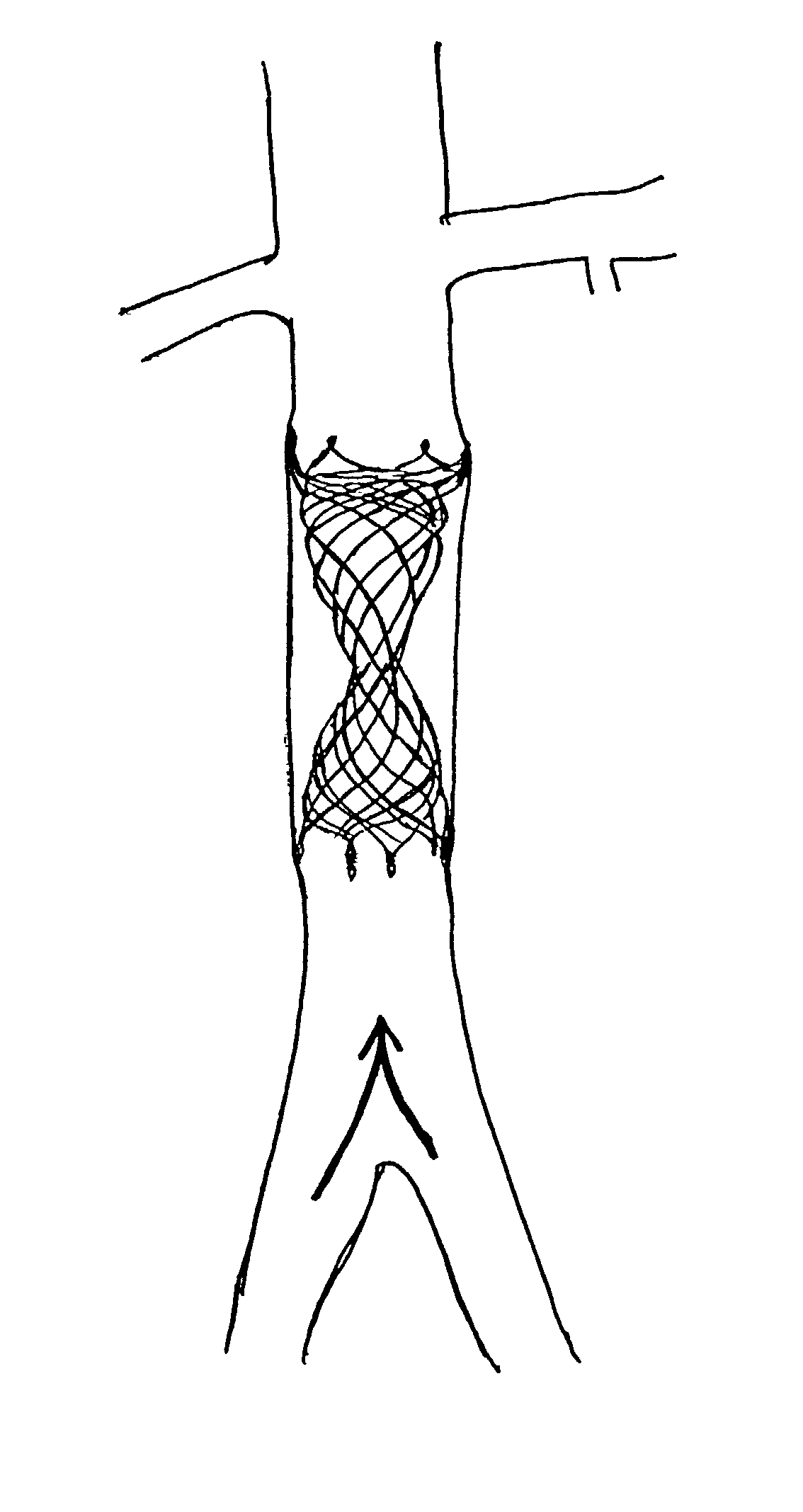
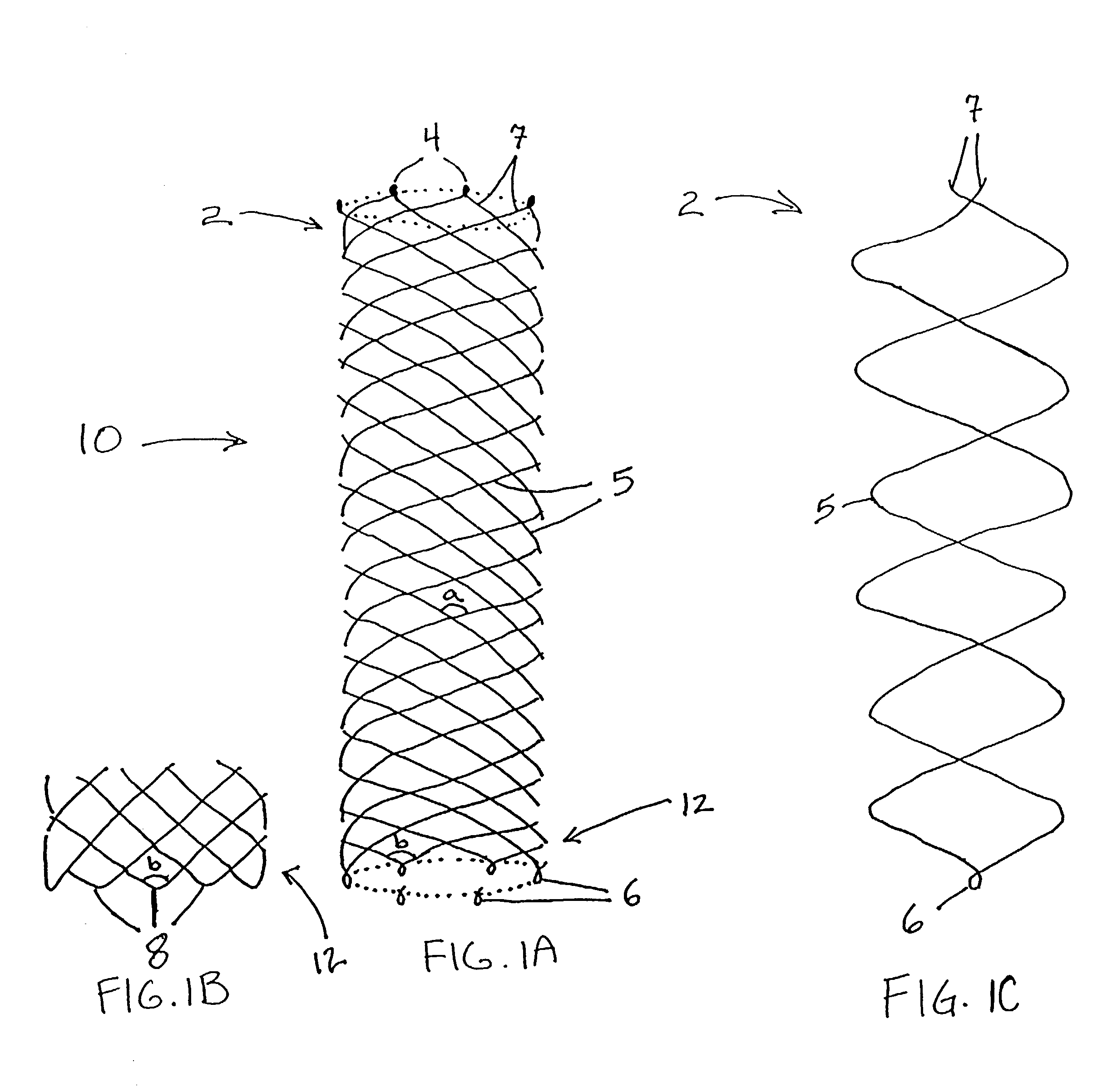
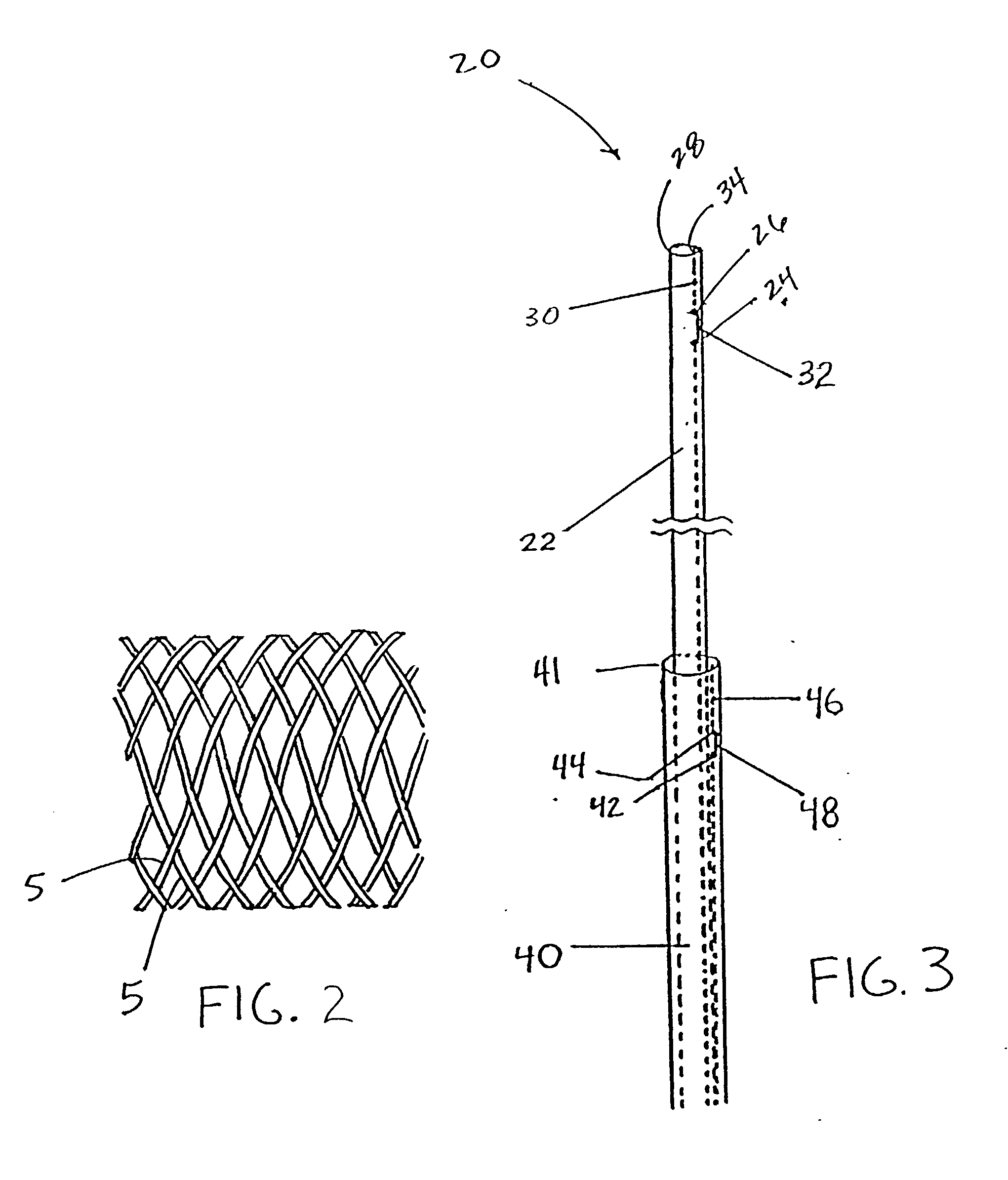
Any discrepancy between the diameters of the
stent and the vessel can result in a considerable elongation of the
stent.
The
disadvantage of a propensity for elongation is that great care must be taken when delivering such a stent in a vessel or non-vascular tubular structure in order to properly position it.
A further
disadvantage of intravascular devices formed using a
plain weave, is that they are often incapable of maintaining their shape when bent.
Consequently, the
diameter of the stent may exceed the
diameter of the vessel or structure through which it is traveling, impeding the delivery of the stent or causing the stent to lodge in the vessel.
This problem may be due in part to the use of weave materials such as stainless steel, which exhibit poor shape memory.
This problem may also be due to the free, unclosed wires used to form the stent.
The free sharp ends can create potential complications by penetrating, or perforating the wall of the tubular structure where such a stent is placed.
Further, steps that have been taken to eliminate the free, sharp ends, such as connection with U-shaped members using
welding, glue or the like (Wallsten, 1987) are time-consuming and expensive.
The delivery systems for such devices have also suffered from problems relating to the repositionability of the devices as they are delivered into position in the
living creature.
In stenting long arterial segments, the contiguously decreasing diameter of the arterial
system from the center to the periphery may
pose problems.
The aforementioned designs, however, are not capable of catching thrombi effectively at the periphery of the lumen so the patients will practically be unprotected against subsequent
peripheral embolization (Xian, 1995; Jaeger, 1998).
Further, most of filters tend to be tilted in the cava which can deter their
thrombus-capturing
efficacy.
Although coil type
occlusion devices have shown at least a degree of utility, they have a number of drawbacks that could be significant in some applications.
Moreover, a long vascular segment is often obliterated because of the frequent need for multiple coils and the coils often remain elongated within the vessel because their unconstrained diameter is larger than the
vascular lumen.
However, since optimal arrangement of the coil alone may not prevent migration in some cases, such as high flow conditions or venous placement, a coil anchoring
system has been devised (Knya et al., 1998).
Although an anchoring
system may stabilize a coil
conglomerate within the vasculature, significantly reducing or eliminating the possibility of coil migration, such a system may render the coil non-repositionable.
Although such non-coil devices may be repositionable, they too exhibit drawbacks.
For instance, the quadruple-disc device is several centimeters long in an elongated fashion, making difficult to keep the superselective position of the
catheter tip during deployment.
The multiple rigid connections between the
layers and the relative long and rigid connection between the occluder and the delivery cable further increase this drawback.
A common
disadvantage of both designs is that they lack guidewire compatibility.
Another relative disadvantage of both devices is their cost of manufacturing.
Because of the size of the delivery sheath, such a device is not suitable for the treatment of patients with a
body weight of less than 8 kg.
Using even a larger umbrella, this procedure is not recommended for the treatment of the lesions with a diameter of 8 mm or above (Latson, 1991).
One of the main drawbacks of the Rashkind umbrella is that it is not suitable for
occlusion of all types of PDA.
Longer PDA may hinder the discs to be positioned in the proper way, that is, parallel to each other, thereby deteriorating its self-anchoring.
Another disadvantage of the umbrella is that the occluding capacity of the design depends exclusively on the
thrombogenicity of the porous Dacron material, frequently resulting in partial and lengthy occlusion.
Formerly, detachable and non detachable balloons were used for this purpose, but they did not cause satisfactory ureteral occlusion.
Migration as well as deflation of the balloons occurred relatively frequently (Gunter, 1984; Papanicolau, 1985) leading to recurrence of the
urine leakage.
A
silicone ureteral occluder was developed and used with only limited success because of
device migration (Sanchez, 1988).
This resulted in repositioning and consequent incomplete ureteral occlusion.
The lack of appropriate self-anchoring results in coil migration which eventually deteriorates the occlusive effect.
Problems pointed out in the foregoing are not intended to be exhaustive but rather are among many that tend to impair the effectiveness of previously known stents, occluders and filters.
Other noteworthy problems may also exist; however, those presented above should be sufficient to demonstrate that previous techniques appearing in the art have not been altogether satisfactory, particularly in providing flexible, self-expanding, repositionable stents, occluders and filters.
As a result of only partially covering a body, blood or other bodily fluids may flow through the bare portion of the body relatively unimpeded by the graft material.
 Login to View More
Login to View More