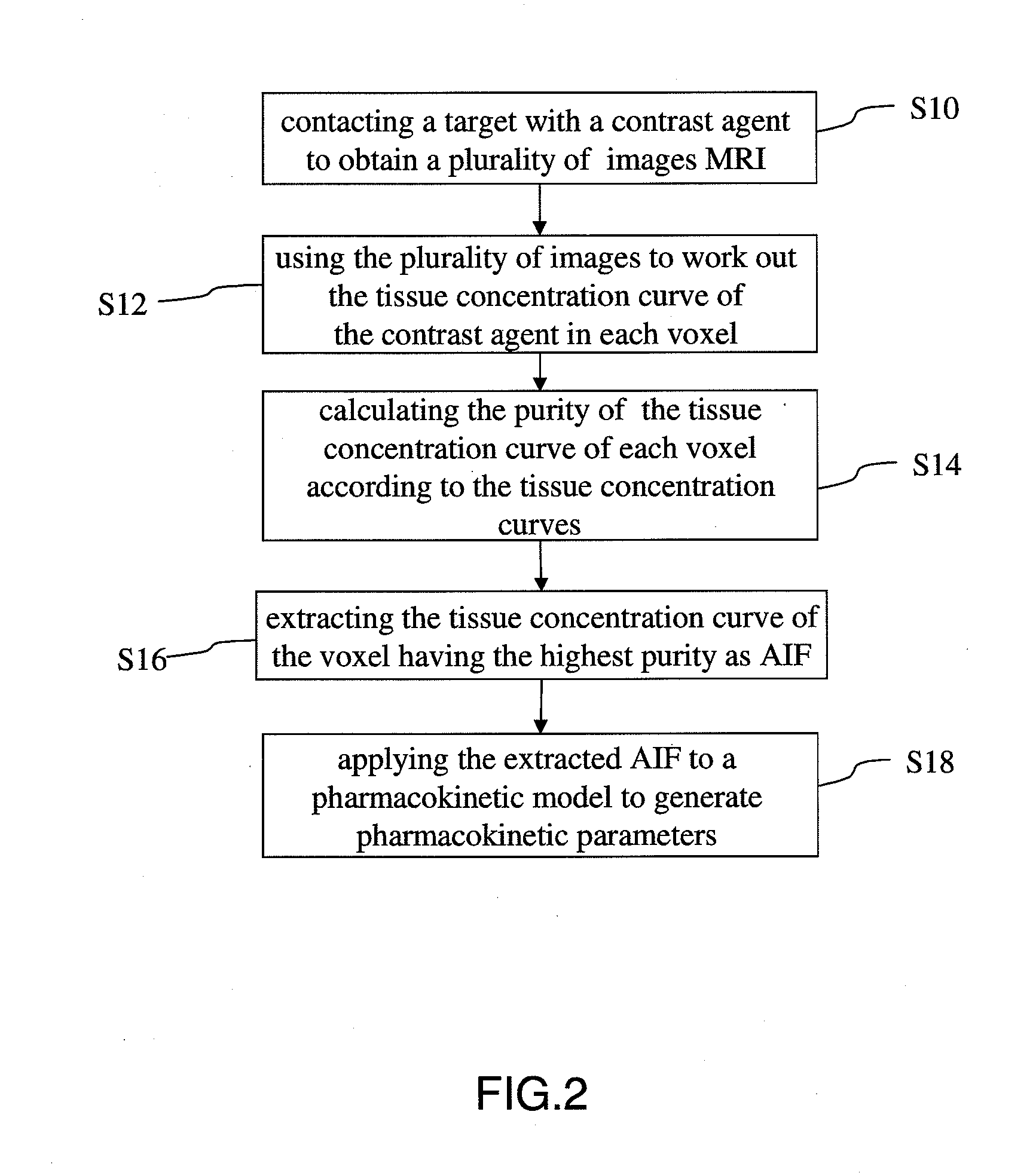
Method for extracting arterial input function and application thereof to dynamic contrast enhanced magnetic resonance imaging
ActiveUS20140241604A1Improve efficiencyImprove reliabilityImage enhancementImage analysisTissue concentrationsVoxel
The present invention discloses a method for extracting AIF and an application thereof to DCE-MRI. The method comprises steps: contacting a target tissue with a contrast agent to obtain a plurality of images; using the plurality of images to work out the tissue concentration curve of the contrast agent in each voxel; calculating the purity of the tissue concentration curve of each voxel according to the tissue concentration curves; and extracting the voxel having the highest purity as the optimized arterial location; and extracting the tissue concentration curve of the voxel having the highest purity as the arterial input function. The extracted AIF is applied to a pharmacokinetic model to obtain associated pharmacokinetic parameters. The present invention not only improves the accuracy and reliability of the derived quantitative indexes but also promotes the efficiency of the quantitative analysis.
Owner:CHANG GUNG UNIVERSITY
Protease inhibitor combination with therapeutic proteins including antibodies
ActiveUS9597379B1High activityEnhances intactHybrid immunoglobulinsPeptide/protein ingredientsTumor targetTherapeutic protein
Protease inhibitors together with protease sensitive therapeutics or diagnostics are provided, which may be ionically or covalently bound, or unbound. The protease inhibitors and / or protease sensitive moiety may be provided in monomeric, homopolymeric, heteropolymeric (for each of the protease and agent) and / or block copolymeric (combining polymers of agent and inhibitor) form. The inhibitors may be native active or e.g., protease activated. Multiple protease inhibitor peptides may be used in-frame with multiple protease cleavage signals (polymeric protease activated protease inhibitors). Combination with the protease inhibitors with the protease sensitive therapeutic enhances the intact, active molecule local-regional or targeted cell or tissue concentration, peak concentration and / or duration of the therapeutic exposure, thereby increasing its therapeutic efficacy. The protease inhibitors are particularly useful for tumor-targeted therapies and for vaccines.
Owner:BERMUDES DAVID G DR
Use of oral heparin preparations to treat urinary tract diseases and conditions
An improved method of treating lower urinary dysfunctional epithelium (LUDE) or a disease, condition, or syndrome associated with LUDE, including interstitial cystitis, comprises the step of administering orally a pharmaceutically effective quantity of heparin to a patient in need of treatment for LUDE or a disease, condition, or syndrome associated with LUDE in order to treat LUDE or a disease, condition, or syndrome associated with LUDE. The heparin can be administered together with a quantity of a penetration enhancer that is sufficient to result in a tissue concentration of heparin that is sufficient to treat LUDE or a disease, condition, or syndrome associated with LUDE. A suitable penetration enhancer is sodium N-[8-(2-hydroxybenzoyl)amino]caprylate. The method can further comprise the administration of at least one additional pharmaceutical composition to treat LUDE or a disease, condition, or syndrome associated with LUDE The invention further includes a pharmaceutical composition comprising: (1) a quantity of heparin that is pharmaceutically sufficient for the treatment of LUDE or a disease, condition, or syndrome associated with LUDE; and (b) at least one filler, excipient, or carrier; wherein the pharmaceutical composition is formulated for the treatment of LUDE or a disease, condition, or syndrome associated with LUDE.
Owner:URIGEN PHARMA INC
Calciparine/sodium salt nano oral preparation and preparation technique thereof
InactiveCN101249063AImprove stabilityHigh nano encapsulation efficiencyOrganic active ingredientsAntineoplastic agentsHigh concentrationHigh absorption
The invention relates to a calcium heparin / sodium salt nano-sized oral preparation and a preparation method thereof, and a calcium heparin / sodium salt nano-sized liposome with high encapsulation rate for preparing the calcium heparin / sodium salt nano-sized oral preparation. The calcium heparin / sodium salt nano-sized liposome is obtained by the following steps: dissolving chitosan in 1-10% diluted solution of acetic acid (the usage amount of chitosan is 0.5-5g per 100ml acetic acid solution) to obtain an acidified chitosan solution, filtering, collecting filtrate, adding dropwise 1-99% aqueous solution of calcium heparin / sodium salt into the filtrate under magnetic stirring until milk white precipitate occurs, centrifugating the resulating suspension, collecting precipitates, and freezes-drying to obtain calcium heparin / sodium salt nano-sized liposome. The calcium heparin / sodium salt nano-sized oral preparation is characterized in that the preparation has good stability, high encapsulation rate (up to 98%), and high in vitro release rate (up to 25% at hour 4); the preparation is absorbed in the intestinal tract with high absorption rate and high concentration; the half life is prolonged upon the increase of oral dosage, showing zero-order kinetics; the tissue concentration is larger than blood concentration; the preparation is released sustainedly; and study in rabbits (20mg oral intake) shows that the blood concentration in vivo is kept high for over 50 hours, thereby proving sustained release.
Owner:褚红女
Specific magnetic resonance contrast agent for brain tumor and neurodegenerative diseases and preparation method thereof
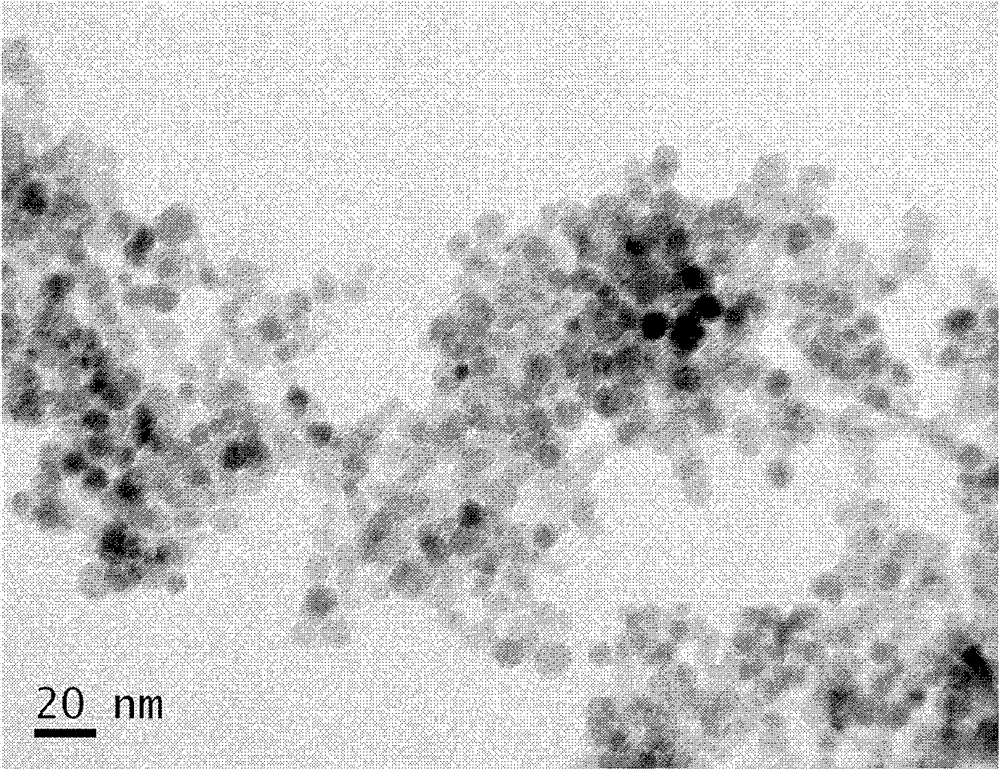
The invention provides a contrast agent capable of specifically imaging tumor and neurodegenerative diseases (including Alzheimer's disease (AD), Parkinson's disease (PD) and the like) in magnetic resonance imaging and a preparation method thereof by using the high affinity between lactoferrin and cells which express a lactoferrin receptor on surface to realize target-directed imaging. The magnetic resonance contrast agent is formed by conjugating super-paramagnetic ferric oxide nanoparticles with lactoferrin, the particle size of the magnetic resonance contrast agent is below 20 nanometers, and the conjugate has the characteristics of small particle size, no toxicity, high specificity, high selectivity, high contrast effect, tissue concentration enough for in-vivo imaging, and the like.
Owner:HUAZHONG UNIV OF SCI & TECH
Method of enhancing the antimicrobial action of systemically administered antibiotics
PendingUS20210030874A1Improve bactericidal powerGood curative effectAntibacterial agentsSenses disorderAntimicrobial actionTissue concentrations
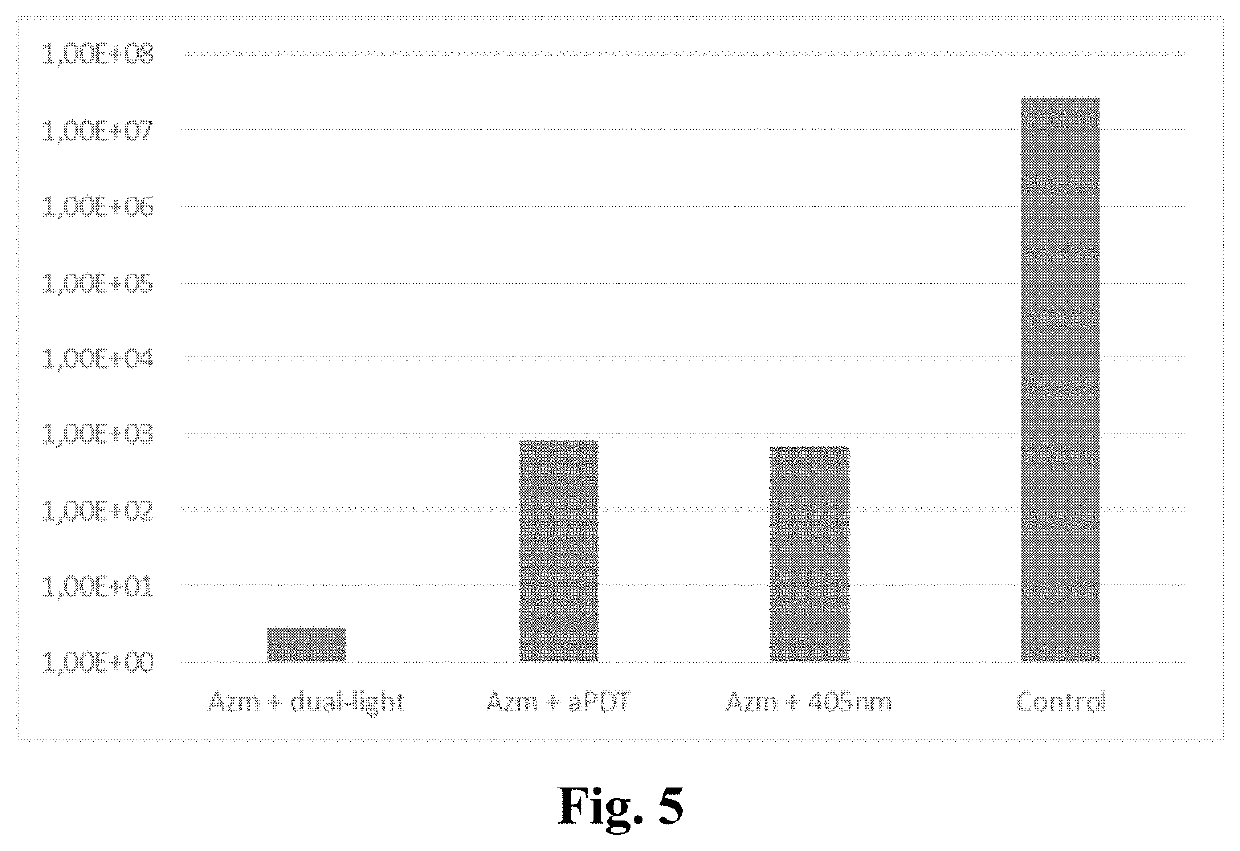
Antibiotic for use in a method comprising systemically administering a photosensitive antibiotic to a subject having a microbial infection in a target tissue to provide a blood plasma concentration of the antibiotic in the subject; allowing the plasma or tissue concentration of the antibiotic increase to a predetermined level; and subjecting the target tissue to photodynamic therapy in order to increase the antimicrobial action of the antibiotic in the target tissue. With light activation the efficacy of the photosensitizer antibiotic can be enhanced, the spectrum of the microbial effect broadened and even an antiviral effect can be obtained. Generally, the present invention can be exploited when there is a need for enhancement of the local antimicrobial effect of antibiotics.
Owner:KOITE HEALTH OY
Cholesteryl ester vesicles loading peptides, proteins and nucleic acids into chylomicrons and body cells
InactiveUS20190175515A1Improve oral bioavailabilityImprove surface propertiesLyophilised deliveryAntineoplastic agentsIntestinal structureChylomicron
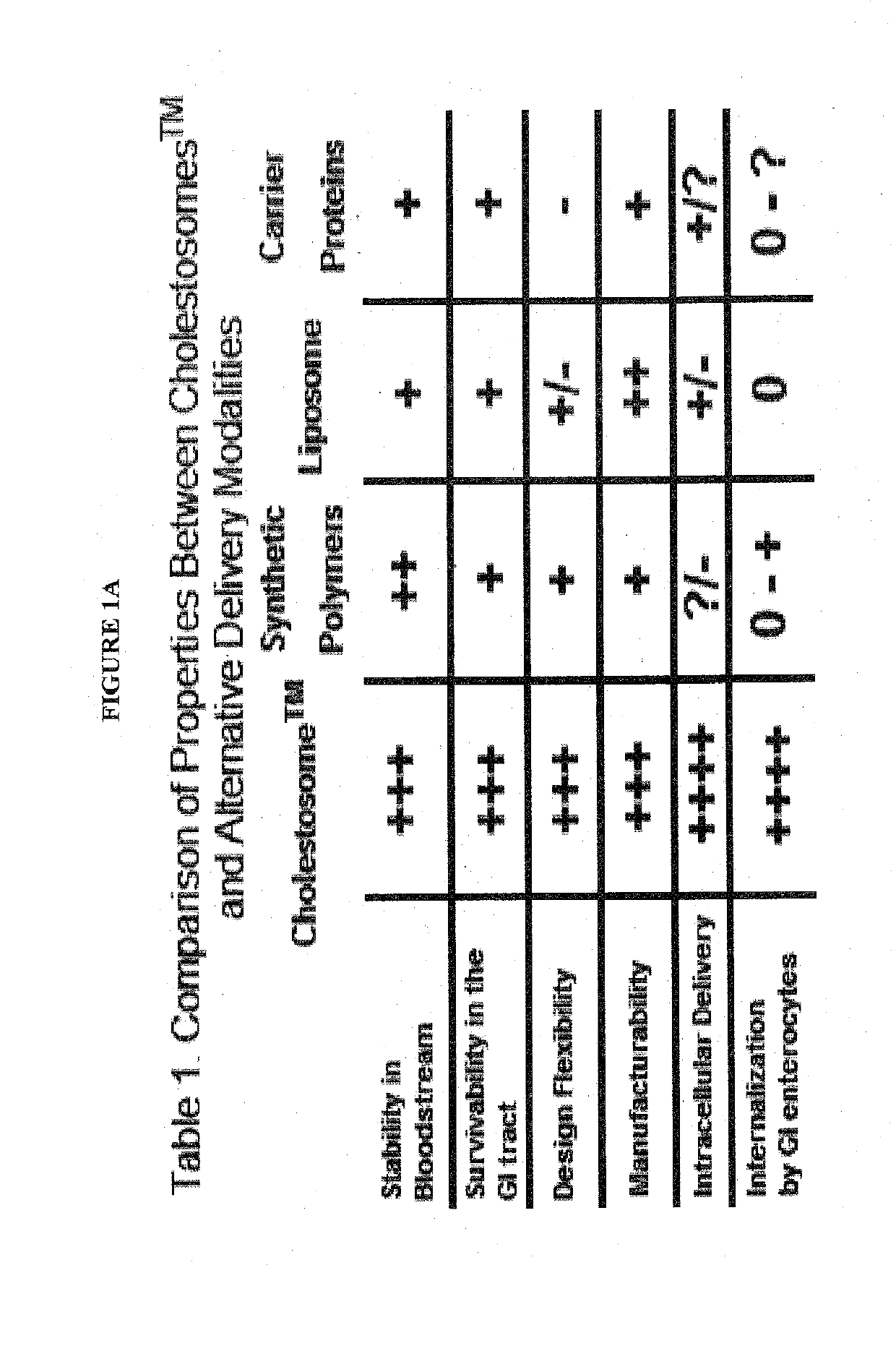
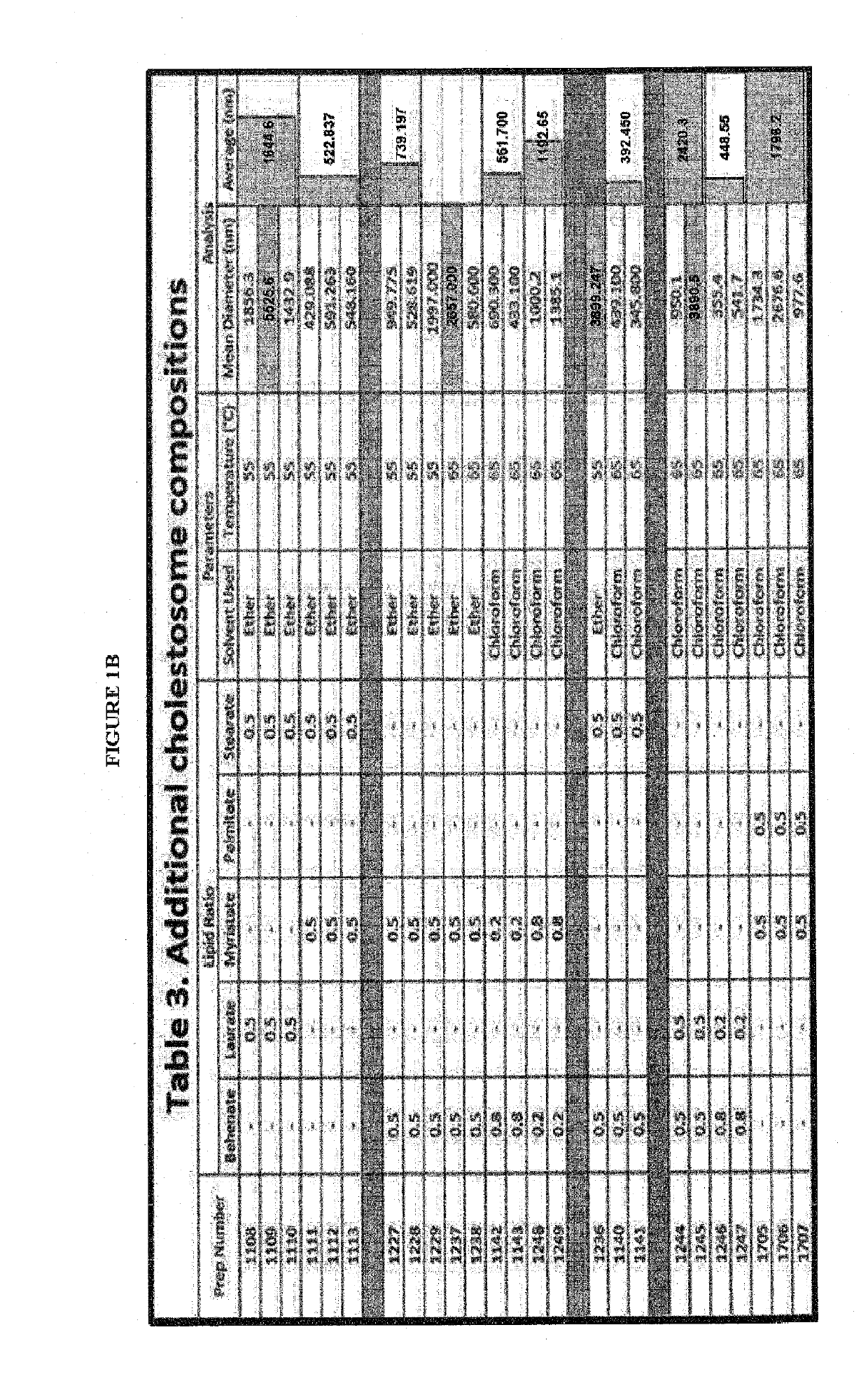
The present invention is directed to one or more macromolecules in a lipid vesicle oral formulation which targets intracellular receptors, in particular for peptides, proteins, nucleic acids and mixtures thereof, optionally in combination with small molecules. The invention encapsulates said macromolecules in a neutral lipid vesicle comprised of one or more cholesteryl esters. Unique properties of macromolecules encapsulated in said vesicles include high oral bioavailability, defined herein as in at least 50%, i.e., often in excess of 50% on the basis of oral to parenteral AUC. Non-limiting examples are provided, for large hydrophilic molecules such as peptides, proteins and nucleic acids which heretofore have been very poorly absorbed by the mammalian intestine. In prior art; said molecules are generally less than 25% bioavailable, even with protective coatings and optionally absorption enhancing component substances in the formulation. An additional feature of the present invention is high tissue concentrations after oral use, a result of rapid uptake of cholestosomes delivered by chylomicrons to body cells. A preferred embodiment is disclosed for insulin, where with cholestosome encapsulation oral bioavailability is at least 66%. Prior to the present invention, oral bioavailability of insulin and other peptides and proteins was maximally 25% and usually between 5% and 10%. Additional preferred examples are provided for one or more macromolecules useful in the treatment of cancer and in particular intracellular targeting in the practice of cancer immunotherapeutics.
Owner:THERASYN SENSORS
Externally used loratadine formulation
InactiveCN1634053AEffectively antagonizes sensitizationReduce adverse reactionsAerosol deliveryOintment deliveryDiseasePassive Cutaneous Anaphylaxis
The pharmacological experiment shows that, the externally used preparation of Loratadine has very good allergy resisting and anti-inflammatory action to the rat passive cutaneous anaphylaxis (Rat PCA) model and dimethylbenzene caused mouse otitis model, in the experiment, it is found by chance that, the externally used preparation of Loratadine also has very fine inhibitory action to dinitrofluorobenzene caused mouse porphyria hypersensitivity (PTH), the novel pharmacological action mechanism of the Loratadine shows that the Loratadine external preparation has good therapeutic action to skin inflammations which mainly include porphyria hypersensitivity.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for testing absolute volume of concentration of oxidized hemoglobin and reduced hemoglobin in human tissue
ActiveCN100518640CLuminous power is stableSimple designMaterial analysis by optical meansDiagnostic recording/measuringHemoglobin FHEMOGLOBIN I
Owner:TSINGHUA UNIV
Method and pharmaceutical composition for treating inflammation
InactiveUS20050169887A1Improve inflammationAvoid unwanted side-effectsPeptide/protein ingredientsAntipyreticTissue concentrationsWhole body
A method of treating an inflammation in a subject in need thereof is disclosed. The method comprises locally or systemically administering to the subject IFN-gamma in an amount so as to achieve an IFN-gamma bulk tissue concentration at a site of inflammation of 1-8,000 units per kilogram body weight, thereby ameliorating the inflammation.
Owner:YEDA RES & DEV CO LTD
Brain tumor magnetic resonance contrast agent and its preparation method
InactiveCN102784400ALow costEasy to commoditizeEmulsion deliveryIn-vivo testing preparationsSide effectPhosphate
The invention provides a brain tumor magnetic resonance contrast agent. The contrast agent is transferrin coupled super-paramagnetic iron oxide nanoparticles and contains a physiologically acceptable diluent, wherein the diluent can be PBS (phosphate buffer solution) or normal saline; the weight / volume application ratio of the transferrin coupled super-paramagnetic iron oxide nanoparticles to the diluent is 10-20mg:1mL; the super-paramagnetic iron oxide nanoparticles can be super-paramagnetic ferric oxide nanoparticles or super-paramagnetic ferriferrous oxide nanoparticles; and the particle sizes of the super-paramagnetic iron oxide nanoparticles are 10-20nm. The contrast agent utilizes the high affinity between the transferrin and transferrin acceptors expressed on surfaces of cells to realize the targeting developing, and has the characteristics of high specificity, good selectivity, strong contrast effect, low toxic side effect, enough tissue concentration for the in-vivo imaging, and the like.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Methods for Reducing the Occurrence of Hot Flashes
InactiveUS20160166535A1Reduce in quantityReduce severityOrganic active ingredientsBiocideTissue concentrationsPresent method
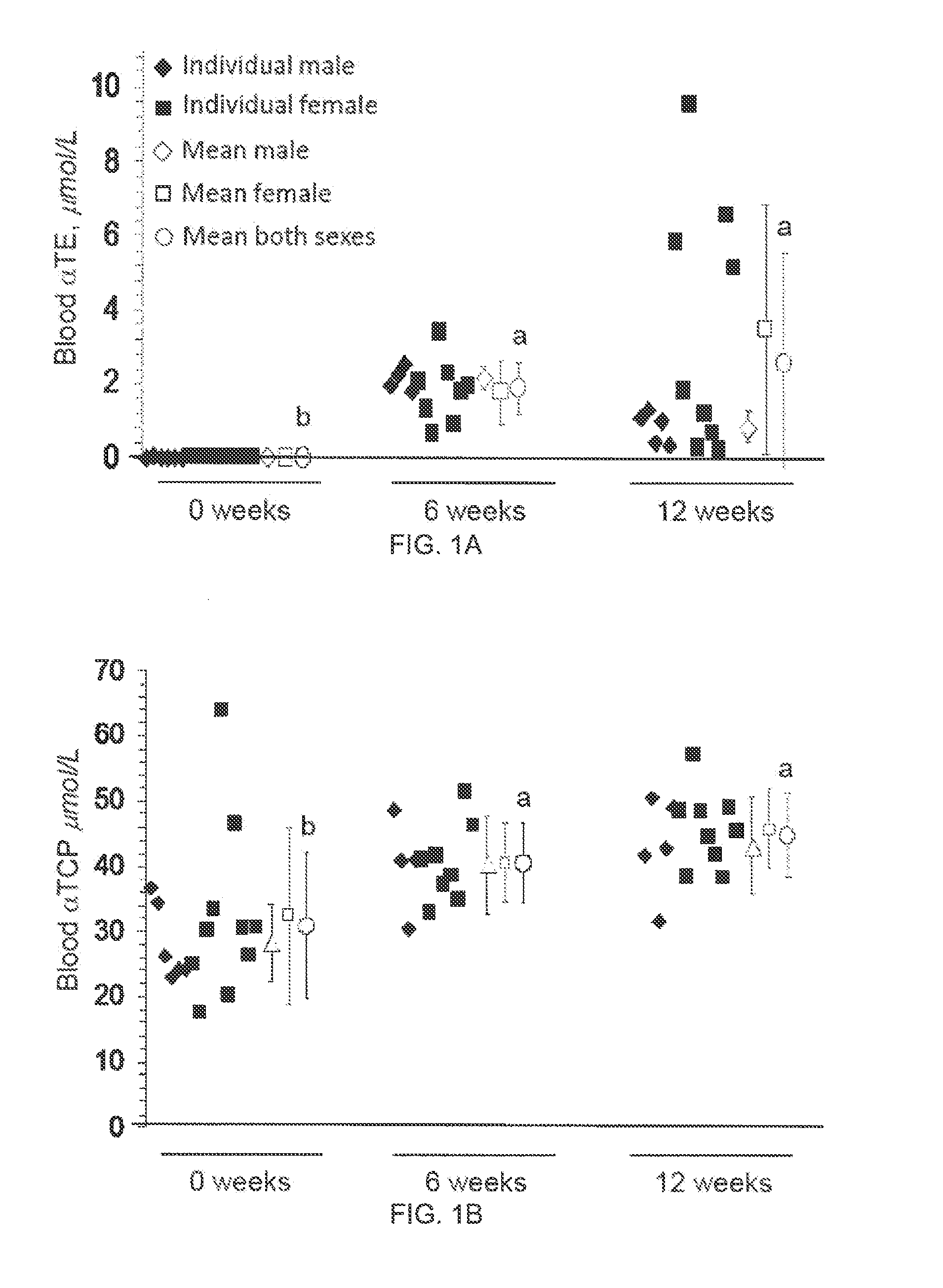
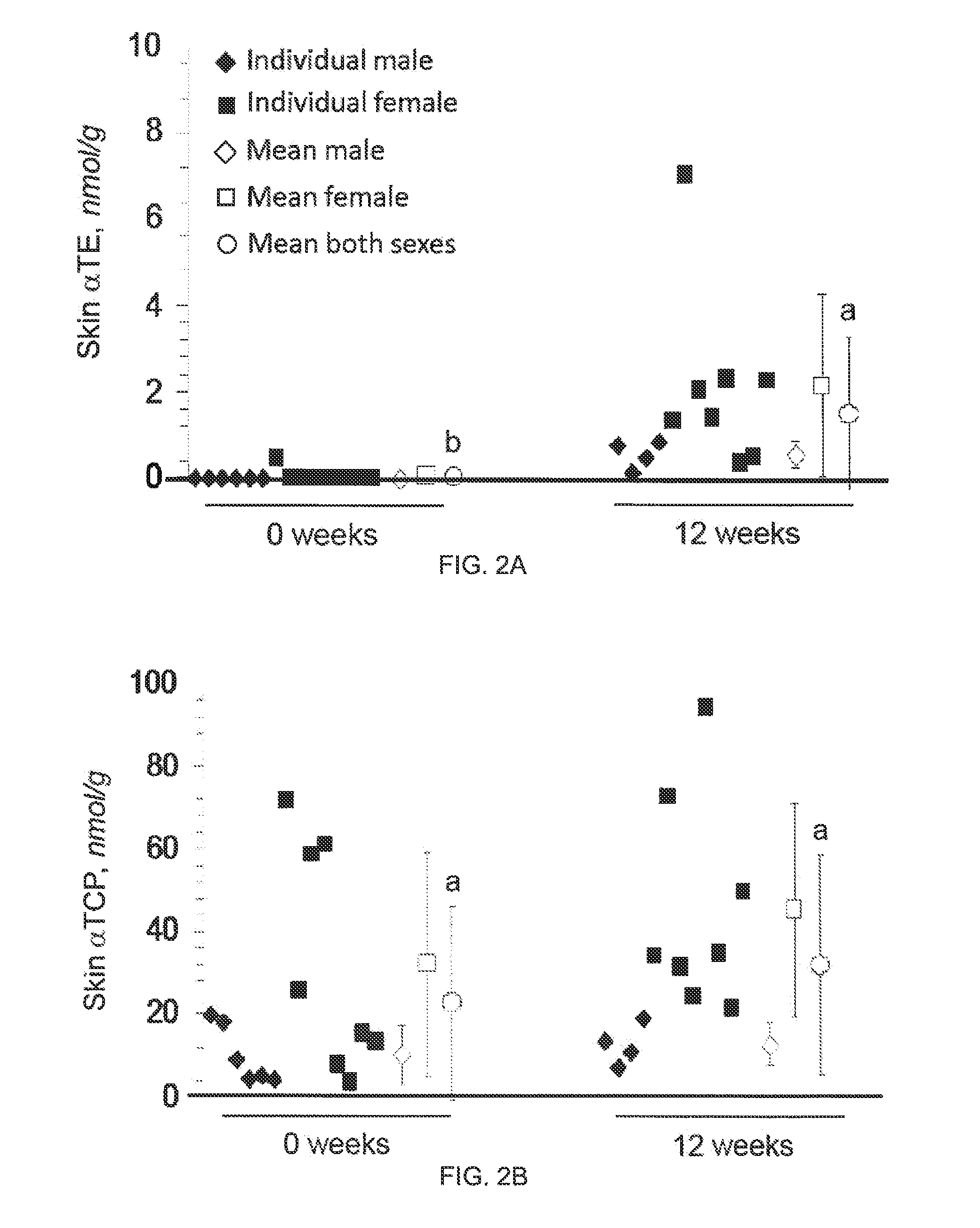
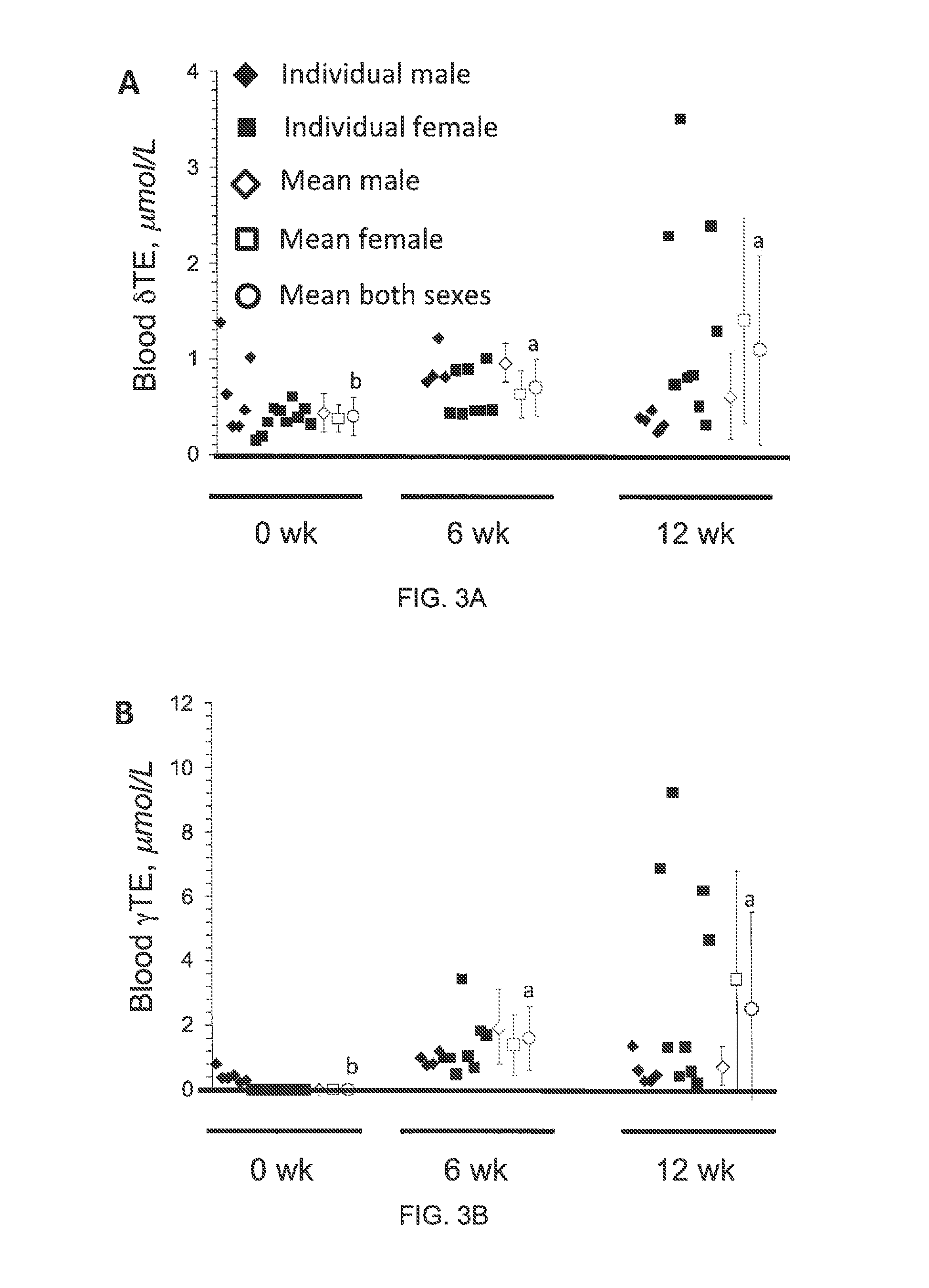
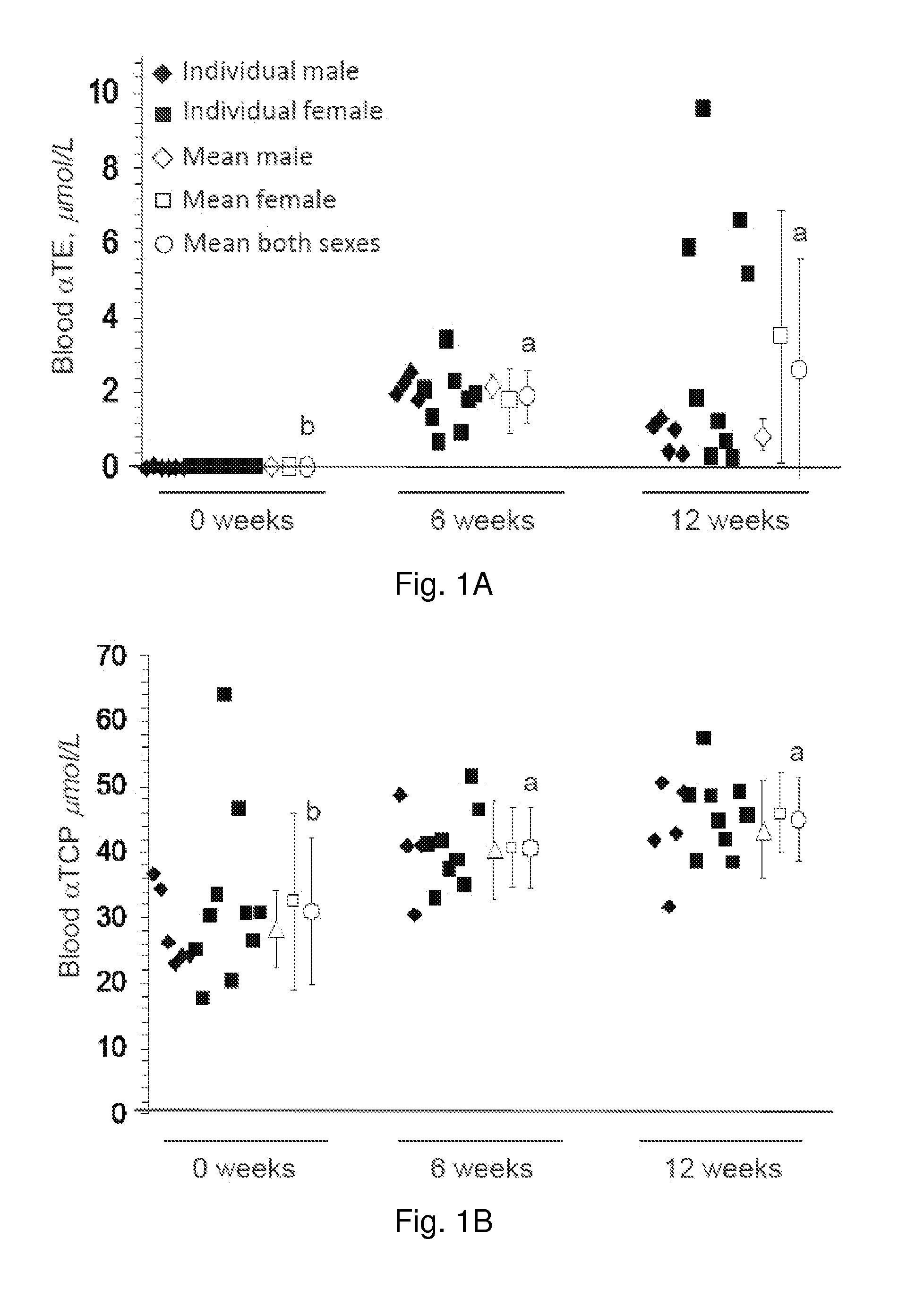
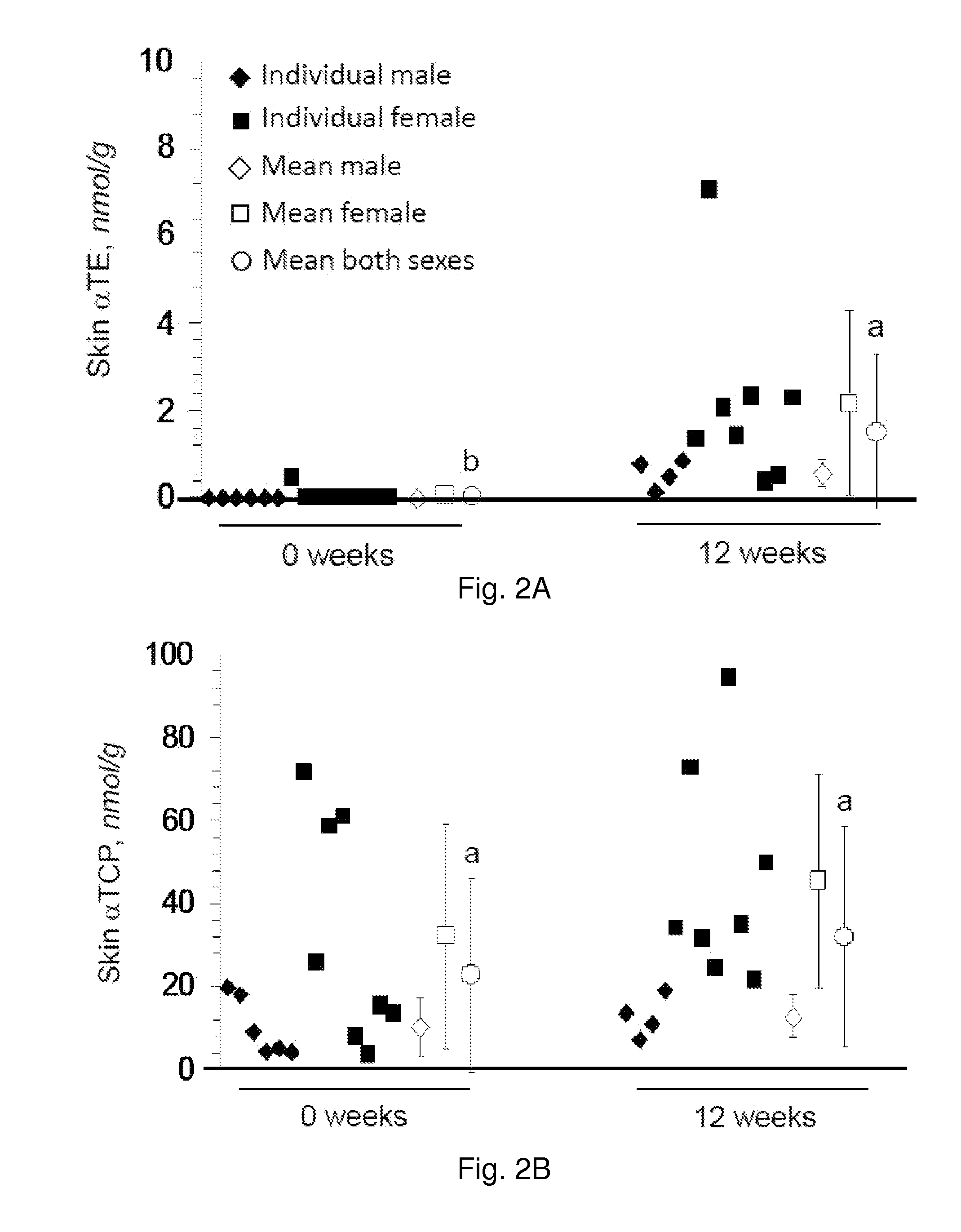
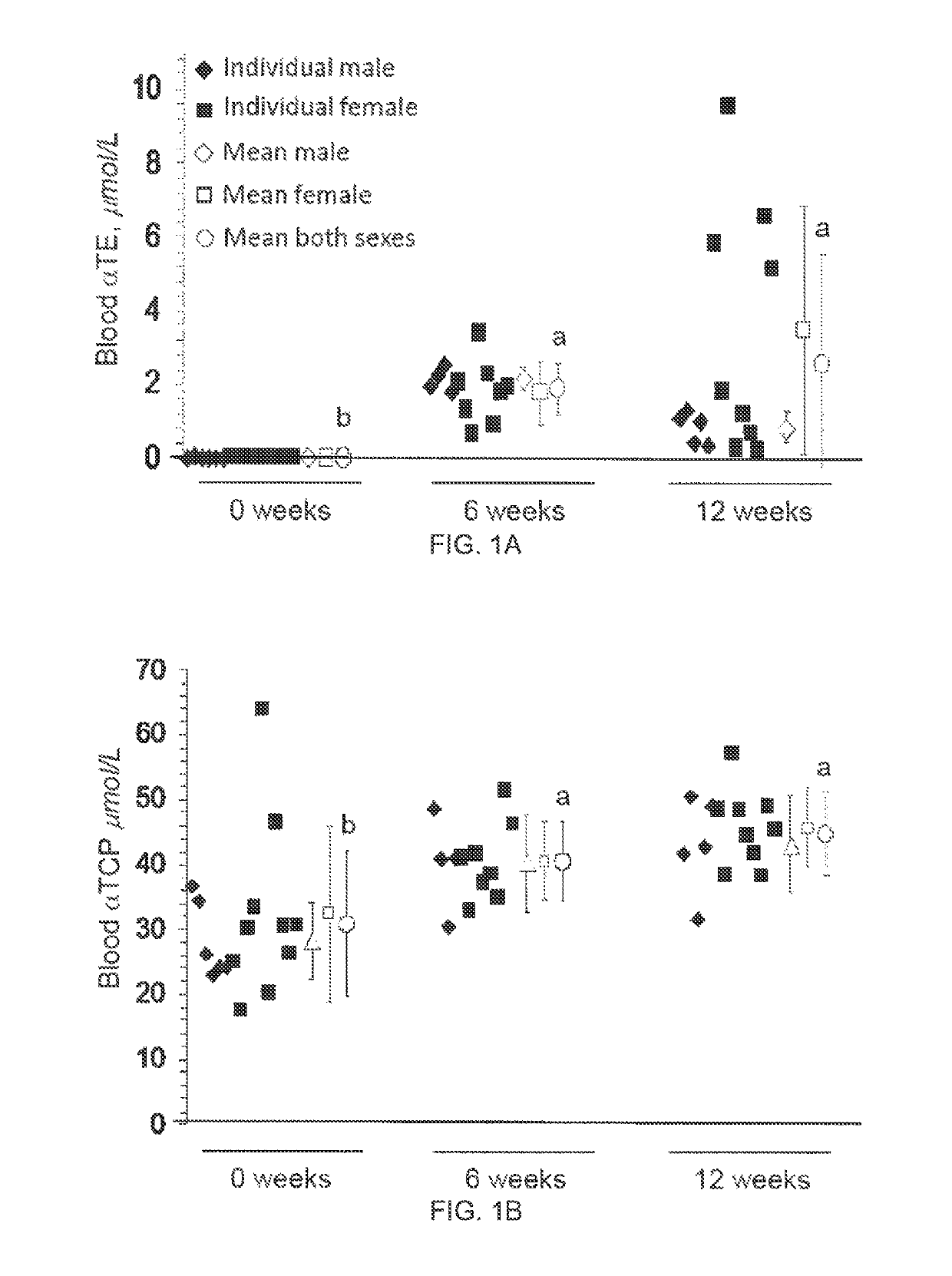
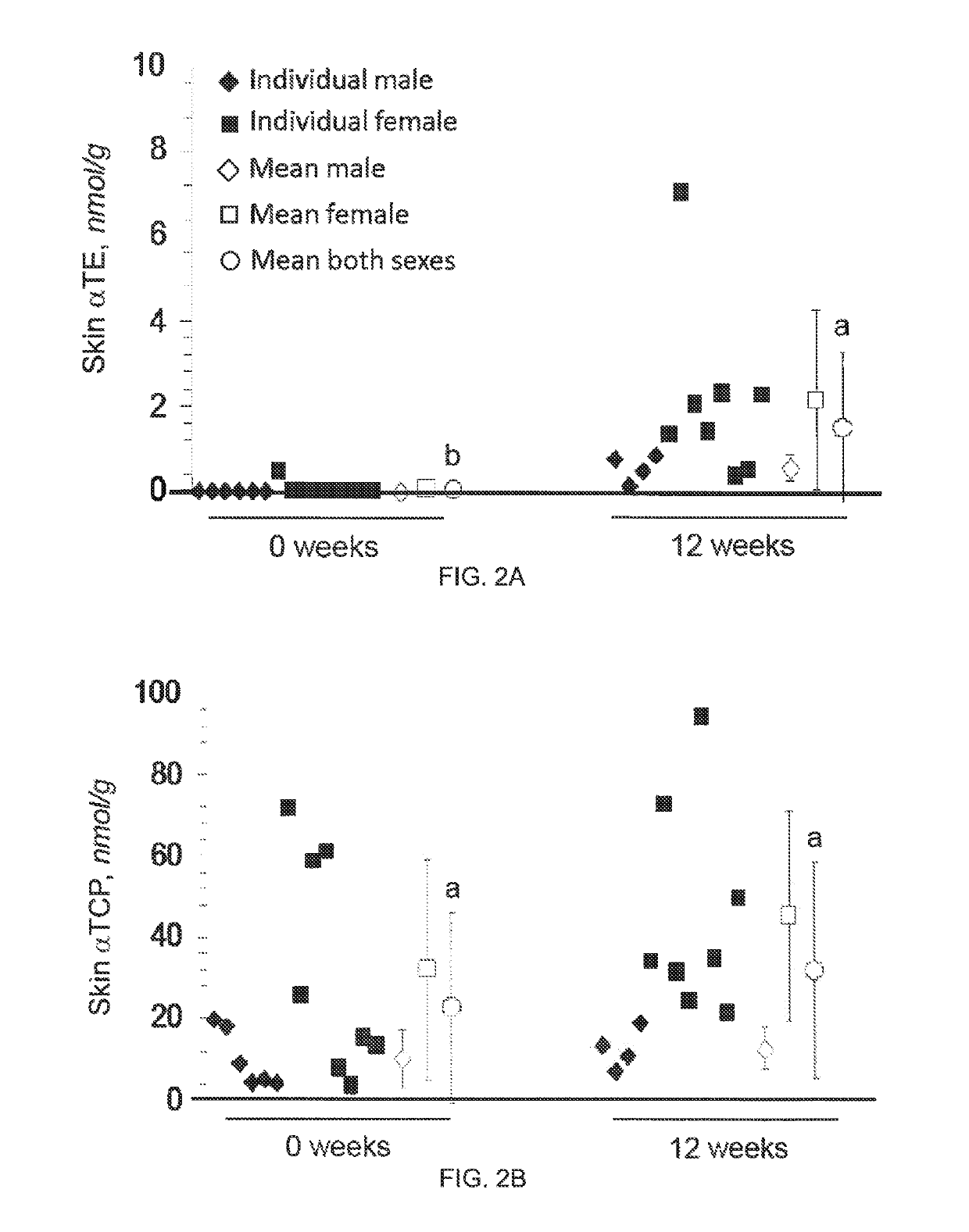
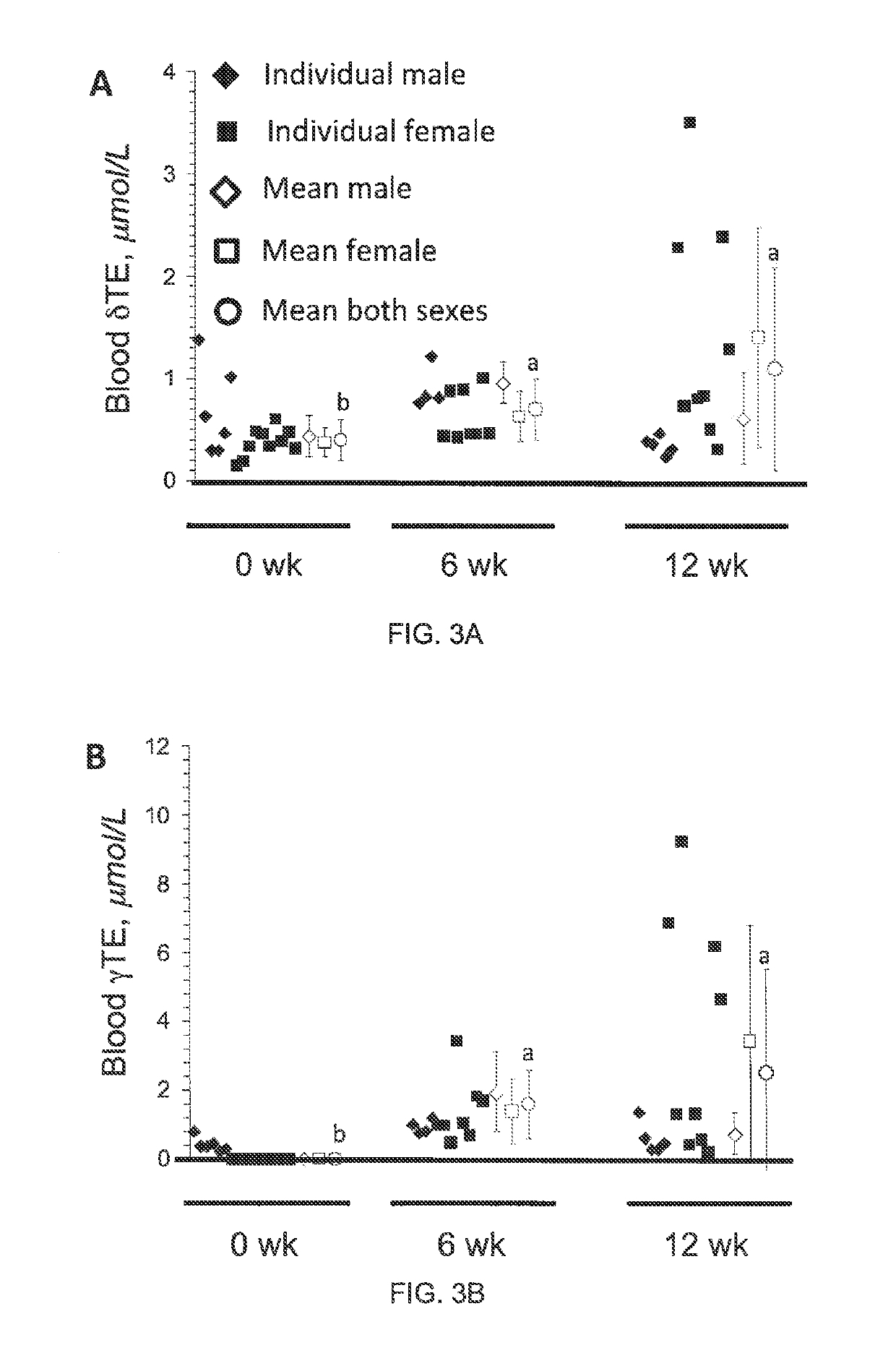
The present invention provides methods to reduce the number and severity of hot flashes utilizing tocotrienols. In particular, symptoms of perimenopause and menopause may be treated using the present methods. The present invention also provides methods to increase tissue concentrations of tocotrienols.
Owner:OHIO STATE INNOVATION FOUND
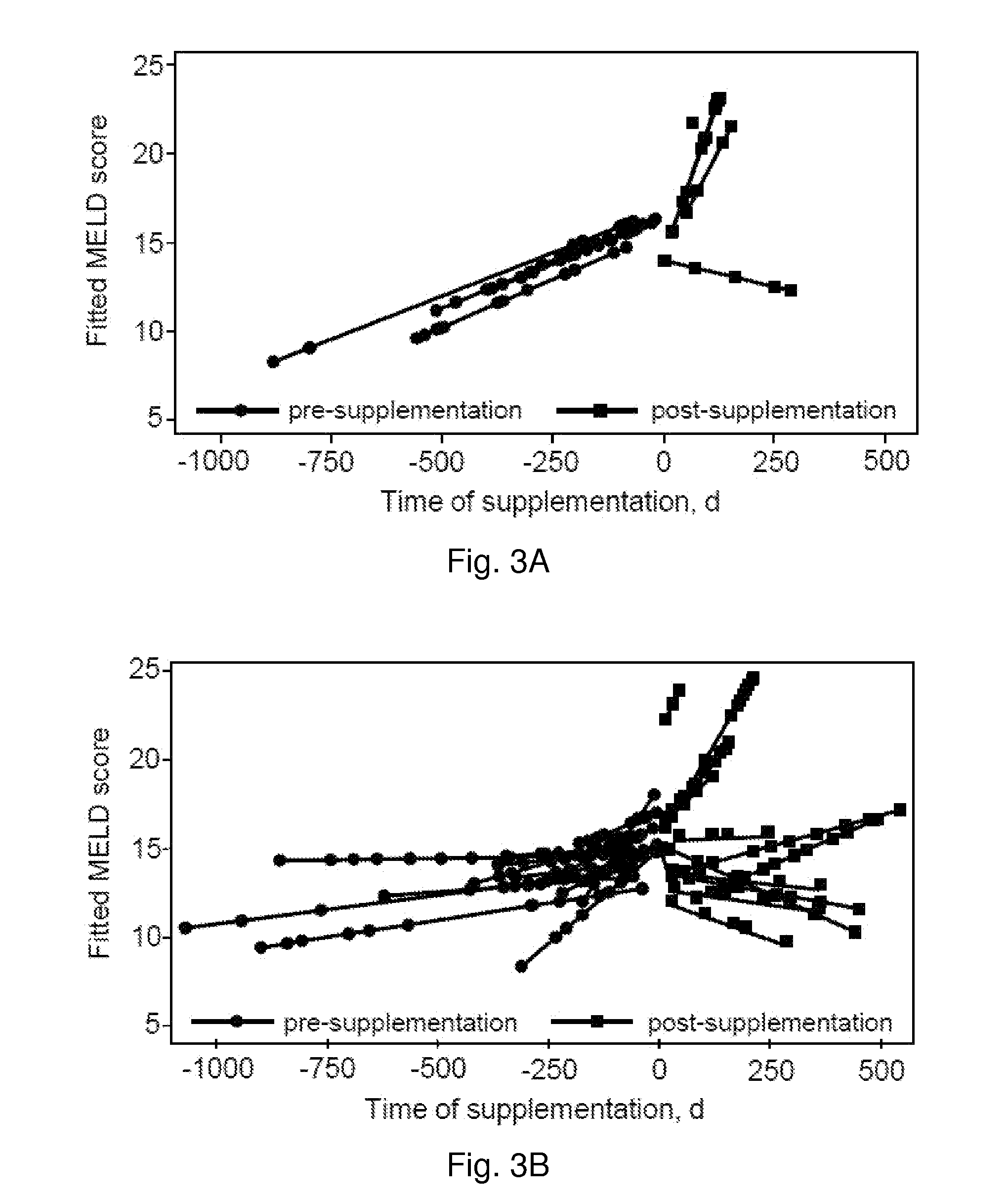
Methods for Improving Liver Function
InactiveUS20130309202A1Improve liver functionSlow riseBiocidePeptide/protein ingredientsTissue concentrationsPresent method
The present invention provides methods to improve liver function utilizing tocotrienols. In particular, various liver pathologies may be treated using the present methods, including cirrhosis, hepatitis, and sclerosing cholangtitis. The present invention also provides methods to increase tissue concentrations of tocotrienols.
Owner:OHIO STATE INNOVATION FOUND
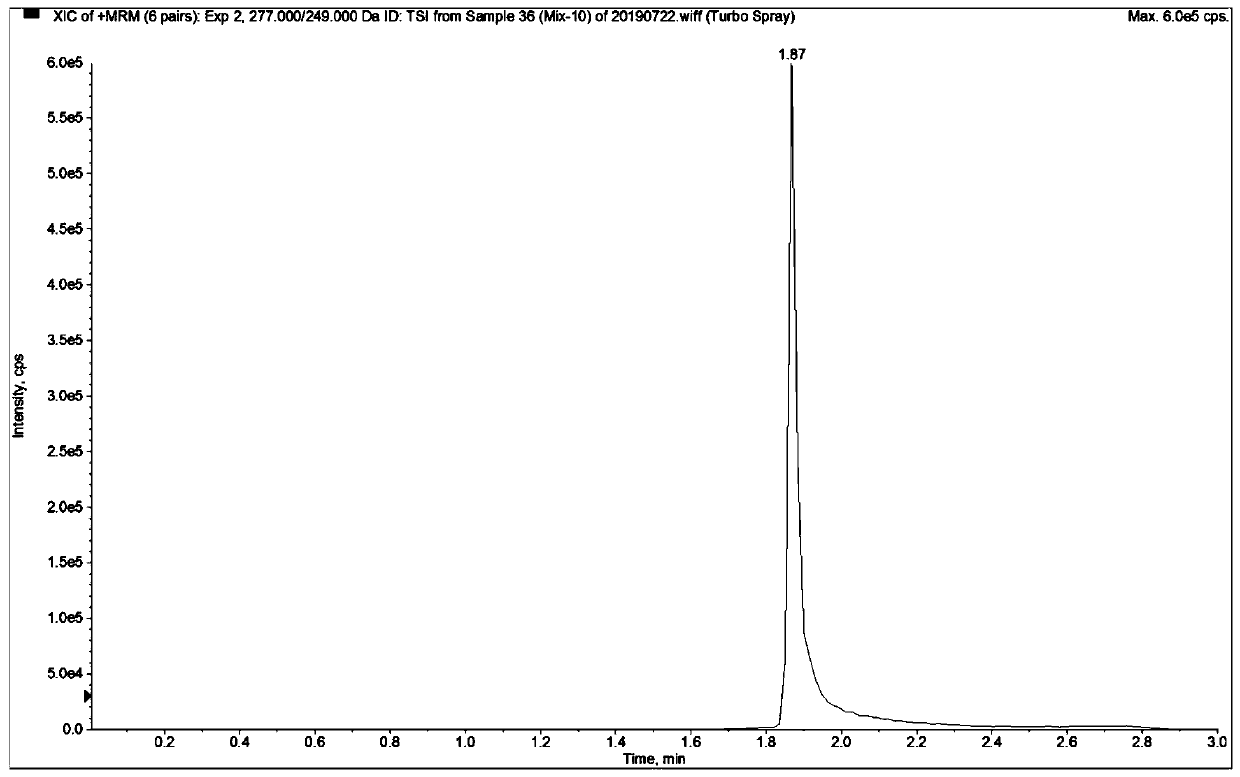
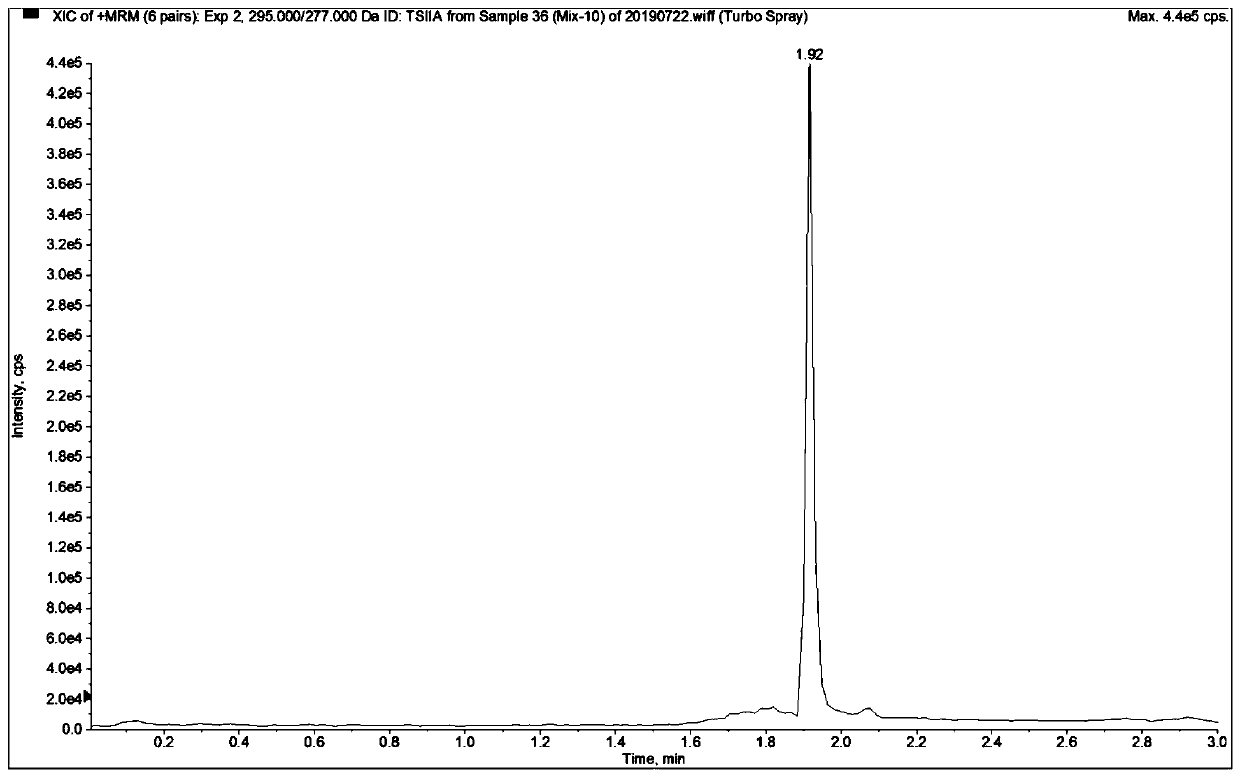
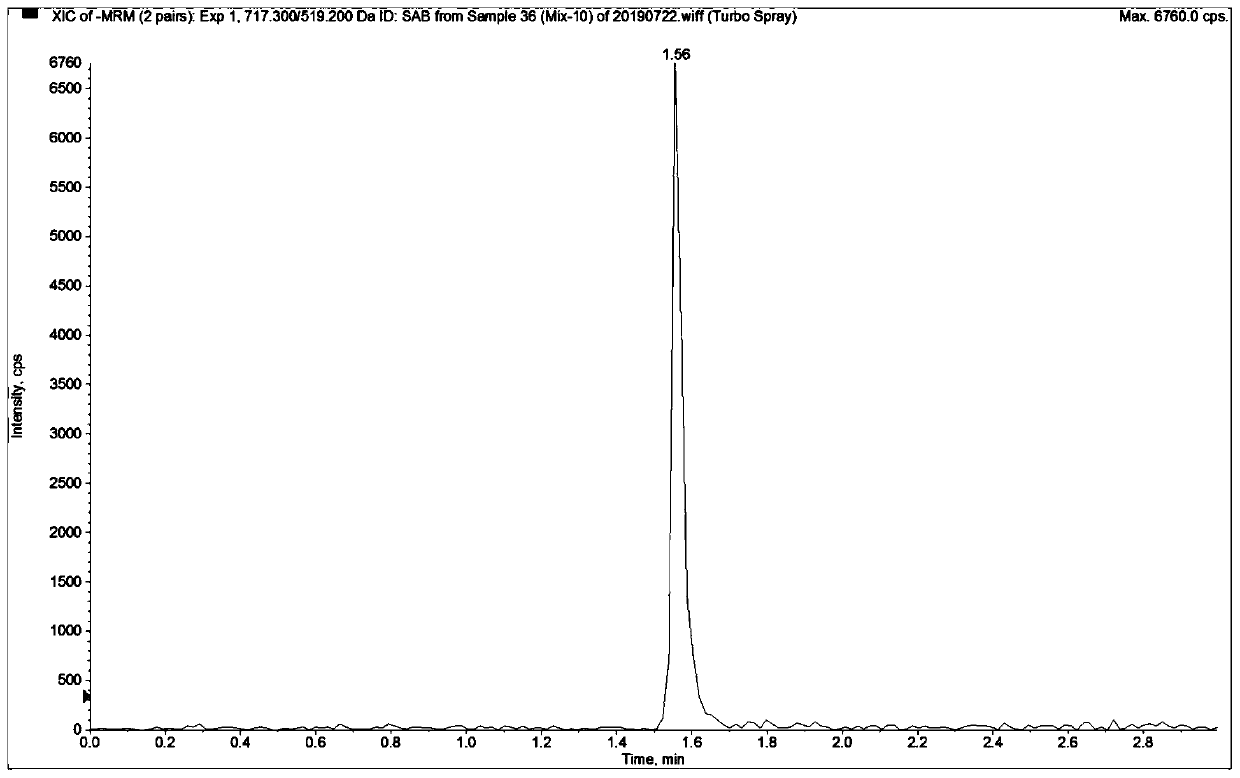
Mass spectrometric detection method for plasma or tissue concentrations of seven components of compound salvia miltiorrhiza preparation
ActiveCN110907581AThe pre-processing process is simpleGood peak shapeComponent separationSalvianolic acid BTanshinone IIA
The invention relates to a mass spectrometric detection method for plasma or tissue concentrations of seven components of a compound salvia miltiorrhiza preparation. The method comprises the followingsteps of (1) preparing a stock solution, namely respectively dissolving standard substances tanshinone I, tanshinone IIA, salvianolic acid B, tanshinol, notoginsenoside R1, ginsenoside Rg1 and ginsenoside Rb1 in a (80 + / -5)% methanol aqueous solution to prepare the standard substance stock solution; dissolving carbamazepine in a (80 + / -5)% methanol aqueous solution to prepare a carbamazepine stock solution; (2) preparing a working solution; (3) preparing a to-be-detected sample solution; (4) carrying out liquid chromatography separation on the to-be-detected sample solution obtained in the step (3); and carrying out mass spectrometric analysis on the sample after liquid chromatography separation. In the detection process of the method, the chromatographic peak shape is excellent, a channel of each compound only has a unique target peak, the interference of other effects, such as solvent effects, etc., is avoided, and the method is easy to operate and rapid to analyze, and is high in specificity and high in separation degree.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
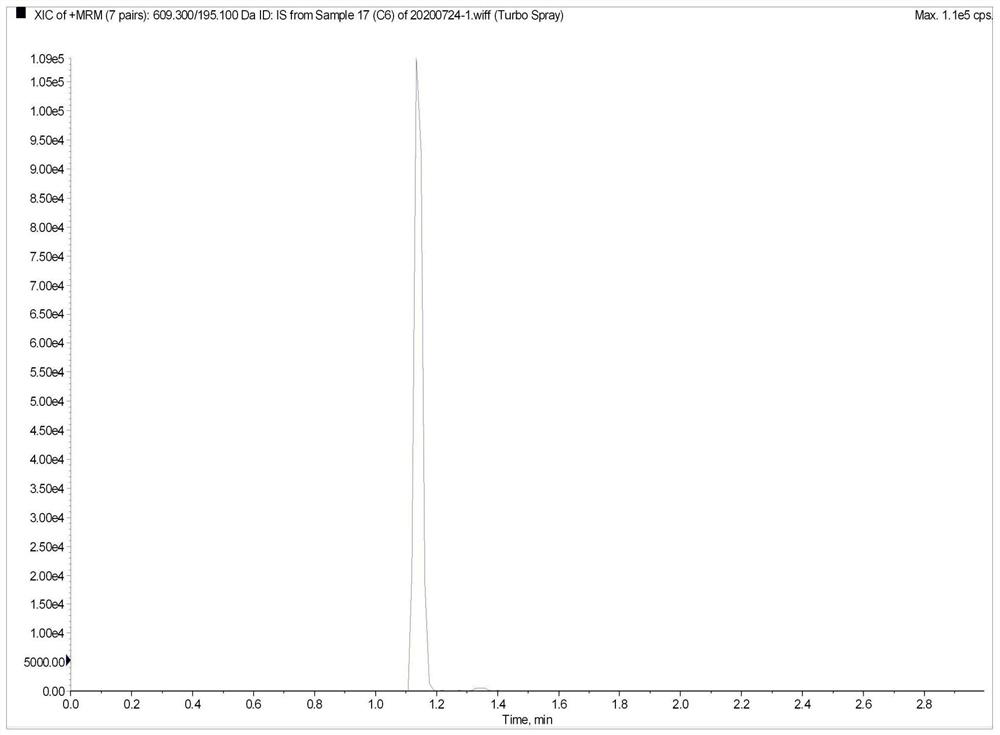
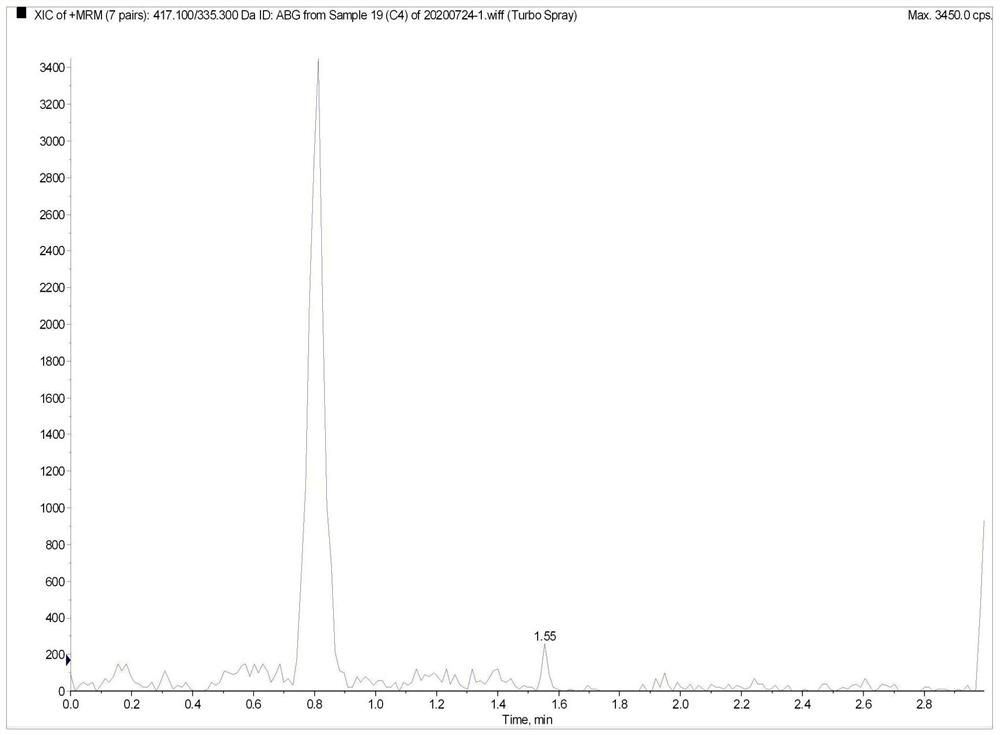
Method for the determination of plasma or tissue concentrations of six bufastenolide components in bufa skin-containing medicinal preparations
ActiveCN112198251BMeet quantitative requirementsEasy to operateComponent separationMedicinal herbsReserpine
The present invention relates to a method for detecting the concentration of six bufastenolide components in plasma or tissue in preparations containing bufa skin. The method comprises the following steps: (1) preparation of a stock solution: lipobufaxin, bufalin, cinobufogen, cinobufulin, celinobufagin and xenobufalin are respectively dissolved in (50 ±5)% acetonitrile aqueous solution to prepare standard stock solution; dissolving reserpine in (50 ± 5)% acetonitrile aqueous solution to prepare reserpine stock solution; (2) preparation of working solution; (3) Preparation of the sample solution to be tested; (4) subjecting the sample solution to be tested obtained in step (3) to liquid chromatography separation; subjecting the sample separated by liquid chromatography to mass spectrometry analysis. In the detection process of the method of the invention, the chromatographic peak shape is excellent, and the channel of each compound has only a unique target peak, which avoids other interferences such as solvent effects, and has the advantages of simple operation, rapid analysis, high specificity and high resolution.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
Mass spectrometry detection method of plasma or tissue concentration of seven components of compound salvia miltiorrhiza preparation
ActiveCN110907581BThe pre-processing process is simpleGood peak shapeComponent separationSalvianolic acid BTanshinone IIA
The invention relates to a mass spectrometry detection method for plasma or tissue concentrations of seven components of a compound salvia miltiorrhiza preparation. The method includes the following steps: (1) Preparation of stock solution: take standard products Tanshinone I, Tanshinone IIA, salvianolic acid B, Danshensu, and Panax notoginseng saponins R 1 , Ginsenoside Rg 1 and Rb 1 , were dissolved in (80±5)% methanol aqueous solution to prepare standard stock solution; carbamazepine was dissolved in (80±5)% methanol aqueous solution to prepare carbamazepine stock solution; (2) working solution (3) preparation of the sample solution to be tested; (4) separation of the sample solution to be tested obtained in step (3) by liquid chromatography; and mass spectrometry analysis of the sample separated by liquid chromatography. In the detection process of the method of the invention, the chromatographic peak shape is excellent, and the channel of each compound has only a unique target peak, which avoids other interferences such as solvent effects, and has the advantages of simple operation, rapid analysis, high specificity and high resolution.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
Methods for reducing the occurrence of hot flashes
InactiveUS10238629B2Reduce in quantityReduce severityOrganic active ingredientsCapsule deliveryTissue concentrationsPresent method
The present invention provides methods to reduce the number and severity of hot flashes utilizing tocotrienols. In particular, symptoms of perimenopause and menopause may be treated using the present methods. The present invention also provides methods to increase tissue concentrations of tocotrienols.
Owner:OHIO STATE INNOVATION FOUND
Calciparine/sodium salt nano oral preparation and preparation technique thereof
InactiveCN101249063BImprove stabilityHigh nano encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsHigh concentrationHigh absorption
The invention relates to a calcium heparin / sodium salt nano-sized oral preparation and a preparation method thereof, and a calcium heparin / sodium salt nano-sized liposome with high encapsulation rate for preparing the calcium heparin / sodium salt nano-sized oral preparation. The calcium heparin / sodium salt nano-sized liposome is obtained by the following steps: dissolving chitosan in 1-10% dilutedsolution of acetic acid (the usage amount of chitosan is 0.5-5g per 100ml acetic acid solution) to obtain an acidified chitosan solution, filtering, collecting filtrate, adding dropwise 1-99% aqueoussolution of calcium heparin / sodium salt into the filtrate under magnetic stirring until milk white precipitate occurs, centrifugating the resulating suspension, collecting precipitates, and freezes-drying to obtain calcium heparin / sodium salt nano-sized liposome. The calcium heparin / sodium salt nano-sized oral preparation is characterized in that the preparation has good stability, high encapsulation rate (up to 98%), and high in vitro release rate (up to 25% at hour 4); the preparation is absorbed in the intestinal tract with high absorption rate and high concentration; the half life is prolonged upon the increase of oral dosage, showing zero-order kinetics; the tissue concentration is larger than blood concentration; the preparation is released sustainedly; and study in rabbits (20mg oral intake) shows that the blood concentration in vivo is kept high for over 50 hours, thereby proving sustained release.
Owner:褚红女
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com